
Enriched environment alleviates adolescent visceral pain, anxiety- and depression-like behaviors induced by neonatal maternal separation
Introduction
Neonatal exposure to stressful environments is widely believed to be associated with impairments in brain development and adult behavior. Early life stress significantly increases the risk of visceral hypersensitivity and psychiatric disorders (1-4). Both physical abuse and mental neglect in childhood are key environmental risk factors associated with the development of visceral pain and multiple psychiatric disorders, such as major depressive disorder and anxiety disorders (5). Animal models of early life stress are established by disrupting the mother-infant interaction through repeating the maternal separation (MS) paradigm (6,7), altering the normal response of the hypothalamic-pituitary-adrenal (HPA) axis and neuroimmune response to stress (7,8). This modeling leads to enhancement of the neuroendocrine and neuroinflammation responses of the rodents and has adverse effects on rodent brain anatomy and function, synaptic plasticity, emotional responses, and cognition (7,9,10) in adulthood. However, the role of early life stress in chronic pain and mental illness in adolescence remains underexplored.
Neuroinflammation is essential for the development of chronic pain, including chronic visceral pain (11). Recent studies have shown that neuroinflammation is a key factor in early life stress-induced visceral hypersensitivity in adults (12). In visceral pain conditions, microglia have been shown to produce and secrete the cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and other pro-inflammatory factors are increased through a myeloid differentiation factor 88 (MyD88)-dependent signaling cascade in the central nervous system including medial prefrontal cortex (mPFC), basolateral amygdala (BLA), and paraventricular nucleus (PVN) (13-15). Likewise, neuroinflammation in these brain regions is also involved in anxiety and depression, consistent with the well-known comorbidities of chronic pain and psychiatric disorders (7,8). Therefore, in this study, we examined the changes of neuroinflammatory factors in these brain regions.
Environmental enrichment (EE), that is, optimization of housing conditions to promote social interaction, sensorimotor and cognitive stimulation, as well as novel stimuli and physical exercise, can counteract the negative impact of early stress by promoting neurogenesis, synaptic plasticity, and modulate neuroinflammatory and neuroendocrine responses of the hypothalamic-pituitary-adrenal (HPA) axis to stress (15). However, whether EE can alleviate chronic visceral pain, anxiety, and depression remains unexplored. In this study, we hypothesized that EE could alleviate chronic visceral pain, anxiety, and depression by attenuating neuroinflammatory responses in the brain during adolescence.
The aim of this study was to verify the possible beneficial effects of EE intervention from birth onwards in the adolescence of MS mice. To this end, we performed a series of behavioral tests (visceral pain, anxiety- and depression-like behaviors), and also detected brain neuroinflammatory factors using enzyme-linked immunosorbent assay (ELISA). We present the following article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-410/rc).
Methods
Animals
Pregnant female C57BL/6 J mice were provided by the Experimental Animal Research Center of Xuzhou Medical University (Jiangsu, China). Weaned male mice, 3–5 per cage, were housed in a humidity- and temperature-controlled environment on a 12-h light/dark cycle with ad libitum access to food and water. The research team monitored the animals once daily. Mice were euthanized immediately if they developed the following symptoms: hunchback, lethargy, shortness of breath, skin discoloration or irregularity, paraplegia, significantly enlarged lymph nodes, and visible subcutaneous solid tumors, and autopsies were performed to determine the cause of the disease and recorded as “adverse events”. After behavioral testing, the mice were euthanized. All experimental procedures were approved by the Animal Care and Use Committee of Xuzhou Medical University (No. 202010A132) in compliance with the national guidelines for the care and use of animals. This experiment followed the guidelines of the International Association for the Study of Pain (IASP).
Maternal separation
The birth status of the mice was checked daily, and the date of birth was recorded as postnatal day 0 (P0). In the neonatal maternal separation (NMS) group, pups were removed from their dams and home cages for 6 hours every day from postnatal day 1 (P1) to P21. Then, the pups were reunited with their dams in a cage. After weaning on P21, males were selected for subsequent experiments.
Enriched environment (EE)
The EE consisted of free-turning running wheels, pipes, stairs, and various colored ocean balls, etc. Toys are changed once a week, while paying attention to color changes.
Experimental design
A total of 144 mice were randomized into the study, and all were included in the analysis. According to the authoritative literature, 8 mice in each group were used (16). All researchers who performed the animal experiments and analyzed the data were blinded to the treatment conditions. The behavioral tests were conducted from 8:00 am to 11:30 am, and the order of the tests was random each day, with each animal being tested at a different time on each test day. The primary endpoint of this study was defined as visceral pain, anxiety- and depression-like behaviors, which was measured by visceral pain threshold test (PPT), open field test (OFT), and sucrose preference test (SPT), respectively. The protocol (including the research question, key design features, and analysis plan) was prepared before the study but was not registered.
The study consisted of 2 experiments as shown in Figure 1.
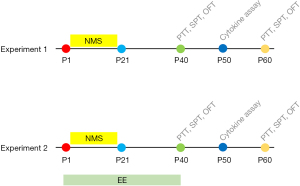
In experiment 1, male mice were randomly and blindly separated into 2 groups according to random number table method. The NMS group: mice pups were removed from their dams and home cages for 6 hours every day from P1 to P21; control group: mice pups stayed with their dams and home cages until P21. In adolescence, mice underwent behavior tests (visceral pain: visceral pain threshold; anxiety- and depression-like behaviors: center time, center distance, total distance, and sucrose preference) on P40 and P60 (n=8 mice per group) or were sacrificed on P50 for inflammatory cytokines assays (TNF-α, IL-1β, and IL-10) of the mPFC, BLA, and PVN (n=8 mice per group).
In experiment 2, male mice were randomly and blindly separated into 4 groups according to random number table method. The NMS+EE group: mice pups in the NMS group were removed from their dams and home cages for 6 hours every day from P1 to P21, and were housed in EE from P1 to P40; MS group: mice pups were removed from their dams and home cages for 6 hours every day from P1 to P21; EE group: mice pups stayed with their dams until P21 and in EE from P1 to P40; control: control group: mice pups stayed with their dams and home cages until P21 and in a standard environment from P1 to P40. In adolescence, mice underwent behavioral tests (visceral pain: visceral pain threshold; anxiety- and depression-like behaviors: center time, center distance, total distance and sugar preference) on P40 and P60 (n=8 mice per group) or were sacrificed on P50 for inflammatory cytokines assays (TNF-α, IL-1β, and IL-10) of mPFC, BLA, and PVN (n=8 mice per group).
Visceral PTT
Mice were anesthetized with sevoflurane, and then an uninflated balloon coated with paraffin oil was introduced into the colorectum with the end of the balloon 0.5 cm from the anal verge. After 15 minutes of acclimatization, pressure was given by inflating the balloon, and the pressure value when the mouse abdominal wall muscles contracted and was not in contact with the table was regarded as the visceral pain threshold.
OFT
The OFT was used to assess the severity of anxiety. The device for the open field experiment is a box with a white background, which is evenly divided into 9 squares. The middle square is named the central area and the surrounding 8 square are peripheral areas. Briefly, at the beginning of each experiment, mice were gently placed in the central area and then mice behaviors were recorded for 5 minutes. The observation indicators included the following: total movement distance, time in the central area, and distance in the central area.
SPT
The SPT was used to assess the severity of depression. Mice were raised in single cages and given a bottle of 1% sucrose solution and a bottle of tap water for 48 hours. Care was taken to avoid water bottle leakage. In order to avoid positional influence, 2 water bottles were placed at the same level, and the positions of the 2 water bottles were exchanged regularly. The sugar water preference experiment was carried out after 24 hours of water deprivation. Each mouse was simultaneously given 2 pre-weighed bottles of water, one was 1% sucrose solution and the other was plain water. In order to prevent the rats from preferring to drink water on 1 side, the positions of the 2 water bottles were switched regularly. After 2 hours, the 2 bottles of solution were taken out and weighed, and the percentage of sucrose preference of each mouse was calculated [sucrose solution consumption/(sucrose solution consumption + water consumption) ×100%].
ELISA
The levels of TNF-α, IL-1β, and IL-10 were quantified using mice-specific ELISA kits according to the manufacturers’ instructions (R&D, San Clara, CA, USA). Mice were sacrificed after prior anesthesia and then brain tissue (mPFC, BLA, and PVN) were collected and homogenized in a buffer containing protease inhibitor phenylmethylsulfonyl fluoride (PMSF; 1 mmol/L). Homogenates were centrifuged (8,000 g) at 4 ℃ for 5 minutes. The concentrations of TNF-α, IL-1β, and IL-10 in the resulting supernatants were assessed using ELISA kits according to the manufacturers’ instructions and were expressed as pg/mL of protein.
Statistical analysis
All statistical analyses were carried out with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). All measurement data were expressed as mean ± standard deviation (SD). Comparisons between two groups were performed by the t-test. Comparison among multiple groups was performed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A P value <0.05 was considered statistically significant.
Results
NMS induced adolescent visceral pain, anxiety- and depression-like behaviors
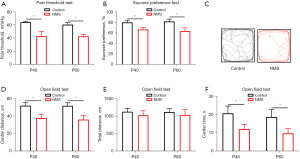
In adolescence, the visceral pain, anxiety- and depression-like behaviors of the mice were measured. The results showed that compared with the control group, the mice in the NMS group had significantly lower visceral pain thresholds on P40 and P60 (Figure 2A); compared with the control group, the mice in the NMS group had significantly lower sucrose intake percentage on P40 and P60 (Figure 2B), which indicated that stress in early life could induce depression-like behaviors; the OFT showed no significant changes in the total distance, and a significant decrease in center time and center distance in the NMS group (Figure 2C-2F), exhibiting anxiety-like behavior.
NMS increased adolescent mPFC, BLA, and PVN inflammatory cytokines
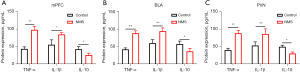
The results of ELISA showed that NMS increased the expression of TNF-α and IL-1β in mPFC, BLA, and PVN compared to control group (Figure 3A,3B). In contrast, NMS decreased the expression of IL-10 in the mPFC, BLA, and PVN compared to the control group (Figure 3C). These results suggested that NMS contributed to a predisposition to pro-inflammatory features in adolescent brains.
EE alleviated NMS-induced adolescent visceral pain, anxiety- and depression-like behaviors
Compared with the group without EE, NMS mice in the EE group had increased visceral pain thresholds on P40 and P60 (Figure 4A). The SPT showed that MS mice in the EE group had an increased percentage of sucrose intake on P40 and P60 compared to the group without EE (Figure 4B) Furthermore, in the OFT, compared with the group without EE, NMS mice in the EE group had greatly increased center distance and center time on P40 and P60 (Figure 4C-4E), with no difference in total distance (Figure 4F). These results suggested that EE alleviated NMS-induced adolescent visceral pain, anxiety- and depression-like behaviors.

EE biased adolescent brain mPFC, BLA, and PVN towards anti-inflammatory features
After postnatal EE intervention, samples were obtained from 3 brain regions on P50 to detect inflammatory factors by ELISA. The results of ELISA showed that NMS mice in the EE group decreased the expression of TNF-α and IL-1β in mPFC, BLA, and PVN, and increased the expression of IL-10 in mPFC, BLA, and PVN compared with the group without EE on P40 and P60 (Figure 5A-5C).

Discussion
In this study, we performed a series of behavioral tests (visceral pain, anxiety- and depression-like behaviors), and also detected brain neuroinflammation factors using ELISA to verify the possible beneficial effects of EE intervention from birth onwards in adolescent NMS mice. Our study showed that NMS induced visceral pain, anxiety- and depression-like behaviors in adolescence, whereas EE intervention alleviated visceral pain, anxiety- and depression-like behaviors and depressed brain neuroinflammation in adolescence.
Previous study has found that NMS animals had significantly reduced grooming behavior and impaired inhibitory avoidance memory but not object recognition memory, suggesting that NMS altered learning and memory in a task-specific manner and that EE during two critical ontogenic periods (early life and peripuberty) reversed the effects of NMS. In addition, NMS had no significant effect on the expression of Fos and glucocorticoid receptor (GR) in the hippocampus, but EE increased the expression of Fos and the total number of GR-positive cells in the hippocampus (16). These findings suggest that different environmental qualities during two critical ontogenic periods were effective way to study the effects of early experiences on behavioral and physiological plasticity. The effects of NMS can persist into adulthood, but a favorable environment early life and peripuberty can reverse some of these effects through neuroplasticity. However, the impact of different environments qualities on adolescent behavior and their mechanisms remains poorly underexplored. There is growing evidence that early life stress may permanently damage the developing brain, adversely affect behavior in adulthood, and increase the risk of chronic pain and psychopathology (17-23). However, little is known about the effects on puberty. In this study, we found that NMS can cause visceral pain, anxiety- and depression-like behaviors in adolescence from P40 to P60, combined with previous reports that NMS also can cause visceral pain, anxiety- and depression-like behaviors in adulthood, indicating that damage to the brain by early life stress may be immediate and persistent. This should prompt us to pay attention to the mental health of adolescents who have had unfortunate childhoods, and if possible, try to help children avoid unfortunate childhoods so as to avoid irreversible neuropsychiatric trauma. Given that neuroinflammation was key to the development of visceral pain, anxiety- and depression-like behaviors, and these symptoms had already occurred by adolescence, it was clear that neuroinflammation had become uncontrolled by this time. Consistent with this, our results showed increased expression of TNF-α and IL-β and decreased expression of IL-10 in mPFC, BLA, and PVN on P50.
Early life stress was closely associated with the development of chronic pain and psychiatric disorders (17-23). Besides, early life stress can affect neuroinflammation, and neuroinflammation plays a role in chronic pain and psychiatric pathology (24-26). This process provides a new target for improving neuroinflammation and potentially preventing/relieving chronic pain and psychiatric symptoms. Given the limited treatment options currently available for chronic pain and psychiatric disorders, along with significant side effects, there is an urgent need to develop new and improved treatments. The EE is an underexplored but promising approach for ameliorating neuroinflammation to alleviate/prevent early life stress-induced chronic pain and psychiatric disorders.
Cognitive-behavioral therapy (CBT) is a clinically adjunctive therapy for neurological disorders such as anxiety and depression (27-30). Since chronic visceral patients are often accompanied with similar psychiatric disorders, it may not be surprising that CBT improves the quality of life of those with chronic visceral pain (31). Vachon et al. and van Praag et al. showed that EE, the animal analog of CBT, can improve pain behavior after insults (32,33). However, whether CBT improves visceral pain remains unclear. Our experimental data demonstrate that EE alleviated NMS-induced adolescent visceral pain as well as anxiety- and depression-like behaviors, supporting the notion that CBT can be an alternative for the treatment of visceral pain. Since the damage of stress to the developing brain of neonatal mice was immediate and long-term, in this experiment, the mice were given EE intervention from the first day after birth, which may maximally protect the fragile nervous system, and eventually attenuate the central inflammatory response and relieve visceral pain and anxiety- and depression-like behaviors in adolescence. This suggests that EE intervention should be carried out as early as possible to prevent/alleviate the development of neurological diseases in adolescence. At the same time, we believe that this assumption can be reasonably extended to adulthood.
Data from animal studies suggest that EE has considerable behavioral and physiological anxiolytic effects on psychological and neurogenic stressors. The mechanisms by which EE can reverse the effects of stress range from cognitive/behavioral to cellular and molecular processes. Findings on social isolation suggest that the interaction of two factors, inanimate enrichment and social stimuli, contribute to the beneficial effects of EE (34). The enhancing effects of EE on cognition have been attributed to its induction of hippocampal neurogenesis, which may simultaneously affect the processing of emotions and stress responses in interconnected circuits connecting the amygdala and hypothalamus, respectively. At the same time, EE-induced hippocampal GR expression levels were also relatively increased, which may reduce plasma corticosterone levels for anxiolytic effects (35,36). EE has also been shown to increase the total weight of the brain and cortex, while increasing neuronal density, the number of dendritic branches synapses and neurotrophic factor levels. Any or all of these EE-induced changes may modulate its effect on stress (37). Here, our study shows that environmental enrichment reverses CNS neuroinflammation and improves behavior, providing a new explanation for the mechanism of environmental enrichment.
Currently, tricyclic antidepressants, selective 5-HT reuptake inhibitors (SSRIs), γ-GABA mimics (pregabalin), к opioid receptor agonists (asimadoline), corticotropin-releasing hormone antagonists or physical therapy are commonly used in clinical treatment for the comorbidities of pain and mental illness. EE is created by keeping animals in an environment with different objects that collectively provide sensory, cognitive, motor (e.g., running wheels) and social stimuli that promote animal movement, sensory, social interaction and cognitive activities in a voluntary or stress-free manner. The biggest advantage of EE over traditional methods is that it can improve behavior from a variety of mechanisms without trauma and side effects.
Our study had some limitations. Firstly, the indicators chosen for this study were too limited to investigate the dynamic changes of visceral pain, anxiety- and depression- like behaviors in adolescence. Secondly, the exact role of TNF-α, IL-β, and IL-10 in visceral pain, anxiety, and depression-like behaviors in adolescence, respectively, is unclear. In the future, we will focus on signaling pathways that regulate neuroinflammation, such as the TRL4/NF-κB signaling pathway in microglia. Thirdly, effects of different environments qualities on visceral pain, anxiety and depression in middle-aged and elderly people need to be further explored. Finally, therapeutic window of EE deserves further study. Previous study found the potential beneficial effects of environmental manipulations decrease with age (38). Thus, we placed postnatal 1-day-old mice under EE intervention until puberty for behavioral testing. We believe that the earlier the intervention, the better the treatment effect, so in the future, we will study the difference in the initiation of intervention at different ages to determine the optimal time window.
In conclusion, we examined the adverse effects induced by early life stress in mice during adolescence, and confirmed that MS induced visceral pain, anxiety- and depression-like behaviors, accompanied by an imbalance of neuroinflammation in the central nervous system. Early EE interventions reversed MS-induced adolescent neuroinflammatory imbalances and subsequently prevented or ameliorated visceral pain, anxiety- and depression-like behaviors. Therefore, our findings show that EE may have important applications in the clinical treatment of early life stress-induced adolescent visceral pain, anxiety- and depression-like behaviors.
Acknowledgments
Funding: This study was supported by the Talent Construction and Research Start-up Fund of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (to Ming Xia).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-410/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-410/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experimental procedures were approved by the Animal Care and Use Committee of Xuzhou Medical University (No. 202010A132), in compliance with the national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ventriglio A, Gentile A, Baldessarini RJ, et al. Early-life stress and psychiatric disorders: epidemiology, neurobiology and innovative pharmacological targets. Curr Pharm Des 2015;21:1379-87. [Crossref] [PubMed]
- Carr CP, Martins CMS, Stingel AM, et al. The Role of Early Life Stress in Adult Psychiatric Disorders. J Nerv Ment Dis 2013;201:1007-20. [Crossref] [PubMed]
- Nakamura T, Kurosaki K, Kanemoto M, et al. Early-life experiences altered the maturation of the lateral habenula in mouse models, resulting in behavioural disorders in adulthood. J Psychiatry Neurosci 2021;46:E480-9. [Crossref] [PubMed]
- Tsotsokou G, Nikolakopoulou M, Kouvelas ED, et al. Neonatal maternal separation affects metabotropic glutamate receptor 5 expression and anxiety-related behavior of adult rats. Eur J Neurosci 2021;54:4550-64. [Crossref] [PubMed]
- Nemeroff CB. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016;89:892-909. [Crossref] [PubMed]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 2011;214:131-40. [Crossref] [PubMed]
- van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci 2017;11:87. [Crossref] [PubMed]
- Lippmann M, Bress A, Nemeroff CB, et al. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 2007;25:3091-8. [Crossref] [PubMed]
- González-Pardo H, Arias JL, Vallejo G, et al. Influence of environmental enrichment on the volume of brain regions sensitive to early life stress by maternal separation in rats. Psicothema 2019;31:46-52. [PubMed]
- Maghami S, Zardooz H, Khodagholi F, et al. Maternal separation blunted spatial memory formation independent of peripheral and hippocampal insulin content in young adult male rats. PLoS One 2018;13:e0204731. [Crossref] [PubMed]
- Lumertz FS, Kestering-Ferreira E, Orso R, et al. Effects of early life stress on brain cytokines: A systematic review and meta-analysis of rodent studies. Neurosci Biobehav Rev 2022;139:104746. [Crossref] [PubMed]
- Chen ZY, Zhang XW, Yu L, et al. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur J Pain 2015;19:176-86. [Crossref] [PubMed]
- Lee H, Lee S, Cho IH, et al. Toll-like receptors: sensor molecules for detecting damage to the nervous system. Curr Protein Pept Sci 2013;14:33-42. [Crossref] [PubMed]
- O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev 2008;226:10-8. [Crossref] [PubMed]
- Francis DD, Diorio J, Plotsky PM, et al. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci 2002;22:7840-3. [Crossref] [PubMed]
- Vivinetto AL, Suárez MM, Rivarola MA. Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behav Brain Res 2013;240:110-8. [Crossref] [PubMed]
- Alvarez P, Bogen O, Green PG, et al. Nociceptor Overexpression of NaV1.7 Contributes to Chronic Muscle Pain Induced by Early-Life Stress. J Pain 2021;22:806-16. [Crossref] [PubMed]
- Green PG, Alvarez P, Levine JD. Sexual dimorphic role of the glucocorticoid receptor in chronic muscle pain produced by early-life stress. Mol Pain 2021;17:17448069211011313. [Crossref] [PubMed]
- Fuentes IM, Jones BM, Brake AD, et al. Voluntary wheel running improves outcomes in an early life stress-induced model of urologic chronic pelvic pain syndrome in male mice. Pain 2021;162:1681-91. [Crossref] [PubMed]
- Green PG, Alvarez P, Levine JD. A role for gut microbiota in early-life stress-induced widespread muscle pain in the adult rat. Mol Pain 2021;17:17448069211022952. [Crossref] [PubMed]
- Huang ST, Song ZJ, Liu Y, et al. BNSTAV GABA-PVNCRF Circuit Regulates Visceral Hypersensitivity Induced by Maternal Separation in Vgat-Cre Mice. Front Pharmacol 2021;12:615202. [Crossref] [PubMed]
- Qin X, He Y, Wang N, et al. Moderate maternal separation mitigates the altered synaptic transmission and neuronal activation in amygdala by chronic stress in adult mice. Mol Brain 2019;12:111. [Crossref] [PubMed]
- Tang HL, Zhang G, Ji NN, et al. Toll-Like Receptor 4 in Paraventricular Nucleus Mediates Visceral Hypersensitivity Induced by Maternal Separation. Front Pharmacol 2017;8:309. [Crossref] [PubMed]
- Park HJ, Kim SA, Kang WS, et al. Early-Life Stress Modulates Gut Microbiota and Peripheral and Central Inflammation in a Sex-Dependent Manner. Int J Mol Sci 2021;22:1899. [Crossref] [PubMed]
- Dutcher EG, Pama EAC, Lynall ME, et al. Early-life stress and inflammation: A systematic review of a key experimental approach in rodents. Brain Neurosci Adv 2020;4:2398212820978049. [Crossref] [PubMed]
- Desplats P, Gutierrez AM, Antonelli MC, et al. Microglial memory of early life stress and inflammation: Susceptibility to neurodegeneration in adulthood. Neurosci Biobehav Rev 2020;117:232-42. [Crossref] [PubMed]
- Matsumoto K, Hamatani S, Shimizu E. Effectiveness of Videoconference-Delivered Cognitive Behavioral Therapy for Adults With Psychiatric Disorders: Systematic and Meta-Analytic Review. J Med Internet Res 2021;23:e31293. [Crossref] [PubMed]
- Eimontas J, Pakalniškienė V, Biliunaite I, et al. A tailored Internet-delivered modular intervention based on cognitive behavioral therapy for depressed older adults: a study protocol for a randomized controlled trial. Trials 2021;22:925. [Crossref] [PubMed]
- Hofmann SG, Wu JQ, Boettcher H. Effect of cognitive-behavioral therapy for anxiety disorders on quality of life: a meta-analysis. J Consult Clin Psychol 2014;82:375-91. [Crossref] [PubMed]
- Hans E, Hiller W. A meta-analysis of nonrandomized effectiveness studies on outpatient cognitive behavioral therapy for adult anxiety disorders. Clin Psychol Rev 2013;33:954-64. [Crossref] [PubMed]
- Lackner JM, Jaccard J, Keefer L, et al. Improvement in Gastrointestinal Symptoms After Cognitive Behavior Therapy for Refractory Irritable Bowel Syndrome. Gastroenterology 2018;155:47-57. [Crossref] [PubMed]
- Vachon P, Millecamps M, Low L, et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav Brain Funct 2013;9:22. [Crossref] [PubMed]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 2000;1:191-8. [Crossref] [PubMed]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Behav 2002;73:193-207. [Crossref] [PubMed]
- Morley-Fletcher S, Rea M, Maccari S, et al. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci 2003;18:3367-74. [Crossref] [PubMed]
- Ickes BR, Pham TM, Sanders LA, et al. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol 2000;164:45-52. [Crossref] [PubMed]
- Parent-Vachon M, Vachon P. Environmental enrichment alleviates chronic pain in rats following a spared nerve injury to induce neuropathic pain. A preliminary study. Vet Med (Auckl) 2018;9:69-72. [Crossref] [PubMed]
- Chandler K, Dosso H, Simard S, et al. Differential Effects of Short-term Environmental Enrichment in Juvenile and Adult Mice. Neuroscience 2020;429:23-32. [Crossref] [PubMed]


