
Novel pathogenic variant combination in LPL causing familial chylomicronemia syndrome in an Asian family and experimental validation in vitro: a case report
Introduction
Familial chylomicronemia syndrome (FCS) is a rare autosomal recessive genetic disorder, with an estimated prevalence of one per million (1,2). FCS is characterized by aberrantly high triglyceride level (>10 mmol/L) in the blood, specifically chylomicronemia, due to the impaired hydrolysis of triglycerides by lipoprotein lipase (LPL), which could lead to the potentially fatal recurrent acute pancreatitis. Clinically, the patients with FCS could present with varying symptoms including nausea, vomiting, abdominal pain, and different clinical features such as eruptive xanthomas, lipemia retinalis, hepatosplenomegaly, pancreatitis, and failure to thrive (3). Eruptive xanthomas are small erythematous or yellow papules localized on the extensor surfaces of extremities, buttocks, and the back (4), which serve as a clue for severe hypertriglyceridemia. Histologically, accumulation of lipid-laden macrophages in the skin can be identified in eruptive xanthomas, which were known to be generated by endothelial cell mediated intracellular hydrolysis of internalized chylomicrons, subsequent release of lipids and uptake by skin macrophages (5). In contrast to multifactorial chylomicronemia syndrome, the clinical manifestations tend to appear in early life in FCS patients (6).
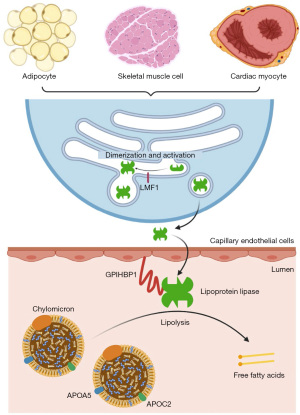
There are five causal genes in FCS: lipoprotein lipase (LPL), apolipoprotein C2 (APOC2), apolipoprotein A5 (APOA5), glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1), and lipase maturation factor 1 (LMF1). Biallelic pathogenic variants in LPL are the predominant cause of FCS. LPL encodes lipoprotein lipase in various cell types, including adipocytes, skeletal muscle cells and cardiac myocytes. After folding and assembly (i.e., maturation) with the help of LMF1 (7), lipoprotein lipase is released extracellularly and binds to GPIHBP1 expressed on capillary endothelial cells, which shuttles lipoprotein lipase into the capillary lumen (8). There, lipoprotein lipase catalyzes the hydrolysis of triglycerides (9), with the help of two regulators: apolipoprotein C-II (apoC-II) (10) and apolipoprotein A-V (apoA-V) (11) (Figure 1). While apoC-II serves as a cofactor for lipoprotein lipase, apoA-V affects plasma triglyceride levels possibly by stabilizing the lipolytic machinery via binding to lipoproteins, endothelial proteoglycans, and lipoprotein lipase (12). We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-15/rc).
Case presentation
A 4-year-old girl was referred to our Thrombosis and Hemostasis center for follow-up findings of extremely prolonged activated partial thromboplastin time (APTT) value (>120 s), prothrombin time (PT) value (>80 s), and thrombin time (TT) value (>50 s) at another hospital, and incidental findings of splenomegaly and lipemia during routine health examination for kindergarten. The patient had no history of bleeding problems or physical findings of coagulopathy.
Physical examination revealed that her spleen was enlarged with its lower edge palpated 2.5 cm below the left costal margin, and splenomegaly was confirmed by B ultrasound, showing a spleen size of 10.5 cm × 3.5 cm. No obvious abnormality in liver, gallbladder or pancreas was found by B ultrasound examination.
A lipid profile showed significantly increased triglyceride (TG) level of 22.5 mmol/L (reference range: 0–1.7 mmol/L) (Figure 2A), and marginally increased level of total cholesterol (5.44 mmol/L, reference range: 0–5.2 mmol/L). Levels of HDL-C, LDL-C, and apolipoprotein A-I were slightly decreased, while apolipoprotein B level was normal (Table 1). Patient’s liver function was normal, except for marginally increased ALT (Table 1).
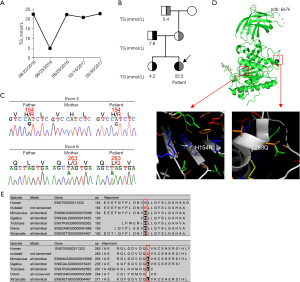
Table 1
| Tests | Values | Reference ranges |
|---|---|---|
| Triglyceride | 22.5 mmol/L | 0–1.7 mmol/L |
| Total cholesterol | 5.44 mmol/L | 0–5.2 mmol/L |
| HDL-C | 0.44 mmol/L | 0.9–1.81 mmol/L |
| LDL-C | 1.53 mmol/L | 2.07–3.36 mmol/L |
| Apolipoprotein A-I | 0.9 g/L | 1–1.76 g/L |
| Apolipoprotein B | 0.83 g/L | 0.6–1.14 g/L |
| AST | 35 IU/L | 10–67 IU/L |
| γ-GT | 8 IU/L | 7–32 IU/L |
| ALT | 42 IU/L | 5–35 IU/L |
| APTT (repeated) | 38.6 s | 28–40 s |
| PT (repeated) | 14.1 s | 11–14.5 s |
| TT (repeated) | 13.9 s | 14–21s |
| Fibrinogen | 1.96 g/L | 2–5 g/L |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; γ-GT, gamma-glutamyl transferase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time.
Because chylomicronemia can increase plasma turbidity and interfere with coagulation assay using optical analyzer (13), a repeat coagulation assay was performed using magnetic bead method instead. The results showed a normal coagulation profile: APTT 38.6 s (reference range 28–40 s), PT 14.1 s (reference range 11–14.5s), TT 13.9 s (reference range 14–21s), fibrinogen: 1.96 g/L (reference range 2–5 g/L).
A detailed family history revealed that the patient’s father, elder sister, and grandfather also had hypertriglyceridemia (father: TG 7.78 mmol/L; sister: TG 4.2 mmol/L; maternal grandfather: TG 5.4 mmol/L) (Figure 2B). The great grandmother had a medical history of diabetes mellitus. The family pedigree could represent a pedigree of an autosomal recessive disorder of hypertriglyceridemia.
Therefore, whole exome sequencing was performed using samples from the patient, her father, mother, elder sister and grandfather. The results showed that the patient had compound heterozygous pathogenic variants in the LPL gene: NM_000237.2, c.461A>G (p.H154R, rs1563574212) and c.788T>A (p.L263Q). Both variants have not been functionally characterized before. The LPL p.H154R variant was inherited from patient’s father, and it was also identified in her elder sister. The LPL p.L263Q variant was inherited from patient’s mother and found in her grandfather. Sanger sequencing was performed to validate the findings (Figure 2C). During the disease course, the patient had no eruptive xanthomas, lipemia retinalis, abdominal pain, nausea, or vomiting. As chylomicronemia increases the risk of recurrent acute pancreatitis, the patient was switched to strict low-fat diet during hospital stay, in which fat was kept below 15% of her daily energy take. The strict low-fat diet helped to reduce TG levels and the lowest level observed during the disease course was 4.91 mmol/L. After the patient was discharged, her TG levels increased due to the altered diet at home. She was administered daily fenofibrate (100 mg), which failed to affect the high TG levels, so the therapy was discontinued.
To predict the potential effects of these variants on the function of LPL protein, we downloaded the crystal structure of LPL from the protein data bank (PDB), the code of which is PDB: 6e7k (2). The localizations of the two variants in the LPL protein were visualized in Figure 2D using the pymol software. Interestingly, the p.H154R variant was two residues away from Asp-156, which makes the catalytic triad together with Ser-132 and His-241 (14). Homologous protein alignment using MutationTaster2 (15) showed that both His-154 and Leu-263 were conserved among different species (Figure 2E), indicating the potential harmful effects of the two LPL pathogenic variants. The pathogenicity of these two variants was predicted using PANTHER (Protein ANalysis THrough Evolutionary Relationships) Classification System (16) and SIFT (Sorting Intolerant From Tolerant) (17), both of which (Figure S1 and Figure S2, respectively), predicted that the two variants had high probability to be damaging.
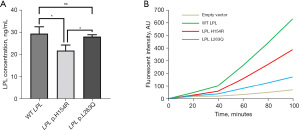
In vitro experiments were carried out to validate the pathogenicity of these two LPL variants. First, the human LPL gene was cloned into pcDNA3.1 vector for expression of wild-type (WT) lipoprotein lipase. Site-directed mutagenesis was performed to construct expression vectors with LPL p.H154R or p.L263Q variant. Human embryonic kidney 293 cells were transfected with empty vector, WT, LPL p.H154R or LPL p.L263Q plasmids using lipofectamine 3000 (Invitrogen). The expression levels of WT and mutant proteins were quantified by ELISA (Cloud-Clone Corp). The results showed that the p.H154R variant caused significantly reduced expression of lipoprotein lipase, while the protein expression levels were comparable between WT and LPL p.L263Q (Figure 3A). To determine how the two mutants affected protein function, we quantified the lipolytic activity of WT and mutant proteins using an LPL activity assay kit (Sigma-Aldrich). The results showed that both mutants displayed significantly reduced catalytic activity compared to the WT vector (p.L263Q < p.H154R < WT) (Figure 3B).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Fredrickson type 1 hyperlipoproteinemia, also known as familial chylomicronemia syndrome, has an estimated prevalence of 1 per million, which is one of the lowest among all hyperlipoproteinemia subtypes (18). In this family, the patient showed early presentation of severe hypertriglyceridemia (22.31 mmol/L) and splenomegaly. Three other family members who had heterozygous variant also presented with increased TG levels, hinting a familial inheritance pattern. Whole exome sequencing revealed the patient had compound heterozygous variants in the LPL gene (p.H154R and p.L263Q). LPL p.H154R was inherited from her father whereas LPL p.L263Q was inherited from her mother. The clinical and genetic findings corroborated the diagnosis of FCS in the patient. Acute pancreatitis is a characteristic of FCS. According to the APPROACH study, the percentage of FCS patients with the first pancreatitis event before 5 years old was around 15% (19). In addition, diet variation is an important factor in the initial timing of acute pancreatitis. This may explain why our patient did not have pancreatitis when diagnosed.
During the diagnosis of this patient, it is worth noting that the patient was referred to our center due to abnormal coagulation profile. Optical analyzers are widely used in coagulation profiling. Lipemia, especially the increased chylomicron level, is an important factor during testing as it can increase plasma turbidity (13). Therefore, caution should be taken when interpretating the coagulation profile of FCS patients due to increased plasma turbidity caused by the high chylomicron levels. If there are abnormal coagulation profiles measured by turbidity-based assay, the possibility of chylomicronemia should be considered and a repeat coagulation assay should be performed using other methods that are not based on turbidity, such as magnetic bead method.
Although some family members had hypertriglyceridemia for a long time, the underlying genetic defects were only discovered due to the workup for a compound heterozygous patient with significantly abnormal findings. Interestingly, both LPL pathogenic variants (p.H154R and p.L263Q) in this family have not been functionally characterized before. Further in vitro testing supported the pathogenicity of these variants as we found that the LPL p.H154R variant reduced the expression of lipoprotein lipase and decreased its lipolytic activity, while the LPL p.L263Q variant mainly impaired its lipolytic activity. Our analyses do not explain the mechanism of reduced LPL level in the p.H154R mutant cell line. However, we might speculate that this variant might alter the structure of LPL and led to protein instability. To validate this hypothesis, further studies such as hydrogen-deuterium exchange (HDX) coupled with mass spectrometry (HDXMS) could be performed. One limitation of our study is that LPL activity and mass level were not measured using post-heparin plasma sample of the patient, which had been performed by other groups to validate FCS (20). The prevalence of loss-of-function (LOF) LPL variant carrier is varying among different studies, which is affected by the study populations and LPL variant types (21-23). Due to founder effects, some populations have higher carrier frequencies of LOF LPL variants than others. For example, the carrier frequency of defective LPL alleles is as high as 1 in 40 in some regions of the French Canadian population, which has the highest prevalence of homozygous LPL deficiency around the world (24,25). Unfortunately, such carrier frequency data was not available for the Chinese population. The impact of LOF LPL variant can also be varying. There is great heterogeneity in lipoprotein lipase activity and plasma TG levels (ranged from normal to severe) among heterozygous carriers with LOF LPL variants, which could be affected by factors such as underlying polygenic risk variants of hypertriglyceridemia, hyperinsulinemia and abdominal obesity (26-29). The contributing role of triglycerides and triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease is supported by mutational analyses, genome-wide association studies, and Mendelian randomization studies (30,31). Studies are ongoing to identify effective measures to lower triglyceride-rich lipoproteins and remnants to reduce the risk of atherosclerosis (30,31).
Regarding treatment, strict low-fat diet, with fat kept below 15% of daily energy take, helped to reduce the patient’s TG levels. However, the patient’s response to fenofibrate was minimal and the therapy was discontinued. For the treatment of FCS, a therapeutic lifestyle change including restricted fat intake should be initiated first. Pharmacologically, current lipid-lowering therapies include statins, fibrates, niacin, and omega-3 fatty acids (32). Unfortunately, these drugs are not quite effective in FCS patients, who may still have high TG levels and recurrent acute pancreatitis. Apolipoprotein C-III (apoC-III) and angiopoietin-like 3 (ANGPTL3) are physiological lipoprotein lipase activity inhibitors. Therefore, antisense oligonucleotide targeting apoC-III and monoclonal antibody against ANGPTL3 are developed and being tested in clinical trials to see whether they can effectively preserve LPL-mediated lipolysis of TG (33,34). Alipogene tiparvovec (Glybera) is a novel gene therapy which uses the adeno-associated virus type I vector encoding a hyper-functional version of lipoprotein lipase to reduce TG levels in FCS patients (35,36). However, the therapeutic effect of alipogene tiparvovec cannot be sustained over 6 months, and this drug was discontinued in the European market (33).
In summary, we present a family of familial chylomicronemia syndrome caused by compound heterozygous LPL pathogenic variants (p.H154R and p.L263Q). The LPL p.H154R variant reduced the expression of lipoprotein lipase and decreased its lipolytic activity, while the novel LPL p.L263Q variant mainly impaired its lipolytic activity.
Acknowledgments
Funding: This study was funded by Jiangsu Provincial Special Program of Medical Science (No. BL2012005); Jiangsu Province’s Key Medical Center (No. ZX201102); The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-15/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-15/coif). The authors report that this study was funded by Jiangsu Provincial Special Program of Medical Science (BL2012005); Jiangsu Province’s Key Medical Center (ZX201102); The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldberg RB, Chait A. A Comprehensive Update on the Chylomicronemia Syndrome. Front Endocrinol (Lausanne) 2020;11:593931. [Crossref] [PubMed]
- Baass A, Paquette M, Bernard S, et al. Familial chylomicronemia syndrome: an under-recognized cause of severe hypertriglyceridaemia. J Intern Med 2020;287:340-8. [Crossref] [PubMed]
- Brahm AJ, Hegele RA. Chylomicronaemia--current diagnosis and future therapies. Nat Rev Endocrinol 2015;11:352-62. [Crossref] [PubMed]
- Marogi EP, Ohiomoba RO, Stone NJ. Eruptive Xanthomas: Importance of Recognition to Reduce Delay of Effective Triglyceride Reduction. Am J Med 2022;135:444-7. [Crossref] [PubMed]
- Cabodevilla AG, Tang S, Lee S, et al. Eruptive xanthoma model reveals endothelial cells internalize and metabolize chylomicrons, leading to extravascular triglyceride accumulation. J Clin Invest 2021;131:145800. [Crossref] [PubMed]
- D'Erasmo L, Di Costanzo A, Cassandra F, et al. Spectrum of Mutations and Long-Term Clinical Outcomes in Genetic Chylomicronemia Syndromes. Arterioscler Thromb Vasc Biol 2019;39:2531-41. [Crossref] [PubMed]
- Wolska A, Dunbar RL, Freeman LA, et al. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017;267:49-60. [Crossref] [PubMed]
- Beigneux AP, Davies BS, Gin P, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 2007;5:279-91. [Crossref] [PubMed]
- Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol 2016;27:233-41. [Crossref] [PubMed]
- Baggio G, Manzato E, Gabelli C, et al. Apolipoprotein C-II deficiency syndrome. Clinical features, lipoprotein characterization, lipase activity, and correction of hypertriglyceridemia after apolipoprotein C-II administration in two affected patients. J Clin Invest 1986;77:520-7. [Crossref] [PubMed]
- Priore Oliva C, Pisciotta L, Li Volti G, et al. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2005;25:411-7. [Crossref] [PubMed]
- Ramasamy I. Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med 2014;52:1695-727. [Crossref] [PubMed]
- Gardiner C, Lane P, Tailor H, et al. A practical method for reducing the interference due to lipaemia in coagulation tests. Int J Lab Hematol 2020;42:140-4. [Crossref] [PubMed]
- Emmerich J, Beg OU, Peterson J, et al. Human lipoprotein lipase. Analysis of the catalytic triad by site-directed mutagenesis of Ser-132, Asp-156, and His-241. J Biol Chem 1992;267:4161-5. [Crossref] [PubMed]
- Schwarz JM, Cooper DN, Schuelke M, et al. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014;11:361-2. [Crossref] [PubMed]
- Tang H, Thomas PD. PANTHER-PSEP: predicting disease-causing genetic variants using position-specific evolutionary preservation. Bioinformatics 2016;32:2230-2. [Crossref] [PubMed]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009;4:1073-81. [Crossref] [PubMed]
- Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients 2013;5:981-1001. [Crossref] [PubMed]
- Blom DJ, O'Dea L, Digenio A, et al. Characterizing familial chylomicronemia syndrome: Baseline data of the APPROACH study. J Clin Lipidol 2018;12:1234-1243.e5. [Crossref] [PubMed]
- Ariza MJ, Rioja J, Ibarretxe D, et al. Molecular basis of the familial chylomicronemia syndrome in patients from the National Dyslipidemia Registry of the Spanish Atherosclerosis Society. J Clin Lipidol 2018;12:1482-1492.e3. [Crossref] [PubMed]
- Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res 2002;43:1997-2006. [Crossref] [PubMed]
- Nordestgaard BG, Abildgaard S, Wittrup HH, et al. Heterozygous lipoprotein lipase deficiency: frequency in the general population, effect on plasma lipid levels, and risk of ischemic heart disease. Circulation 1997;96:1737-44. [Crossref] [PubMed]
- Hall S, Chu G, Miller G, et al. A common mutation in the lipoprotein lipase gene promoter, -93T/G, is associated with lower plasma triglyceride levels and increased promoter activity in vitro. Arterioscler Thromb Vasc Biol 1997;17:1969-76. [Crossref] [PubMed]
- Gagné C, Brun LD, Julien P, et al. Primary lipoprotein-lipase-activity deficiency: clinical investigation of a French Canadian population. CMAJ 1989;140:405-11. [PubMed]
- Minnich A, Kessling A, Roy M, et al. Prevalence of alleles encoding defective lipoprotein lipase in hypertriglyceridemic patients of French Canadian descent. J Lipid Res 1995;36:117-24. [Crossref] [PubMed]
- Julien P, Vohl MC, Gaudet D, et al. Hyperinsulinemia and abdominal obesity affect the expression of hypertriglyceridemia in heterozygous familial lipoprotein lipase deficiency. Diabetes 1997;46:2063-8. [Crossref] [PubMed]
- Babirak SP, Iverius PH, Fujimoto WY, et al. Detection and characterization of the heterozygote state for lipoprotein lipase deficiency. Arteriosclerosis 1989;9:326-34. [Crossref] [PubMed]
- Dron JS, Hegele RA. Genetics of Hypertriglyceridemia. Front Endocrinol (Lausanne) 2020;11:455. [Crossref] [PubMed]
- Ueda M, Burke FM, Remaley AT, et al. Familial Chylomicronemia Syndrome with a Novel Homozygous LPL Mutation Identified in Three Siblings in Their 50’s. Ann Intern Med 2020;172:500-2. [Crossref] [PubMed]
- Ginsberg HN, Packard CJ, Chapman MJ, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur Heart J 2021;42:4791-806. [Crossref] [PubMed]
- Budoff M. Triglycerides and Triglyceride-Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am J Cardiol 2016;118:138-45. [Crossref] [PubMed]
- Tatsuno I, Saito Y, Kudou K, et al. Long-term safety and efficacy of TAK-085 in Japanese subjects with hypertriglyceridemia undergoing lifestyle modification: the omega-3 fatty acids randomized long-term (ORL) study. J Clin Lipidol 2013;7:615-25. [Crossref] [PubMed]
- Hegele RA, Tsimikas S. Lipid-Lowering Agents. Circ Res 2019;124:386-404. [Crossref] [PubMed]
- D'Erasmo L, Bini S, Arca M. Rare Treatments for Rare Dyslipidemias: New Perspectives in the Treatment of Homozygous Familial Hypercholesterolemia (HoFH) and Familial Chylomicronemia Syndrome (FCS). Curr Atheroscler Rep 2021;23:65. [Crossref] [PubMed]
- Scott LJ. Alipogene tiparvovec: a review of its use in adults with familial lipoprotein lipase deficiency. Drugs 2015;75:175-82. [Crossref] [PubMed]
- Salmon F, Grosios K, Petry H. Safety profile of recombinant adeno-associated viral vectors: focus on alipogene tiparvovec (Glybera®). Expert Rev Clin Pharmacol 2014;7:53-65. [Crossref] [PubMed]


