
Application of IgG antibody titer and subtype in diagnosis and severity assessment of hemolytic disease of the newborn
Introduction
Hemolytic disease of the newborn (HDN) can occur in the fetal or early neonatal period (1). The etiology is complex, but the most common cause is ABO and rhesus (Rh) blood group incompatibility (2). Different ethnicity, regions, and medical settings can affect the incidence of ABO-HDN (3). Among Asian populations, in India there is a high correlation between the rates of prenatal ABO incompatibility and postnatal ABO-HDN incidence. In Singapore, the correlation between prenatal ABO incompatibility rates and postnatal ABO-HDN occurrence is low, and in China ABO incompatibility accounts for 20–25% of mothers and infants (4). Data on the incidence of postnatal ABO-HDN also vary, which is believed to account for the 10–20% of ABO incompatibility between mothers and infants, while the incidence of ABO-HDN is reported to be between 2% and 5% (5-7). In China, the RhD-negative blood type is uncommon, much lower than in the white population, most cases of ABO-HDN occur in pregnant women with type O. Due to the use of anti-D immunoglobulin in Western countries for HDN caused by Rh blood incompatibility, the incidence and mortality of ABO-HDN have been greatly reduced from 1% to 0.02% and from 25% to 8–9%, respectively.
The pathogenesis of HDN is alloimmunization because of Rh or ABO incompatibility between maternal and fetal blood. The maternal antibodies attack the fetal red blood cells (RBCs) after sensitization. Hemolysis can be alleviated or suppressed by preventing the continuous formation of immune complexes (8-10). C1q in C3 complement also plays an important role in this reaction. The titer of maternal blood group antibody is considered as relevant in prenatal diagnostic workup according to domestic clinical guidance. The incidence of HDN is high in pregnant women with blood type O, but also in newborns born to mothers with blood type A containing anti-B antibodies. The severity of the clinical manifestation of ABO-HDN is related to the following factors: the amount of maternal IgG antibody and the degree of binding of antibody and antigen (11), as well as the fetal or neonatal compensatory ability. ABO-HDN can cause death, so it should be paid more attention. The combination of prenatal determination of ABO blood group, serological examination and neonatal hemolysis tests for risk assessment and corresponding treatment can effectively reduce the risk of HDN (12).
Nowadays, ABO-HDN is mainly diagnosed by three hemolysis tests, including direct anti-human globulin test (DAT), antibody release test and free antibody test (13). The DAT and antibody release test are confirmatory test, while the free antibody test is a supplementary test. However, all of these tests are qualitative, and unable to accurately assess the severity of ABO-HDN. There are few studies investigating the value of quantitative detection of IgG antibody titer in the assessment of ABO-HDN, and the results need to be further verified. This study investigated the titer of IgG antibody of pregnant women to explore its correlation with the severity of ABO-HDN, with the times of pregnancy taken into consideration as well. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-385/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanxi Provincial Children’s Hospital (No. 2021-Tk034) and informed consent was taken from all the participants (pregnant women and the newborn’s guardians).
General clinical data of study patients
Our study group comprised 725 pregnant women with type O RhD-positive blood group underwent regular prenatal examination in Shanxi Provincial Children’s Hospital: (I) age range 20–45 years; (II) 382 primigravidae (52.7% of the total cases), and 343 non-primigravidae (47.3%); (III) husbands were RhD-positive for non-type O (284 cases in blood group A, 265 cases in blood group B, 176 cases in blood group AB); (IV) no previous blood transfusion record, normal liver and kidney function, and autoimmune hemolytic diseases or other hemolytic diseases were excluded.
The diagnostic criteria of HDN are: (I) maternal and infant ABO or Rh blood group incompatibility; (II) maternal blood group IgG antibody positive; (III) hyperbilirubinemia; (IV) of the three hemolysis tests of the newborn (direct resistance, free and release), the direct test or release test are positive; if negative the diagnosis is pathological jaundice.
Anti-A (B) titer test was initiated at 16 weeks of gestation and retested every 4 weeks. If postpartum hemolysis symptoms were detected, the newborn underwent ABO, Rh blood group, three hemolysis and serum indirect bilirubin tests.
Measurement technique
Changchun Bosun Biotechnology, Ltd. provided 0.2 mol/L 2-mercaptoethanol (2-ME), normal saline, anti-A, anti-B, anti-D, multi-specific anti-globulin reagents, antibody screening RBCs, neonatal hemolytic disease detection card 1 (ABO/RhD blood group, DAT), microcolumn gel method neonatal hemolytic disease detection card 2 (free antibody test, antibody release test), ABO standard RBCs, FYQ immune microcolumn incubator, TD-3A blood group serology centrifuge, low-speed centrifuge, incubators, acid release reagent and other instruments.
Detection of blood group antibody titer in pregnant women
The ABO antibody titer of pregnant women with type O RhD-positive (whose husband was not type O RhD-positive) was measured from 16 weeks of gestation (as the base titer) and reviewed monthly. Irregular antibody screening was only done once, as it is effective throughout the pregnancy. Prepare 10 dry test tubes labeled 1:2 [1], 1:4 [2], 1:8 [3], 1:16 [4], 1:32 [5], 1:64 [6], 1:128 [7], 1:256 [8], 1:512 [9], 1:1,024 [10], and add 0.1 mL of normal saline to each tube. Add 0.1 mL of the treated serum to the first tube and mix well, then remove 0.1 mL of the diluted serum from the first tube and add it to the second tube, and so on, and discard the last 0.1 mL when double diluted to the 10th tube. Finally, centrifuged for 5 min. The reciprocal of the maximum dilution of RBCs in the gel or microcolumn was used as the antibody titer.
Serological test of neonatal blood group
Neonatal ABO and RhD blood groups were detected after delivery, and hemolysis tests (direct resistance, free and release) were performed.
ABO/RhD blood typing and erythrocyte DAT
The ABO/RhD blood group card contains six microcolumn gel pores, the first 3 of which are anti-A gel, anti-B gel and anti-D gel, respectively, while the fourth, fifth and sixth wells are neutral gel. Neonatal blood is centrifuged, 1 mL hemolysized blood is removed, and the RBCs are diluted, mixed and washed with 9% normal saline. Finally, a 0.8–1% concentration is prepared and added into the first, second, third and sixth wells, respectively. The standard RBC reverse shaping reagent is prepared in the same way to a concentration of 0.8–1%, then added to the fourth and fifth wells. Finally, remove 25 µL of neonatal plasma and add it to the fourth, fifth and sixth wells, and mix for 5 min, before recording the results.
Free IgG antibody test
The free IgG antibody test is used for the absorption of autoantibodies, the separation and identification of more than two types of specific antibodies in a serum, confirmation of weak antigens and the concentration of low concentrations of antibodies. The procedure is as follows: three test tubes are labeled Ac, Bc and Oc respectively, to which are added 100 µL subserum and corresponding type A and type B reagents to each tube. Next, 50 µL of the RBCs and O-type screening reagent cells are placed in a 37 °C water bath for 30 min, removed and washed three times with normal saline for the last time. Finally, 100 µL of anti-human globulin reagent is added to each tube, centrifugation at 3,400 rpm for 15 s, and the results were interpreted.
Determination of serum bilirubin
The determination of serum bilirubin is by spectrophotometer. As the fetal age increases and the severity of HDN increases, amniotic fluid will accordingly become more yellow. Therefore, detecting the absorbance of amniotic fluid at different stages of pregnancy can help determine the severity of the disease and the required treatment.
Statistical analysis
The data were analyzed by SPSS 20.0. The results are expressed as mean ± standard deviation. Two-sample or two-group comparisons were performed using the t-test or the Mann-Whitney U test, and multi-sample or multi-group comparisons using the F test or Kruskal-Wallis test. Counting data were compared using the χ2 test. And P<0.05 was considered as statistical significant.
Result
ABO/RhD blood type determination
The DAT is used to detect RBCs sensitized by antibodies. The daughter cells were washed three times with normal saline, and the supernatant was removed before 100 µL anti-human globulin reagent was added and centrifuged at 3,400 rpm for 15 s. The results were observed under a microscope, positive for agglutination and negative for non-agglutination. ABO/RhD blood type was determined by the agglutination results of antibody A, antibody B, antibody D, antibody Ac, and antibody Bc (14).
Analysis of free IgG antibody in the serum of newborns
A positive free IgG antibody test indicates corresponding IgG antibody in neonatal serum, indicating that there is still unsensitized IgG antibody in the RBC blood group (Table 1).
Table 1
| Type | Result | ||
|---|---|---|---|
| A | B | O | |
| + | − | − | Free anti-A antibodies are present |
| − | + | − | Free anti-B antibodies are present |
| + | + | − | Free anti-AB antibodies are present |
| − | − | − | None |
+, positive; −, negative.
Diagnostic criteria for HDN
According to the diagnostic criteria of HDN, 116 of the 725 neonates tested positive and 609 were judged negative (Figure 1).
Comparative analysis of IgG antibody titer in the pregnant women
Among the primigravidae, there were 284 cases (74.3%) of antibody titer <1:64, 45 (11.8%) had a titer of 1:64, 26 (6.8%) had a titer of 1:128, 20 (5.2%) had a titer of 1:256, 4 (1.0%) had a titer of 1:512, and 3 (0.8%) had a titer >1:512. In the non-primigravida group, there were 93 cases (27.1%) of antibody titer <1:64, 85 cases (24.7%) of antibody titer 1:64, 78 cases (22.7%) of antibody titer 1:128, and 46 cases (13.4%) of antibody titer 1:256. Twenty-eight cases (8.2%) with a potency of 1:512, and 13 cases (3.8%) with a potency of >1:512, as shown in Table 2.
Table 2
| No. of pregnancies | Cases | Maternal IgG anti-A (B) antibody titer, n (%) | |||||
|---|---|---|---|---|---|---|---|
| <1:64 | 1:64 | 1:128 | 1:256 | 1:512 | >1:512 | ||
| First pregnancy | 382 | 284 (74.3) | 45 (11.8) | 26 (6.8) | 20 (5.2) | 4 (1.0) | 3 (0.8) |
| Non-first pregnancy | 343 | 93 (27.1) | 85 (24.7) | 78 (22.7) | 46 (13.4) | 28 (8.2) | 13 (3.8) |
| Total | 725 | 377 | 130 | 104 | 66 | 32 | 16 |
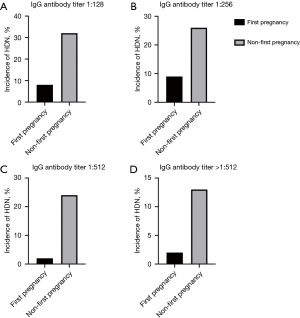
Relationship between number of HDN incidence
Figure 2 shows that the incidence of ABO-HDN in non-primigravidae was higher than the primigravidae.
Correlation analysis of conjugal blood group matching and IgG antibody titer in pregnant women
According to the ABO blood group of the husbands, they were divided into the wife-to-husband O-A group (n=284), the wife-to-husband O-B group (n=265) and the wife-to-husband O-AB group (n=176) as shown in Table 3. A total of 15.5% (45/284), 15.1% (40/265) and 17.4% (31/176) of husbands with blood type O-A, O-B and O-AB, respectively, had newborns with ABO-HDN (Table 3). There was no statistical significance in the positive rate of husbands with blood type distribution (P>0.05) (Table 4).
Table 3
| Wife-husband blood group matching | Maternal and infant blood group |
Cases | Maternal IgG antibody titer, n (%) | |||
|---|---|---|---|---|---|---|
| ≤1:64 | 1:128 | 1:256 | ≥1:512 | |||
| O-A | O-A | 284 | 198 (69.7) | 40 (14.1) | 27 (9.5) | 19 (6.7) |
| O-B | O-B | 265 | 188 (70.9) | 38 (14.3) | 23 (8.7) | 16 (6.1) |
| O-AB | O-AB | 176 | 121 (68.7) | 26 (14.8) | 16 (9.1) | 13 (7.4) |
| Total | 725 | 507 | 104 | 66 | 48 | |
Table 4
| Wife-husband blood type | Maternal and infant blood group | Cases | Number of HDN cases | Positive rate (%) |
|---|---|---|---|---|
| O-A | O-A | 284 | 45 | 15.50 |
| O-B | O-B | 265 | 40 | 15.10 |
| O-AB | O-AB | 176 | 31 | 17.40 |
| Total | 725 | 116 | 48.00 |
HDN, hemolytic disease of the newborn.
Discussion
HDN can be a serious diseases of the fetus and newborn if not detected early and treated appropriately (15,16). Due to incompatibility between the ABO blood group of the mother and the fetus (17), there is passive sensitization of maternal antibodies, mainly the mother’s IgG blood group antibody, which on later contact with fetal RBCs causes a series of antigen-antibody reactions and HDN (18-20). This often accompanied by different degrees of clinical symptoms: anemia, jaundice, hyperbilirubinemia, hepatosplenomegaly and other complications, even death. ABO-HDN has the highest incidence rate of neonatal immune hemolytic diseases (21). Pregnant women with type O blood have the highest probability of HDN, but pregnant women with type A blood containing anti-B antibody may also develop neonatal hemolytic disease (22). ABO-HDN can be treated prenatally and postnatally. The prenatal treatment includes early delivery, intrauterine plasma exchange, intrauterine blood transfusion, and intravenous drops of human immunoglobulin to inhibit hemolysis. The postnatal treatment involves blue light, immunoglobulin, human serum albumin, exchange transfusion, etc. (23-27).
In the present study, there were 21 cases of HDN in 382 pregnant women, and the risk of HDN increased with the number of pregnancies. Therefore, even in a primigravida, the fetus can develop HDN (28). At the end of the first pregnancy, the blood group antigen carried on the surface of neonatal RBCs enters the maternal circulation, stimulating the production of antibodies (29). With second and subsequent pregnancies, the titer of maternal IgG antibodies increases, and consequently the risk of HDN. Therefore, type O non-primigravidae should be given enhanced attention in the prenatal examination. For these pregnant women, prenatal treatment for ABO-HDN should be taken according to the maturation of the fetal lungs. If the fetal lungs were maturated, early delivery could be considered. If the fetal lungs were not maturated, repeated plasma exchange, intrauterine blood transfusion and phenobarbital could be considered (30-33).
The serum indirect bilirubin level of neonates with HDN confirmed by three hemolysis tests after delivery was detected, and a correlation analysis was conducted between the serum indirect bilirubin and the maternal blood group IgG antibody titer (34). The neonatal serum indirect bilirubin level increased with increasing maternal blood group antibody titer, indicating that the maternal blood group IgG antibody titer can predict the newborn’s serum indirect bilirubin level (35). However, women with normal antibody titer during pregnancy can also develop the disease, but it is also related to the antigenicity of fetal and fetal erythrocyte surface antigens (36-38). Blood group and IgG subtype antibodies correlated with the severity of HDN (not with IgG4 subtype, but with the amount of IgG1 and IgG3) (39).
For newborns at risk of ABO-HDN, blood group identification, serological examination and hemolysis using cord blood at birth (40), early detection and diagnosis of HDN, timely infusion of albumin or immunoglobulin C, blue light therapy or blood exchange therapy can avoid serious hemolytic disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-385/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-385/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-385/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Shanxi Provincial Children’s Hospital (No. 2021-Tk034) and informed consent was taken from all the participants (pregnant women and the newborn’s guardians).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Myle AK, Al-Khattabi GH. Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric Health Med Ther 2021;12:491-8. [Crossref] [PubMed]
- Pegoraro V, Urbinati D, Visser GHA, et al. Hemolytic disease of the fetus and newborn due to Rh(D) incompatibility: A preventable disease that still produces significant morbidity and mortality in children. PLoS One 2020;15:e0235807. [Crossref] [PubMed]
- Metcalf RA, Khan J, Andrews J, et al. Severe ABO Hemolytic Disease of the Newborn Requiring Exchange Transfusion. J Pediatr Hematol Oncol 2019;41:632-4. [Crossref] [PubMed]
- Routray SS, Behera R, Mallick B, et al. The Spectrum of Hemolytic Disease of the Newborn: Evaluating the Etiology of Unconjugated Hyperbilirubinemia Among Neonates Pertinent to Immunohematological Workup. Cureus 2021;13:e16940. [Crossref] [PubMed]
- Zheng YL, Hong Q, Wang QM. Investigation and Analysis of Non-ABO Hemolytic Disease of the Newborn. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021;29:1330-3. [PubMed]
- Bi SH, Jiang LL, Dai LY, et al. Rh-incompatible hemolytic disease of the newborn in Hefei. World J Clin Cases 2019;7:3202-7. [Crossref] [PubMed]
- Li S, Mo C, Huang L, et al. Hemolytic disease of the fetus and newborn due to alloanti-M: three Chinese case reports and a review of the literature. Transfusion 2019;59:385-95. [Crossref] [PubMed]
- Chen X, Feng J, Jiang Y. Hemolytic Disease of the Fetus and Newborn Caused by Maternal Autoantibody with Mimicking Anti-E Specificity. Lab Med 2021;52:399-402. [Crossref] [PubMed]
- Wang R, Li Y, Tong Y, et al. Hemolytic Disease of the Fetus and Newborn Caused by Anti-Group A IgG From a Group B Mother. J Pediatr Hematol Oncol 2021;43:e785-7. [Crossref] [PubMed]
- Krog GR, Donneborg ML, Hansen BM, et al. Prediction of ABO hemolytic disease of the newborn using pre- and perinatal quantification of maternal anti-A/anti-B IgG titer. Pediatr Res 2021;90:74-81. [Crossref] [PubMed]
- Goulet DR, Atkins WM. Considerations for the Design of Antibody-Based Therapeutics. J Pharm Sci 2020;109:74-103. [Crossref] [PubMed]
- Lu L, Zhang H, Zhan M, et al. Antibody response and therapy in COVID-19 patients: what can be learned for vaccine development? Sci China Life Sci 2020;63:1833-49. [Crossref] [PubMed]
- Nagano K, Tsutsumi Y. Phage Display Technology as a Powerful Platform for Antibody Drug Discovery. Viruses 2021;13:178. [Crossref] [PubMed]
- Kondzior M, Grabowska I. Antibody-Electroactive Probe Conjugates Based Electrochemical Immunosensors. Sensors (Basel) 2020;20:2014. [Crossref] [PubMed]
- Remmel JL, Ackerman ME. Rationalizing Random Walks: Replicating Protective Antibody Trajectories. Trends Immunol 2021;42:186-97. [Crossref] [PubMed]
- Kuramochi T, Igawa T, Tsunoda H, et al. Humanization and Simultaneous Optimization of Monoclonal Antibody. Methods Mol Biol 2019;1904:213-30. [Crossref] [PubMed]
- Li Y. A brief introduction of IgG-like bispecific antibody purification: Methods for removing product-related impurities. Protein Expr Purif 2019;155:112-9. [Crossref] [PubMed]
- Dean AQ, Luo S, Twomey JD, et al. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs 2021;13:1951427. [Crossref] [PubMed]
- Ma H, Ó'Fágáin C, O'Kennedy R. Antibody stability: A key to performance - Analysis, influences and improvement. Biochimie 2020;177:213-25. [Crossref] [PubMed]
- Dovgan I, Koniev O, Kolodych S, et al. Antibody-Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjug Chem 2019;30:2483-501. [Crossref] [PubMed]
- Farahavar G, Abolmaali SS, Gholijani N, et al. Antibody-guided nanomedicines as novel breakthrough therapeutic, diagnostic and theranostic tools. Biomater Sci 2019;7:4000-16. [Crossref] [PubMed]
- Esmaeilzadeh A, Rostami S, Yeganeh PM, et al. Recent advances in antibody-based immunotherapy strategies for COVID-19. J Cell Biochem 2021;122:1389-412. [Crossref] [PubMed]
- Kouhi A, Pachipulusu V, Kapenstein T, et al. Brain Disposition of Antibody-Based Therapeutics: Dogma, Approaches and Perspectives. Int J Mol Sci 2021;22:6442. [Crossref] [PubMed]
- Acheampong DO. Bispecific Antibody (bsAb) Construct Formats and their Application in Cancer Therapy. Protein Pept Lett 2019;26:479-93. [Crossref] [PubMed]
- Cao YJ, Yu C, Wu KL, et al. Synthesis of precision antibody conjugates using proximity-induced chemistry. Theranostics 2021;11:9107-17. [Crossref] [PubMed]
- Zhao P, Gunawardena HP, Zhong X, et al. Microdroplet Ultrafast Reactions Speed Antibody Characterization. Anal Chem 2021;93:3997-4005. [Crossref] [PubMed]
- Tabasinezhad M, Talebkhan Y, Wenzel W, et al. Trends in therapeutic antibody affinity maturation: From in-vitro towards next-generation sequencing approaches. Immunol Lett 2019;212:106-13. [Crossref] [PubMed]
- Norman RA, Ambrosetti F, Bonvin AMJJ, et al. Computational approaches to therapeutic antibody design: established methods and emerging trends. Brief Bioinform 2020;21:1549-67. [Crossref] [PubMed]
- Kanyavuz A, Marey-Jarossay A, Lacroix-Desmazes S, et al. Breaking the law: unconventional strategies for antibody diversification. Nat Rev Immunol 2019;19:355-68. [Crossref] [PubMed]
- Tang F, Shi W, Huang W. Homogeneous Antibody-Drug Conjugates via Glycoengineering. Methods Mol Biol 2019;2033:221-38. [Crossref] [PubMed]
- Lee PS, Chau B, Barman I, et al. Antibody blockade of CD96 by distinct molecular mechanisms. MAbs 2021;13:1979800. [Crossref] [PubMed]
- Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect 2020;9:940-8. [Crossref] [PubMed]
- Teimouri A, Mohtasebi S, Kazemirad E, et al. Role of Toxoplasma gondii IgG Avidity Testing in Discriminating between Acute and Chronic Toxoplasmosis in Pregnancy. J Clin Microbiol 2020;58:e00505-20. [Crossref] [PubMed]
- Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord 2021;14:1756286421997381. [Crossref] [PubMed]
- Yu J, Song Y, Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J Hematol Oncol 2020;13:45. [Crossref] [PubMed]
- Kdimati S, Mullins CS, Linnebacher M. Cancer-Cell-Derived IgG and Its Potential Role in Tumor Development. Int J Mol Sci 2021;22:11597. [Crossref] [PubMed]
- Chen M, Qin R, Jiang M, et al. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int J Infect Dis 2021;104:415-22. [Crossref] [PubMed]
- Hou H, Wang T, Zhang B, et al. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. Clin Transl Immunology 2020;9:e01136. [Crossref] [PubMed]
- Kronimus Y, Dodel R, Galuska SP, et al. IgG Fc N-glycosylation: Alterations in neurologic diseases and potential therapeutic target? J Autoimmun 2019;96:14-23. [Crossref] [PubMed]
- Bockermann R, Järnum S, Runström A, et al. Imlifidase-generated Single-cleaved IgG: Implications for Transplantation. Transplantation 2022;106:1485-96. [Crossref] [PubMed]
(English Language Editor: K. Brown)



