A review of the Nunn modified single patch technique for atrioventricular septal defect repair
Introduction
Atrioventricular septal defect (AVSD) is a commonly encountered and challenging surgical problem, especially when associated with Tetralogy of Fallot (TOF). The definition, morphology and terminology of what constitutes an AVSD (canal defects, septal defects, etc.) are beyond the scope of this review. It is also agreed that the spectrum of AVSD from complete to incomplete have varying degrees of septal defects. The ‘ventricular component’ or ‘ventricular septal defect’ (VSD) of AVSD has numerous variations which are well known to surgeons and requires all of the care and attention to detail that any defect near the valves and the conduction tissue does. This VSD is an anatomical entity and stands in anatomical classifications of VSD’s alongside perimembranous, inlet, muscular, etc. Similarly, the ‘primum defect’ or ‘atrial septal defect’ (ASD) in AVSD is very different to most other isolated ASD’s and certainly again requires a great attention to detail to protect the inlet valves and conduction tissue. Different morphologists use different terminology to describe the ‘atrial’ and ‘ventricular’ components of AVSD. For the purpose of this review, we describe the atrial and ventricular ‘components’ of AVSD as ASD and VSD to make it easier for the reader.
Early surgical intervention is required to prevent future sequelae of mitral annular enlargement, ventricular dilation, pulmonary hypertension and cardiac failure. The goal of surgical repair is to eliminate significant residual shunting, especially any combination of interventricular communication and left atrioventricular valvar regurgitation (LAVVR) (1). Numerous techniques are described in literature based on number of patches used. Traditionally, a single patch or two-patch technique is used for repair of complete AVSD, of which the latter is more widely used. These repairs are complex with steep learning curve prompting Nunn and team to popularize the so-called modified single patch technique, which avoids the use of a VSD patch, thus greatly simplifying the repair. This procedure has different names: no patch technique, simplified single patch technique, Australian technique, modified single patch technique, and Nunn technique. The authors choose to use the term ‘Nunn repair’ as he (Dr. Graham Nunn) demonstrated the universal application of this procedure for all forms of AVSD and popularized the same.
In the last two decades, Nunn repair has revolutionized the operation for complete AVSD and has gained worldwide popularity. Many centers have adopted it as their procedure of choice. This technique involves bringing down the leaflets onto the crest of the ventricular septum, thus obliterating the VSD, and then closing the ASD with autologous pericardial patch. Long-term data is now available from various centers. Initially described for small VSD components, this technique is suitable for most, if not all, balanced AVSDs irrespective of VSD size, borderline left ventricular cavity, and associated TOF. Additionally, this method requires minimal manipulation of the valve leaflets, especially in Rastelli type C lesions. It also provides the advantage of being easily reproducible by surgeons at all levels on the learning curve. This review discusses all aspects of Nunn repair/modified single patch technique including the outcomes.
Historical perspective
Lillehei and colleagues [1955] reported one of the earliest repair of a complete AVSD using cross circulation (2). He directly attached the common atrioventricular (AV) valve leaflets to the crest of the ventricular septum, closing the VSD without a patch. This is probably the earliest account of ‘modified single patch technique’ and should be credited for performing the first procedure. In the late 1950’s and early 1960’s, Lillehei, Kirklin, McGoon, and Cooley all individually described using two separate patches of Ivalon or Teflon sponge to close both ASD and VSD. Maloney described the classic single patch technique in 1962 (3). They used a single Dacron patch to close both the ASD and the VSD and attached the leaflets to this patch (4). In the case of Rastelli type C lesions (which have a common superior leaflet), the leaflet had to be divided and re-attached to the single patch (5).
In 1976, Trusler described the so-called “conventional” two-patch repair consisting of Dacron patch closure of VSD, autologous pericardial patch closure of ASD, and repair of “cleft”/“zone of apposition” (6). The size of the ventricular patch used with the two-patch technique became smaller with time.
For the next two decades, the two-patch technique was the accepted gold standard. In 1997, Wilcox described their report of direct closure of small VSD’s with leaflet tissue and patching the ASD (7). However, Nicholson & Nunn’s team from Australia promoted the use of this technique irrespective of the size of VSD and published their ‘novel’ technique and initial experience in 1999 (8). In this technique, the common AV valves were attached to the crest of the ventricular septum obliterating the VSD and a patch of autologous pericardium was sandwiched between a small Dacron strip and the lowered AV leaflets to close the ASD. Dr. Nunn found that this method was applicable to alteration for variations in leaflet anatomy, sizes of VSD, and ventricular cavity. This technique is popularly known as the Australian repair or Nunn repair (1,9). Elimination of the extra suture line is associated with shorter total bypass and ischemic times (8). They subsequently published more of their experience in 2004 and 2007 (9,10).
Surgical technique
For the sake of this review, we would like to highlight the original steps described by Nunn and his colleagues (8-10).
Set up
The operation is performed through a median sternotomy using conventional full flow cardiopulmonary bypass (CPB) via aortic and bicaval cannulation with moderate systemic hypothermia. The aorta is cross-clamped and antegrade cold blood cardioplegia is delivered at 20-minute intervals. The left ventricle is vented through right upper superior pulmonary vein. A right atriotomy is performed parallel to the AV groove starting from the right atrial appendage to just above the inferior vena cava.
Steps of repair (Figures 1,2)
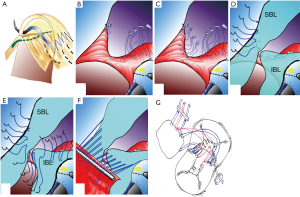
- Interrupted pledgetted 5-0 Polyester (Tevdek, Deknatel Inc, Fall River, MA, USA) mattress sutures are taken from the RV aspect of the inter ventricular septum (IVS) well away from septal crest. The first suture begins at the tricuspid annulus to right of AV node and lies within the leaflet rather than in the annulus (Figure 1A). The subsequent sutures are taken from the RV aspect of the septum starting from under the inferior bridging leaflet (Figure 1B). The sutures are then placed beneath the superior bridging leaflet progressing along the septum towards the middle (Figure 1C).
- These sutures are then taken through the superior and inferior bridging leaflets, thus separating the common valve into the left and right AV valves at the IVS crest. Attempt is made to preserve as much of left AV valve (mitral) as possible. If the bridging leaflets need division, then the suture is taken through the edges of both AV valve components thus approximating them at the septum (Figure 1D,E). Notice that the two ends of middle suture is passed through the apposing edges of the bridging leaflet (Figure 1F,G).
- These sutures are now brought through the pericardial patch edge passing them through the Dacron strip (is measured at 80% of the exposed crest of IVS) (Figure 1F,G). The shorter length of the Dacron strip reduces the annulus size and provides an anchor that allows better leaflet coaptation and acts as a mitral annuloplasty (this seems to be the most neglected step when doing this procedure). The Dacron strip also supports the suture line and aims at better coaptation of the bridging leaflets. The sutures are tied, approximating the atrioventricular leaflets to the crest of IVS thus closing the VSD (Figure 2A). The two ends of the central suture are left uncut (to be used subsequently to close the “cleft”).
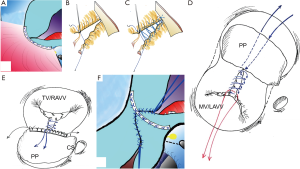
- The “cleft”/“zone of apposition” created by apposition of superior and inferior bridging leaflets is then closed. A nerve hook is used to retract and expose the chordal leaflet junction (Figure 2B). A temporary 7/0 Prolene (Prolene, Ethicon Inc. Somerville, NJ, USA) retraction suture is placed at orifice end of the “cleft” at junction of the chordae and the leaflet and placed on tension. This facilitates apposition of the two leaflet components for suture placement and prevents concertina effect (Figure 2B-D). The two ends of the central interrupted suture, which passes through both the apposing bridging leaflets in the previous step, are passed through the pericardial patch from the right side and used to close the left sided “cleft” in two continuous layers. The suture ends are then passed back through the pericardial patch and tied on the right side (this step ensures that there is no residual leak at the base of the “cleft”) (Figure 2C,D). In addition, if there is a significant leak on the right side, the same suture is used to close the right sided “cleft” and tied together on the right side (Figure 2E,F). To expose the right sided “cleft” a retraction suture is placed at the edge of the “cleft” similar to the one above (Figure 2F).
- The valve is tested by filling the ventricle with saline. If necessary, commissural annuloplasty is performed to improve valve competence (per their report, Nunn and team do not routinely insufflate the ventricle prior to repair).
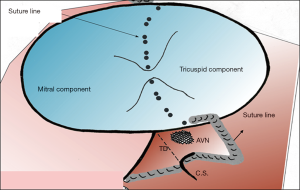
- The primum ASD is now closed with the pericardial patch using a continuous 7/0 prolene (Prolene, Ethicon Inc., Somerville, NJ, USA) suture starting from the annulus of the inferior bridging leaflet. Posteriorly the suture line courses around the AV node ensuring bites through the fibrous tissue, going straight onto the atrial wall and heading towards the anterior lip of the coronary sinus thus ensuring its drainage into the right atrium (Figure 3). The suture continuous around the inferior rim of the primum ASD. A second 7-0 Prolene suture is started from the annulus of the superior bridging leaflet and is used to close the superior rim of the primum ASD.
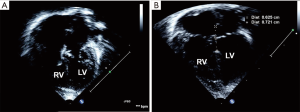
- The atrium is closed in standard fashion with running polypropylene suture in two layers. The patient is re-warmed, heart is de-aired, and cross clamp is removed. The patient is subsequently weaned off CPB. The pre and post-operative ECHO images are shown in Figure 4.
Nunn and colleagues reported the use of modified single patch technique to also successfully repair patients with TOF and AVSD (7). The steps include:
- Prior to septation, the infundibular resection is performed as usual in a case of TOF.
- Care is taken to preserve AV valve competence. More pledgetted sutures are usually taken through the right side of the VSD crest to account for the longer septal crest and to avoid possible left ventricular outflow tract (LVOT) obstruction (Figure 5A).
- A trans-annular patch with monocusp or a RV-PA conduit is commonly used to augment the right ventricular outflow tract (RVOT) (1).
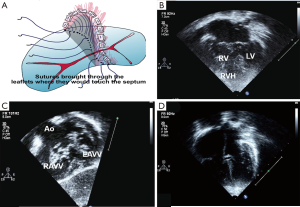
Pre-operative and post-operative ECHO images following TOF-AVSD are shown in Figure 5B-D.
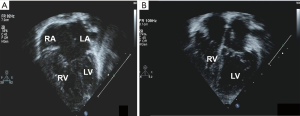
Nunn technique can also be applied for complete AVSD with borderline small LV (Figure 6A). Care is taken to preserve as much of the left AV valve tissue as possible, keeping the suture line on to the right AV valve tissue. The left ventricle cavity seems to improve post operatively (Figure 6B).
Over the years, surgeons all over the world have modified the steps in variance to the original technique described by Nunn and team. The common variations include using different suture materials, non-pledgetted sutures, not using the Dacron strip, using interrupted sutures for the closure of the cleft, redirecting the coronary sinus to the left atrium, using other patch materials for closure of ASD, and plication of the patch on the left side. However, the basic principles of Nunn technique or modified single patch technique remain the same.
Data for all AVSD repairs
McGoon was one of the first to describe a successful repair of AVSD in an infant under 12 months old. Since then, most patients with AVSD are advised repair in the first year of life. Most centers adopted the two-patch technique. The results are variable, with left AV valve regurgitation being one of the main complications. Ginde and team published their long-term follow up of 198 patients who underwent AVSD repair between years 1974 and 2000. The overall survival for the entire cohort was 85% at 10 years, 82% at 20 years, and 71% at 30 years and the freedom from reoperation was 88%, 83%, and 78% at those time intervals. Indications for reoperation included left AV valve regurgitation (7.1%) and LVOT obstruction (3.5%). The authors identified that a reoperation was a risk factor for late mortality (11).
Suzuki and colleagues retrospectively reviewed results of the two-patch technique in 116 patients from February 1997 through October 2002. Survival at 1, 3, and 5 years was 98%, 95%, and 95% respectively. At discharge, 68% had trivial to mild and 3% had moderate to severe left AV valve regurgitation. The overall freedom from reoperation at 1, 3, and 5 years for left AV valve dysfunction was 94%, 89%, and 89%, and for LVOT obstruction was 100%, 93%, and 90%, respectively. They concluded that the traditional two-patch technique is a reproducible and safe technique with low mortality and good midterm outcomes even in very young infants (12).
Other surgeons routinely used the “classic single patch” technique. Early experience with this repair showed difficulty in attaching the bridging leaflets to the patch, which led to post-operative left AV valve dysfunction, residual VSDs, conduction abnormalities, and pulmonary hypertension (4,8). However, there were some favorable long-term studies. Crawford reported a large series of 172 consecutive patients who underwent the above technique. The mean age at operation was 10.8 months. Operative mortality depended on decade of operation, use of crystalloid cardioplegia, and postoperative time on ventilator. Overall mortality was 8.7% and decreased significantly from 16.4% in the first decade to 3.0% in the second decade of performing the operation. Overall survival was 79% at 15 years. Freedom from late re-operation for severe mitral regurgitation was 89.9% at 15 years and did not decrease in the second decade (13).
Draulescu et al. studied 107 patients with complete AVSD who underwent the classic Rastelli one-patch repair between 1984 and 2005. They used a single autologous glutaraldehyde preserved patch for VSD closure, re-suspension of the leaflets, and closure of ASD. In 70 patients, the “cleft” was left open, 11 had RVOT procedure and three had coarctation repair. The overall in-hospital mortality was 13%, however in the latter years this decreased to 4% due to improvements in perioperative management. Early survival was 86%. Five patients had early reoperation (2 for residual VSD closure and 3 for mitral valve repair) and nine patients had late reoperations (4 for sub-aortic stenosis, 5 for mitral valve repair) with success. Overall survival at 10 and 15 years was 84%. Freedom from reoperation for mitral regurgitation was 94% at 10 years, and 91% at 15 and 20 years (14).
Emergence of data supporting the Nunn technique
Since 1999, multiple surgeons have published outcomes related to the Nunn modified single patch technique. Nicholson and colleagues reported in 1999 on 47 patients who underwent the Nunn technique with a mean age at repair of 5.6 months. They found no heart block, no significant residual VSDs, and no LVOT obstruction. There was no mitral valve incompetence in 28% of patients and minimal to mild incompetence in 66%. Only 6% had moderate mitral valve incompetence. They concluded that the modified single-patch technique simplifies the repair with preserved valve function with no LVOT obstruction (8).
Subsequently, Nunn published a more detailed long-term follow up of modified single patch technique for the repair of complete AVSD in 128 patients. Thirty-day mortality was 1.6% and incidence of mitral valve re-operation was 2.3%. There were no patients with residual VSD’s or LVOT obstruction (10).
These results prompted other units to adopt this technique and the published data is positive. In 2010, Jonas and Mora examined 33 patients who underwent Australian repair irrespective of the VSD size (88% had moderate to large VSD). In the balanced canal subgroup, there was no early mortality and two patients required reoperation: one mitral valve repair for cleft dehiscence at 1 year and the other a patient with heterotaxy who required pacemaker implantation. In the unbalanced canal subgroup, two patients (22%) died in perioperative period. There were no late deaths or new LVOT obstruction in either subgroup (5).
In 2010, Halit and colleagues published their data of 26 patients who underwent AVSD repairs. Fourteen had traditional single patch technique (Group 1) and 11 had Australian technique (Group 2). The mean CPB and cross-clamp times in the Australian, technique group was 78.8 and 54.4 minutes, compared to 133.2 and 79.4 minutes in the traditional single patch group, which was a statistically significant reduction. There was no significant difference in early postoperative deaths or incidence of post-operative left AV valve regurgitation (LAVVR) and no patients from either group had LVOT obstruction (15).
Salihoglu and colleagues published their report of thirteen patients who underwent direct closure technique of partial and complete AVSDs. The median age at operation was 13 months for partial AVSDs and 7.5 months with complete type. There was no in-hospital mortality. At discharge, all patients had sinus rhythm with less than moderate AV valve regurgitation (16).
Tagliari et al. also published their results of Nunn technique in 16 patients. They had two hospital deaths (12.5%), three patients required reoperation for the left AV valve, and two patients had heart block requiring pace maker. No patient had LVOT obstruction. At a mean follow-up duration of 55 months, the 14 surviving patients remain asymptomatic and ten had mild LAVVR (71%) (17).
In 2014, Salah reviewed twenty patients with complete AV canal defects who underwent total repair using the modified single patch technique between January 2011 and March 2013. The breakdown by Rastelli type: 75% type A, 10% type B, and 15% type C. Follow up was 1 year. The mean cross-clamp, CPB, and total operative times were 59, 74, and 197 minutes respectively. At 1 year, 88% of patients had follow up echocardiography and none of these patients showed residual shunts or LVOT obstruction. Additionally, 93% had trivial to mild LAVVR and 6% showed moderate regurgitation. He commented that once it was determined that direct closure is feasible; the technical aspects were less daunting. However, the author expresses a concern that any left AV valve deformity, LVOT obstruction, or residual VSD would further deteriorate after repair with the modified single-patch technique (18).
More recently, a study by Li and colleagues early in 2017 included 15 publications involving 1,034 patients. Their results showed that the VSD size was significantly smaller in the modified single patch group. However, they also had lower cross-clamp and CPB times and a shorter length of stay compared to those who underwent two-patch repair. They found no significant differences between the two groups in other postoperative outcomes including mortality, LVOT obstruction, residual VSD, complete heart block, and need for reoperation, though they commented that these outcomes have been improving across all repairs (19).
Comparative literature
Others have compared this technique to the more established techniques and highlighted the ease of surgery, shorter clamp and bypass times and lower complication rates. Backer and colleagues published multiple papers on the topic. In 2007, they described 55 infants who underwent complete AVSD repair, 26 with a modified single patch technique and 29 with a two-patch technique. Rastelli classification breakdown was similar between the two groups, though more Rastelli C lesions underwent the two-patch technique. The mean size of the VSD by trans-esophageal echocardiogram was not significant. Cross-clamp times and CPB times in the modified single-patch group were shorter (97 vs. 123 minutes, P=0.0003; 128 vs. 157 minutes, P=0.03). The median postoperative length of stay was similar (10 vs. 8 days). There was one death in the modified single patch group and none in the other group. One patient from the modified single patch group had mitral valve re-operation in comparison to two in the latter group. No patient had a residual VSD or third degree atrioventricular block in the modified single patch technique group whereas there was one patient each who had the above complications in the two-patch group. No patient in either group had re-operation for LVOT obstruction. They concluded that the results of modified single-patch technique were comparable to the two-patch technique in younger patients with similarly sized VSDs and is performed with significantly shorter cross-clamp and CPB times (20).
In 2009, Backer performed a meta-analysis of modified single patch technique (combined experience of Dr. Nunn, Dr. Richard Jonas and their group) with the data for two-patch and classic single patch techniques. The rate of reoperation for LAVVR was only 2% in the former compared to 7% in the two-patch group and 9.7% in the classic single patch group. Similarly, the rate of reoperation for LVOT obstruction in the two-patch group was 5%. In the long-term follow up of Nunn’s earliest patient, none required reoperation for LVOT obstruction. Backer argues that the prosthetic patch used to close the VSD in the two-patch technique may act as a rigid foreign body causing turbulence of blood flow and promote fibrosis leading on to LVOT obstruction (21).
Pan and colleagues published a comparative study of modified single patch technique (46 patients) and the two-patch technique (59 patients) between 2001 and 2011. There was one death in each of the groups. The aortic cross-clamp and CPB times were shorter in the modified single patch group (70 vs. 83 minutes, P=0.004; 95 vs. 110 minutes, P=0.011). There was no patient with heart block in the first group, but two patients required pacemaker in the two-patch group. Late deaths were one and three in respective groups. Four patients with modified single patch repair developed late LAVVR compared to six patients with two-patch repair. Three and five patients respectively in each group underwent late reoperation. One patient in the modified single patch group required reoperation for a residual VSD compared to no patients in the two-patch group. They concluded that the results in both groups are comparable and the modified single patch repair is a safe and reproducible technique (22).
As more data was available on Nunn technique, Backer published a more detailed meta-analysis of all three approaches: Nunn technique (272 patients), two-patch (889 patients) and classic single patch technique (350 patients). The mortality in the groups was 1.2%, 3.5%, and 4.9% respectively. Left AV valve reoperation rate was 2.2%, 7.2%, and 9.7%. Heart block requiring pacemaker was 0.4%, 1.9%, and 2.3% respectively. Given the shorter clamp and CPB times and associated low mortality and morbidity, the modified single patch technique is their recommended repair method. They also noted that the modified single patch technique is safe in small infants and may decrease the risk of late pulmonary hypertension (23).
Stephens et al. reviewed 139 patients who underwent complete AVSD repair from January 2005 to December 2012. They used a selective approach. Ninety-eight patients had two-patch repair for large VSDs and 41 patients had Nunn technique for “shallow” VSDs. Children with trisomy 21 were more likely to have had a two-patch repair with slightly increased cross clamp and CPB times, but the outcomes were similar. Three patients had in-hospital mortality and one patient had late mortality. Two patients required a permanent pacemaker and 11 patients needed left AV valve reoperation. There was no difference between patients repaired at age over 3 months compared to those repaired prior to 3 months of age (24).
A review of 11 series in 2015 containing 378 patients by El Rassi and colleagues concluded that the modified single patch technique is an alternative method for the repair of complete AV canal. They found reduction in both cross clamp and CPB times, with a mean reduction in both times of 30 minutes. Mortality and LAVVR were low and not different from standard techniques. However, there were no cases of LVOT obstruction needing operation following Nunn technique compared to 15% needing re-operation in standard treatment cohort. They did however comment that with the exception of the series published by Nunn himself, the long-term fate of the LVOT remains unclear following the modified single-patch technique as the follow is limited compared to the standard techniques (25).
Other considerations
TOF and double outlet right ventricle (DORV)
The use of modified single patch technique with associated TOF and DORV is evolving and the literature is scant. Complete AVSD with TOF is a difficult subset of patients. The overall mortality in this group from a meta-analysis of 50 studies is 9% (26). The VSD component is large and with anterior displacement, many worry about possible LVOT obstruction. Therefore, the two-patch technique has been preferred method of repair thus far. Clarke reported a cohort of 88 patients who underwent the Nunn repair and nine of these had TOF or pulmonary atresia anatomy requiring an additional RVOT procedure. There were three early deaths (3.4%) one with TOF anatomy, one with pulmonary atresia who needed an RV to PA conduit, and another with aortic stenosis (9). The Nunn technique could be used for AVSD with TOF, but as previously described; more pledgetted sutures are required on the right side of the VSD to account for the longer septal crest. Left ventricular outflow obstruction has not been a proven problem.
Backer and team used a modification of the modified single patch technique for TOF/DORV AVSD. They only brought the inferior bridging leaflet down and attached to the crest of the VSD as in the standard Nunn technique. A Gore-Tex patch is used to close the VSD under the superior bridging leaflet, which routes the left ventricular blood to the aorta (21).
In 2009, Jeong et al. examined 61 patients who underwent total correction for complete AVSD with either the Nunn technique (18 patients) or the classic single patch technique (43 patients). The aortic cross-clamp time was shorter in the Nunn group (110.8 vs. 134.4 minutes, P=0.03). Two patients needed reoperation and there was one late death in the Nunn group, compared to three reoperations and two late deaths in the classic single patch group. Overall, the outcomes in both groups were similar; however, the Nunn technique was simpler with shorter ischemic times. Despite this, the authors expressed concern that this technique may not be applicable for those with large VSD and associated TOF or valve abnormalities (27).
At this time, the debate continues as to whether Nunn technique is applicable for this subset of population.
Down’s syndrome
Atz et al. published a multicenter observational study (June 2004 to 2006) of 120 children who underwent repair of complete AVSD using the three techniques: single patch (18%), double patch (72%), and a single ASD patch with primary VSD closure (10%). The study demonstrated that the outcomes did not differ by repair type or the presence of trisomy 21 (80% of cohort). The median age at surgery was 3.7 months. In-hospital mortality rate was 2.5% and the 6-month mortality was 4%. The incidence of residual VSD and LAVVR did not differ by repair type. The ICU stay, ventilator support, and total hospital stay were independent of the presence of trisomy 21. Younger age of operation and presence of associated anomalies were associated with longer hospital stay with no increase in the incidence of residual VSD or significant LAVVR (28).
Reoperations for complications
Left AV valve regurgitation
The most common cited reason for re-operation following AVSD repair is LAVVR. Kanani and his group showed that the most common reason for reoperation was dehiscence of the “zone of apposition”/“cleft”. In their study, 33 patients underwent re-operations of the left atrioventricular valve (21 complete and 12 partial). Twenty patients had re-operation within 1 year of primary repair, and 13 patients had repair beyond 1 year. The group, which underwent repair within 1 year, did so because of leaflet tears or dysplastic valves. The latter group had several areas of valvar leakage, which included central regurgitation (29). Repair of LAVVR following previous surgery involves repair of the “zone of apposition” between the left sided bridging leaflets and may require annuloplasty to attain competence. They used a custom flexible partial annuloplasty ring made from a thin-walled 3.5-mm Gore-Tex graft shaped to fit the exact contours and morphologic composition of this annulus to account for the growth potential (29). It is interesting to note that the original Nunn technique may have fewer “cleft” disruptions because a continuous braided suture (Tevdek or Ticron) instead of interrupted polypropylene sutures were used. Additionally, the Dacron strip used to sandwich the leaflets and the pericardial patch would support the leaflets and act as an anterior annuloplasty.
LVOT obstruction
Echocardiography and autopsy studies demonstrate that up to 75% patients with AVSD are at risk for developing LVOT obstruction, of whom 35–45% will need reoperation. It is three times more common following partial AVSD repair compared to complete AVSD repairs. There is theoretical concern for LVOT obstruction following Nunn technique, but long-term studies have not demonstrated an increased incidence. In 2010, Stulak and associates reviewed 146 patients who underwent reoperation after previous repair of partial (n=96) and complete (n=50) AVSD. Reoperation for LVOT obstruction was much higher in the partial AVSD group compared with the complete AVSD group. They concluded that though early results of the Nunn repair did not demonstrate problems with LVOT obstruction, with longer follow-up, the potential for LVOT obstruction might become more evident, particularly with partial AVSD (30).
Other authors worry about LVOT obstruction specifically when the VSD component is large. Adachi and colleagues sought to better define the Nunn repair in hearts with a large ventricular scoop (distance from the ventricular crest to the atrioventricular valve during end-diastole). Thirty-one specimens with complete AVSD and 12 specimens with partial AVSD were evaluated. The scoop had a skewed shape with extension antero-superiorly beyond the AV junction in 16 hearts with complete AVSD. This extension resulted in narrower LVOT compared to complete AVSD with no extension. However, on stratification using the scoop depths there was no obvious differences between the complete with deep scoop vs. shallow scoop. This indicates that the antero-superior extension has more impact on the LVOT rather than the depth of scoop. This skewed extension could potentially lead to asymmetry in the valve leaflets and lead to LVOT obstruction. They concluded that the suitability of simplified single patch technique not only depends on the depth of the scoop but also on the antero-superior extension (31).
Adachi also stated that hearts with DORV are unsuitable for Nunn repair as the only LV outlet is the space between the bridging leaflet and the crest of the ventricular septum. They expressed concern that Nunn technique diminishes this space thus leading to LVOT obstruction. Adachi further quoted Wilcox who in 1997 speculated that if the scoop were too deep it would cause leaflet distortion and increased tension in bringing the leaflets on to the septum. The authors also quoted the 2007 paper by Backer et al. who based their decision to perform the modified single patch technique based on the scoop depth. If the depth were, 10 mm or less they would use the modified single patch technique and use the two-patch technique if the scoop depth was greater than 10 mm (31).
Despite these speculations, the data has not demonstrated increased LVOT obstruction using the Nunn technique. In the study published by Nunn, 30-day mortality was 1.6%, re-operation rate was 2.3% for mitral valve, and there were no residual VSD’s or LVOT obstruction (10). This relative paradox of the perceived LVOT obstruction and the actual figures prompted Kanani and colleagues to reply to the above study in a letter to the editor stating that emerging data on the modified single patch experience indicates a lack of significance in the antero-superior extension demonstrated by Adachi. They instead point to the contribution of an obstructive sub-valvar apparatus where dysplastic leaflet material and papillary muscles extend into the LVOT. They concluded that, “the problem is a complex one in which morphology alone is unlikely to hold all the secrets. Nevertheless, it is gratifying to see, befitting the modern era, that it is the surgeon that is driving the understanding of this issue to cause a more perfect repair” (32).
In a more recent study by Becker and colleagues, modified single patch technique was used in 77 infants with complete AVSD between January 2000 and 2013. Eight patients had a prior coarctation repair in the newborn period. The mean and median follow-up times were 4.6 and 3.7 years, respectively. Only two patients (2.5%) who previously had coarctation repair had late reoperation for LVOT obstruction. The first patient had a discrete fibrous sub aortic membrane and needed reoperation at 3 and 7 years following primary repair. The second patient had LVOT obstruction from accessory chordae from the left AV valve and needed Mitral valve replacement 5 months following primary repair. They concluded that LVOT obstruction is not a significant complication after modified single patch repair of AVSD. Previous operation for coarctation of aorta is an important predictor of late LVOT obstruction following modified single patch technique (33).
The current literature is not clear on the above dichotomy given the fact that Nunn technique creates a ‘primum defect’ but still has a rare incidence of LVOT obstruction in comparison to an isolated ‘primum” defect. The authors can only speculate the reasons for the same. Literature is limited on the influence that the age of repair of the two lesions (complete vs. primum) may have on this issue. If all isolated primum defects were repaired as early as the complete AVSD the incidence of later LVOT obstruction may be completely different and would probably be the same low number as the modified single patch technique post repair. This has not been tested as far as we know but may be the focus of future studies as patients are being operated at a younger age and the mortality for the same is very low. One fact that is clear from the existing literature is that the size of the VSD at repair does not predict the likelihood of LVOT obstruction in the modified single patch technique, and yet a case with a big VSD has a much greater anatomical realignment than a primum defect, which has none, after repair. Often the septum lengthens and the LV grows after complete repair with modified single patch technique. So we can only imagine how much better (different) these two structures would be if the primum defect were repaired at an equally young age. This leads us to a question does late operation (longer duration of ‘goose neck’) lead to future LVOT obstruction?
Summary
The Nunn technique for complete AVSD has universal application. It requires fewer decisions regarding geometry, size, and configuration of patches compared to the two-patch technique. This allows for reproducibility and training of other surgeons. Data has shown good preservation of atrioventricular valve function, no residual VSDs, low incidence of LVOT obstruction, preserved conduction, and improved perioperative and long-term mortality compared to other techniques.
Acknowledgements
Dr. Graham Nunn for the computerized images used in this review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Karl TR, Provenzano SC, Nunn GR, et al. The current surgical perspective to repair of atrioventricular septal defect with common atrioventricular junction. Cardiol Young 2010;20 Suppl 3:120-7. [Crossref] [PubMed]
- Lillehei CW, Cohen M, Warden HE, et al. The direct-vision intracardiac correction of congenital anomalies by controlled cross-circulation: results in thirty-two patients with ventricular septal defects, tetralogy of Fallot, and atrioventricularis comminis defects. Surgery 1955;38:11-29. [PubMed]
- Maloney JV Jr, Marable SA, Mulder DG. The surgical treatment of common atrioventricular canal. J Thorac Cardiovasc Surg 1962;43:84-96. [PubMed]
- Mavroudis C. Atrioventricular Septal Defects (Atrioventricular Canal). In: Mavroudis C, Backer C. editors. Atlas of Pediatric Cardiac Surgery. London: Springer, 2015:117-29.
- Jonas RA, Mora B. Individualized approach to repair of complete atrioventricular canal: selective use of the traditional single-patch technique versus the Australian technique. World J Pediatr Congenit Heart Surg 2010;1:78-86. [Crossref] [PubMed]
- Trusler GA, Yamamoto N, Williams WG, et al. Surgical treatment of pulmonary atresia with intact ventricular septum. Br Heart J 1976;38:957-60. [Crossref] [PubMed]
- Wilcox BR, Jones DR, Frantz EG, et al. Anatomically sound, simplified approach to repair of “complete” atrioventricular septal defect. Ann Thorac Surg 1997;64:487-93. [Crossref] [PubMed]
- Nicholson IA, Nunn GR, Sholler GF, et al. Simplified single patch technique for the repair of atrioventricular septal defect. J Thorac Cardiovasc Surg 1999;118:642-6. [Crossref] [PubMed]
- Clarke A, Nunn GR, Nicholson IA. The simplified single patch repair of complete atrioventricular septal defect. Oper Tech Thorac Cardiovasc Surg 2004;9:233-9. [Crossref]
- Nunn GR. Atrioventricular canal: modified single patch technique. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2007;10:28-31. [Crossref] [PubMed]
- Ginde S, Lam J, Hill GD, et al. Long-term outcomes after surgical repair of complete atrioventricular septal defect. J Thorac Cardiovasc Surg 2015;150:369-74. [Crossref] [PubMed]
- Suzuki T, Bove EL, Devaney EJ, et al. Results of definitive repair of complete atrioventricular septal defect in neonates and infants. Ann Thorac Surg 2008;86:596-602. [Crossref] [PubMed]
- Crawford FA, Stroud MR. Surgical repair of complete atrioventricular septal defect. Ann Thorac Surg 2001;72:1621-8. [Crossref] [PubMed]
- Dragulescu A, Fouilloux V, Ghez O, et al. Complete atrioventricular canal repair under 1 year: Rastelli one-patch procedure yields excellent long-term results. Ann Thorac Surg 2008;86:1599-604. [Crossref] [PubMed]
- Halit V, Oktar GL, Imren VY, et al. Traditional single patch versus the “Australian” technique for repair of complete atrioventricular canal defects. Surg Today 2008;38:999-1003. [Crossref] [PubMed]
- Salihoğlu E, Özkan S, Özçobanoğlu S, et al. Preliminary results of direct closure of an atrioventricular septal defect: revisiting the original technique. Turk J Thorac Cardiovasc Surg 2012;20:699-704. [Crossref]
- Tagliari AP, Schröder DA, Teixeira Filho G, et al. Results of simplified single-patch repair for complete atrioventricular septal defect. Arq Bras Cardiol 2013;100:288-93. [Crossref] [PubMed]
- Salah T. Outcome of Australian Technique in Repair of Complete AV Canal Septal Defects. J Egyp Card Thorac Surg 2014;22:89-96.
- Li D, Fan Q, Iwase T, et al. Modified Single-Patch Technique versus Two-Patch Technique for the Repair of Complete Atrioventricular Septal Defect: A Meta-Analysis. Pediatr Cardiol 2017;38:1456-64. [Crossref] [PubMed]
- Backer CL, Stewart RD, Bailliard F, et al. Complete atrioventricular canal: comparison of modified single-patch technique with two-patch technique. Ann Thorac Surg 2007;84:2038-46. [Crossref] [PubMed]
- Backer CL, Kaushal S, Mavroudis C. Modified single-patch technique: Repairing complete atrioventricular septal defect. Ann Pediatr Cardiol 2009;2:51-4. [Crossref] [PubMed]
- Pan G, Song L, Zhou X, et al. Complete Atrioventricular Septal Defect: Comparison of Modified Single‐Patch Technique with Two‐Patch Technique in Infants. J Card Surg 2014;29:251-5. [Crossref] [PubMed]
- Backer CL, Eltayeb O, Mongé MC, et al. No Ventricular Septal Defect Patch Atrioventricular Septal Defect Repair. Oper Tech Thorac Cardiovasc Surg 2015;20:279-92. [Crossref]
- Stephens EH, Ibrahimiye AN, Yerebakan H, et al. Early complete atrioventricular canal repair yields outcomes equivalent to late repair. Ann Thorac Surg 2015;99:2109-15. [Crossref] [PubMed]
- El-Rassi I, Charafeddine F, Tabbakh A, et al. Surgical repair of complete atrioventricular defect (Nunn technique). Multimed Man Cardiothorac Surg 2015;2015. [Crossref] [PubMed]
- Prifti E, Bonacchi M, Bernabei M, et al. Repair of Complete Atrioventricular Septal Defect with Tetralogy of Fallot. J Card Surg 2004;19:175-83. [Crossref] [PubMed]
- Jeong IS, Lee CH, Lee C, et al. Surgical outcomes of the modified single-patch technique in complete atrioventricular septal defect. Interact Cardiovasc Thorac Surg 2009;8:435-7. [Crossref] [PubMed]
- Atz AM, Hawkins JA, Lu M, et al. Surgical management of complete atrioventricular septal defect: associations with surgical technique, age, and trisomy 21. J Thorac Cardiovasc Surg 2011;141:1371-9. [Crossref] [PubMed]
- Kanani M, Tsang V, Cook A, et al. Re-repair of the left atrioventricular valve in atrioventricular septal defects: the morphologic approach to the role of Gore-tex band reduction annuloplasty. Eur J Cardiothorac Surg 2010;37:273-8. [PubMed]
- Stulak JM, Burkhart HM, Dearani JA. Reoperations after repair of partial and complete atrioventricular septal defect. World J Pediatr Congenit Heart Surg 2010;1:97-104. [Crossref] [PubMed]
- Adachi I, Ho SY, McCarthy KP, et al. Ventricular scoop in atrioventricular septal defect: relevance to simplified single-patch method. Ann Thorac Surg 2009;87:198-203. [Crossref] [PubMed]
- Kanani M, Cook A, Kostolny M. Left ventricular outflow tract obstruction after the modified single patch repair of atrioventricular septal defects: teasing fact from fiction. Ann Thorac Surg 2010;89:1339-40. [Crossref] [PubMed]
- Backer CL, Eltayeb O, Mongé MC, et al. Modified single patch: are we still worried about subaortic stenosis? Ann Thorac Surg 2015;99:1671-5. [Crossref] [PubMed]