
Evaluating fetal heart morphology in hypertensive disorders of pregnancy using the fetal heart quantitative technique
Introduction
Hypertensive disorders of pregnancy (HDP) are a group of diseases associated with elevated blood pressure during pregnancy, are mainly characterized by hypertension and proteinuria, and may be associated with systemic multiple organ dysfunction or failure. In severe cases, convulsions, coma, and even death may occur (1,2). HDP includes chronic hypertension, gestational hypertension, preeclampsia, and eclampsia (3). Studies have shown that HDP have high perinatal morbidity and mortality rates, such as preterm birth, low birth weight, birth asphyxia, stillbirth, and early neonatal death (4,5). Changes in fetal heart function during HDP can reflect the developmental status of the fetus in utero. Thus, accurate evaluations of changes in fetal heart function can be used to evaluate changes in the fetus during pregnancy-induced hypertension,assisting clinicians to understand the intrauterine development of the fetus, implement timely interventions and treatments, and accurately determine patient prognosis.
Fetal heart quantification (HQ) technology is a new technology that combines two-dimensional speckle tracking technology with other technologies applied to fetal heart (6). It can automatically track the border of the endocardium of the left and right ventricles, divide the left and right ventricles into 24 equally spaced segments, and quantitatively evaluate the size, shape and contractility of the fetal heart (7). The sphericity index (SI) is an important parameter for evaluating the morphology of the whole heart or ventricle, and it is currently used to evaluate the changes of heart shape in adults and children (8-10), but it is less commonly used in the evaluation of fetal heart function. A previous study has proved that Fetal HQ can simply and reliably evaluate cardiac SI (11). This study sought to investigate the clinical application value of fetal HQ technology in the analysis of fetal heart form in HDP. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-492/rc).
Methods
Study population
A total of 53 normal pregnant women with a gestational age (GA) of 29.6±2.7 w (the normal group) and 26 HDP pregnant women with a GA of 29.8±2.3 w (the case group) who underwent fetal ultrasonography at the Ningxia Women’s and Children’s Hospital, Beijing University First Hospital and the General Hospital of Ningxia Medical University from December 2021 to March 2022 were included in this study. The pregnant women had an average age of 31.09±0.65 y. The diagnostic criteria for HDP (3) was pregnancy with elevated blood pressure, systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg.
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) be a pregnant women aged ≥18 years; (II) be at 25–35 weeks’ gestation; (III) have a regular menstrual cycle; and (IV) have a single pregnancy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had gestational diabetes, thyroid disease, and other complications during pregnancy, or other diseases that may impact the size, morphology and systolic function of the fetal heart; (II) had a prenatal diagnosis of fetal chromosomal abnormalities; (III) had abnormal intra-cardiac and extra-cardiac structures of the fetus; and/or (IV) had poor quality images.
All the pregnant women signed an informed consent for fetal ultrasonography and were informed of the reliability and limitations of the examination. This study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University (batch No. [202020]0520B) and the Ethics Committee of Ningxia Women’s and Children’s Hospital, Beijing University First Hospital (Ningxia Hui Autonomous Region Maternal and Child Health Hospital) (batch No. KJ-LL-2022-26). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Instruments
A GE Voluson E8/E10 color Doppler ultrasound system (GE Healthcare, Chicago, IL, USA) equipped with C1-6/C2-9 transducers and the fetal HQ software package (GE Healthcare) were used in this study.
Steps
The general clinical data of the pregnant women was collected, including data on age, gestational weeks, blood pressure, last menstrual period (LMP), and past medical history. Each pregnant women was instructed to lie in the supine or lateral position, and routine 2D ultrasonography was performed on the fetus to measure the fetal head circumference (HC), biparietal diameter (BPD), abdominal circumference (AC), femur length (FL), and humerus length (HL), and to estimate fetal ultrasound gestational age and fetal weight.
Next, routine fetal echocardiography was performed, the instrument conditions were adjusted (to reduce the fan angle or depth), and a fetal standard 2D 4-chamber cardiac dynamic map was obtained. The image quality requirements were as follows: not less than 3 s, a frame rate of ≥80 frames/second, and a clearly displayed endocardium border. In this process, the interference of maternal breathing and fetal movement was excluded as much as possible. The acquired 4-chamber cardiac dynamic map was imported into the fetal HQ software package for analysis, the basal-apical length (BAL) and transverse length (TL) of the fetal heart were measured, and the GSI (BAL/TL) of the heart was calculated (see Figure 1).

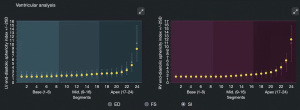
The software automatically tracks the borders of the endocardium of the left and right ventricles, dividing them into 24 equal-spacing segments (see Figure 2). The SI of the 24 segments was obtained; that is, the ratio of the longitudinal meridian of the ventricle to the transverse diameter of each segment. The software was also used to calculate the Z scores of the GSI and 24-segment SI; This study set Z scores >2 or <−2 as abnormal (see Figure 3).

Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, NY, USA) software was used for the statistical analysis. The measurement data with a normal distribution and homogeneous variance are expressed as the . The 24-segment SIs of the left and right ventricles of normal fetuses were compared by paired t-tests. The 2 independent samples t-test was used to compare the fetal GSI and 24-segment SIs between the 2 groups. The chi-square test was used to compare the abnormal rates of the Z scores. A P value <0.05 was considered statistically significant.
Results
Comparison of the SIs of the 24 segments of the left and right ventricles of fetuses in the normal group
The SIs of the 24 segments of the left and right ventricles of the 53 fetuses in the normal group were compared, and the pairwise comparison of segments 1–16 and 20–24 was statistically significant (P<0.05), but the pairwise comparison of segments 17–19 was not statistically significance (P>0.05) (see Table 1).
Table 1
| Location | Segment | LV-SI | RV-SI | t | P value |
|---|---|---|---|---|---|
| Basal | 1 | 1.74±0.38 | 1.51±0.32 | 3.891 | <0.001 |
| Basal | 2 | 1.74±0.38 | 1.48±0.30 | 4.373 | <0.001 |
| Basal | 3 | 1.74±0.38 | 1.46±0.29 | 4.801 | <0.001 |
| Basal | 4 | 1.74±0.38 | 1.44±0.28 | 5.179 | <0.001 |
| Basal | 5 | 1.75±0.38 | 1.43±0.27 | 5.503 | <0.001 |
| Basal | 6 | 1.77±0.38 | 1.44±0.27 | 5.874 | <0.001 |
| Basal | 7 | 1.79±0.37 | 1.45±0.27 | 6.256 | <0.001 |
| Basal | 8 | 1.82±0.36 | 1.47±0.27 | 6.657 | <0.001 |
| Mid | 9 | 1.85±0.36 | 1.50±0.27 | 6.870 | <0.001 |
| Mid | 10 | 1.88±0.36 | 1.54±0.28 | 6.815 | <0.001 |
| Mid | 11 | 1.92±0.36 | 1.58±0.29 | 6.556 | <0.001 |
| Mid | 12 | 1.96±0.37 | 1.63±0.30 | 6.016 | <0.001 |
| Mid | 13 | 2.00±0.39 | 1.69±0.31 | 5.392 | <0.001 |
| Mid | 14 | 2.05±0.40 | 1.76±0.33 | 4.628 | <0.001 |
| Mid | 15 | 2.11±0.42 | 1.85±0.35 | 3.807 | <0.001 |
| Mid | 16 | 2.16±0.43 | 1.96±0.38 | 2.780 | 0.008 |
| Apical | 17 | 2.22±0.43 | 2.10±0.41 | 1.521 | 0.134 |
| Apical | 18 | 2.27±0.43 | 2.27±0.46 | 0.053 | 0.958 |
| Apical | 19 | 2.36±0.43 | 2.50±0.53 | –1.473 | 0.147 |
| Apical | 20 | 2.53±0.45 | 2.84±0.65 | –2.752 | 0.008 |
| Apical | 21 | 2.86±0.51 | 3.38±0.84 | –3.646 | 0.001 |
| Apical | 22 | 3.53±0.64 | 4.33±1.15 | –4.153 | <0.001 |
| Apical | 23 | 5.03±0.94 | 6.33±1.76 | –4.410 | <0.001 |
| Apical | 24 | 9.78±1.84 | 12.45±3.57 | –4.540 | <0.001 |
SI, sphericity index; LV, left ventricle; RV, right ventricle.
Comparison of the fetal GSIs between the 2 groups
The fetal GSI of the 53 normal group was 1.16%±0.10%, and the fetal GSI of the 26 case group was 1.18%±0.13%. There was no statistically significant difference in the fetal GSI between the 2 groups (P>0.05).
Comparison of the 24-segment SIs of the left and right ventricles of the 2 groups of fetuses
There was no statistically significant difference in the SIs of the 24 segments of the left ventricle of the 53 fetuses in the normal group and the 26 fetuses with gestational hypertension in the case group (P>0.05) (see Table 2). There was no statistically significant difference in the SIs of the 1–20 segments of the right ventricle between the 2 groups (P>0.05), but there was a statistically significant difference in the SIs of the 21–24 segments of the right ventricle between the 2 groups (P<0.05) (see Table 3).
Table 2
| Location | Segment | Case group | Normal group | t | P value |
|---|---|---|---|---|---|
| Basal | 1 | 1.80±0.41 | 1.74±0.38 | 0.665 | 0.508 |
| Basal | 2 | 1.79±0.38 | 1.74±0.38 | 0.576 | 0.566 |
| Basal | 3 | 1.78±0.35 | 1.74±0.38 | 0.482 | 0.631 |
| Basal | 4 | 1.77±0.34 | 1.74±0.38 | 0.399 | 0.691 |
| Basal | 5 | 1.78±0.33 | 1.75±0.38 | 0.294 | 0.769 |
| Basal | 6 | 1.78±0.34 | 1.77±0.38 | 0.198 | 0.843 |
| Basal | 7 | 1.80±0.35 | 1.79±0.37 | 0.083 | 0.934 |
| Basal | 8 | 1.82±0.37 | 1.82±0.36 | –0.042 | 0.967 |
| Mid | 9 | 1.84±0.39 | 1.85±0.36 | –0.115 | 0.909 |
| Mid | 10 | 1.87±0.40 | 1.88±0.36 | –0.126 | 0.900 |
| Mid | 11 | 1.91±0.41 | 1.92±0.36 | –0.100 | 0.921 |
| Mid | 12 | 1.96±0.41 | 1.96±0.37 | –0.031 | 0.975 |
| Mid | 13 | 2.01±0.42 | 2.00±0.39 | 0.097 | 0.923 |
| Mid | 14 | 2.07±0.42 | 2.05±0.40 | 0.231 | 0.818 |
| Mid | 15 | 2.14±0.43 | 2.11±0.42 | 0.382 | 0.703 |
| Mid | 16 | 2.22±0.44 | 2.16±0.43 | 0.576 | 0.566 |
| Apical | 17 | 2.30±0.44 | 2.22±0.43 | 0.793 | 0.430 |
| Apical | 18 | 2.38±0.42 | 2.27±0.43 | 1.028 | 0.307 |
| Apical | 19 | 2.49±0.42 | 2.36±0.43 | 1.231 | 0.222 |
| Apical | 20 | 2.68±0.44 | 2.53±0.45 | 1.407 | 0.163 |
| Apical | 21 | 3.04±0.51 | 2.86±0.51 | 1.504 | 0.137 |
| Apical | 22 | 3.77±0.66 | 3.53±0.64 | 1.566 | 0.122 |
| Apical | 23 | 5.40±0.98 | 5.03±0.94 | 1.593 | 0.115 |
| Apical | 24 | 10.50±1.94 | 9.78±1.84 | 1.605 | 0.113 |
SI, sphericity index.
Table 3
| Location | Segment | Case group | Normal group | t | P value |
|---|---|---|---|---|---|
| Basal | 1 | 1.52±0.37 | 1.51±0.32 | 0.191 | 0.849 |
| Basal | 2 | 1.51±0.35 | 1.48±0.30 | 0.360 | 0.720 |
| Basal | 3 | 1.49±0.34 | 1.46±0.29 | 0.521 | 0.604 |
| Basal | 4 | 1.49±0.33 | 1.44±0.28 | 0.666 | 0.507 |
| Basal | 5 | 1.48±0.32 | 1.43±0.27 | 0.738 | 0.463 |
| Basal | 6 | 1.48±0.31 | 1.44±0.27 | 0.696 | 0.488 |
| Basal | 7 | 1.49±0.30 | 1.45±0.27 | 0.550 | 0.584 |
| Basal | 8 | 1.50±0.28 | 1.47±0.27 | 0.401 | 0.690 |
| Mid | 9 | 1.52±0.28 | 1.50±0.27 | 0.252 | 0.802 |
| Mid | 10 | 1.55±0.28 | 1.54±0.28 | 0.103 | 0.918 |
| Mid | 11 | 1.59±0.29 | 1.58±0.29 | 0.059 | 0.953 |
| Mid | 12 | 1.63±0.31 | 1.63±0.30 | –0.010 | 0.992 |
| Mid | 13 | 1.68±0.33 | 1.69±0.31 | –0.096 | 0.923 |
| Mid | 14 | 1.74±0.35 | 1.76±0.33 | –0.269 | 0.788 |
| Mid | 15 | 1.81±0.36 | 1.85±0.35 | –0.453 | 0.652 |
| Mid | 16 | 1.90±0.38 | 1.96±0.38 | –0.679 | 0.499 |
| Apical | 17 | 2.00±0.39 | 2.10±0.41 | –0.985 | 0.328 |
| Apical | 18 | 2.13±0.39 | 2.27±0.46 | –1.331 | 0.187 |
| Apical | 19 | 2.30±0.41 | 2.50±0.53 | –1.689 | 0.095 |
| Apical | 20 | 2.56±0.43 | 2.84±0.65 | –1.980 | 0.051 |
| Apical | 21 | 2.99±0.51 | 3.38±0.84 | –2.164 | 0.034 |
| Apical | 22 | 3.79±0.66 | 4.33±1.15 | –2.245 | 0.028 |
| Apical | 23 | 5.48±0.99 | 6.33±1.76 | –2.276 | 0.026 |
| Apical | 24 | 10.73±1.98 | 12.45±3.57 | –2.286 | 0.025 |
SI, sphericity index.
Comparison of the Z values of the fetal GSIs and 24-segment SIs between the 2 groups
Among the 53 fetuses in the normal group, 7 had incorrect Z values for the fetal GSI, and the incorrect ratio was 13.2%. Among the 26 fetuses in the case group, 4 had abnormal Z values for fetal GSI, and the incorrect ratio was 15.4%. There was no statistically significant difference in the incorrect ratio of Z values of GSI between the 2 groups (P>0.05).
In relation to the left and right ventricles of the 53 normal fetuses, there were 2,544 SI segments, of which 122 segments had incorrect Z values, with an incorrect ratio of 4.8%. In relation to the left ventricles specifically, there were 1,272 SI segments, of which 45 segments had incorrect Z values, with an incorrect ratio of 3.5%. In relation to the right ventricles specifically, there were 1,272 SI segments, of which 77 segments had incorrect Z values, with an incorrect ratio of 6.1%.
In relation to the left and right ventricles of the 26 case group fetuses, there were 1,248 SI segments, of which 51 segments had incorrect Z values, with an incorrect ratio of 4.1%. In relation to the left ventricles specifically, there were 624 SI segments, of which 34 segments had incorrect Z values, with an incorrect ratio of 5.4%. In relation to the right ventricles specifically, there were 624 SI segments, of which 17 segments had incorrect Z values, with an incorrect ratio of 2.7%.
There was no statistically significant difference in the incorrect ratio of the Z values of the fetal left ventricular SIs between the 2 groups (P>0.05). There was a statistically significant difference in the incorrect ratio of the Z values of the SIs in each segment of the fetal right ventricle between the 2 groups (P<0.05).
Discussion
HDP endanger the lives of pregnant women and fetuses, and have a high mortality rate. Increases in the blood pressure of pregnant women leads to the narrowing of placental blood vessels, insufficient fetal blood perfusion, ischemia and hypoxia, which in turn affect the growth and development of the fetus in the uterus (12), resulting in abnormal fetal cardiac function (13).
Previously (14), 2D ultrasound and color Doppler blood flow parameters have been used to evaluate changes in fetal cardiac function in HDP, but it was impossible to quantitatively describe the function of the fetal heart. The fetal HQ technique can quantitatively assess changes in fetal heart size, shape, and function (7), and the operation is fast and simple, with good repeatability. This technique has also been used to quantitatively analyze the changes of fetal heart function in pregnant women with anemia, gestational diabetes, and abnormal fetal heart structure (15-17). To provide a reliable reference for early clinical identification of fetal cardiac morphology or dysfunction. In adult and child patients, the geometric changes of left and right ventricles have been reported as signs of cardiac dysfunction. The latest research shows that fetal growth restriction, gestational diabetes mellitus, gestational anemia, etc. may lead to abnormal shapes of left and right ventricles of the fetus (15,17,18). Traditional evaluation of fetal heart morphology only provides a simple description, and there are no quantitative parameters, let alone the changes of SI in each segment of fetal left and right ventricles. Fetal HQ technology can evaluate the SI of the whole fetal heart and 24 segments of left and right ventricles, which provides a simple and reliable technology for clinical evaluation of the morphological changes of the whole heart and local ventricles.
In this study, we compared the SI of the 24 segments of the left and right ventricles of the normal group and found statistically significant differences between the 1–16 and 20–24 segments (P<0.05). The SI of each segment of the left ventricle in segments 1–16 was significantly higher than that of each segment of the right ventricle, indicating that the right ventricle in segments 1–16 tends to be more spherical than the left ventricle. The SI of each segment of the right ventricle in the 20–24 segments was significantly higher than the SI of each segment of the left ventricle, indicating that the left ventricle in the 20–24 segment tends to be more spherical than the right ventricle. The SI of the apex was significantly higher than that of the basal and middle parts, indicating that the shape of the ventricular cavity tends to be conical.
These results reflect the morphological differences between the left and right ventricles; that is, the left ventricle is mostly conical, while the right ventricle is mostly bullet-shaped, which indirectly confirms the feasibility and accuracy of using the SI to assess ventricular geometry. These results are also consistent with the results of previous studies (19,20). In this study, a total of 2,544 segments of the SI in the left and right ventricles of the fetus in the normal group were calculated. The abnormal rate was 4.8%, and 95.2% fell within the normal range. Thus, the Z score system of fetal HQ is well able to evaluate whether the SI in each stage of the ventricle is normal.
In this study, the GSIs of the fetal hearts between the 2 groups were compared, and the difference was not statistically significant (P>0.05), indicating that gestational hypertension had no significant effect on the overall shape of the fetal heart. A pairwise comparison of the SIs of 24 segments of left and right ventricles of the 2 groups of fetuses showed that there was no significant difference in the SIs of the 24 segments of the left ventricle (P>0.05), and there was no significant difference in the SIs between the 1–20 segments of the right ventricle between the 2 groups (P>0.05), but there was a statistically significant difference in the SIs between the 21–24 segments (P<0.05). Thus, gestational hypertension did not have a significant effect on the shape of the left ventricle of the fetus, but the shape of the right ventricle changed, which had no significant effect on the shape of the basal and middle segments of the ventricle, but did have an effect on the shape of the apical segment of the right ventricle.
In conclusion, we showed that fetal HQ can simply and reliably assess the form of the fetal heart. Thus, this technology has good clinical application feasibility. This study also showed that HDP have no significant effect on the shape of the whole heart and left ventricle but did have an effect on the shape of the apical segment of the right ventricle. Compared to the normal group, the fetal SI was reduced in case group, indicating that the apical segment of the right ventricle tends to be more spherical in the state of HDP, which may be related to the dominance of the right ventricle in cardiovascular circulation during the fetal period, and may also be related to the effect of the anatomical distribution of the cardiovascular blood vessels on ventricular blood supply.
The fetal HQ technique can evaluate the size, morphology and contractility of the fetal heart (7). In this study, only changes in fetal heart morphology under HDP were evaluated. In a follow-up study, the sample size will be increased to comprehensively evaluate the subtle changes of fetal cardiac function under HDP status. Assessing the clinical application value of fetal HQ quantitative analysis of fetal heart function in HDP state, and provide a reference for clinicians to intervene and treat patients.
In conclusion, this study assessed fetal cardiac morphological changes in gestational hypertension using quantitative fetal heart analysis technology, provided a new method for the assessment of fetal cardiac morphological changes in pregnant women with HDP, and improved the clinical application value of prenatal counseling, intervention treatment, and prognosis judgments of pregnant women with HDP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-492/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-492/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-492/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo Z. Epidemiological analysis of pregnancy outcomes and risk factors for adverse pregnancy outcomes in elderly pregnant women. Lanzhou University, 2017.
- Xiao H. Clinical analysis of pregnancy-induced hypertension syndrome complicated with placental abruption. Zhongwai Medical 2014;33:192-3.
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens 2020;38:982-1004. [Crossref] [PubMed]
- Bakker R, Steegers EA, Hofman A, et al. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol 2011;174:797-806. [Crossref] [PubMed]
- Tao S, Liang H, He X, et al. Ultrasound assessment of fetal left ventricular Tei index and its correlation with placental hypoxia in fetuses with hypertensive disorders of pregnancy. Journal of Hainan Medical College 2017;23:1647-50.
- DeVore GR, Klas B, Satou G, et al. Evaluation of Fetal Left Ventricular Size and Function Using Speckle-Tracking and the Simpson Rule. J Ultrasound Med 2019;38:1209-21. [Crossref] [PubMed]
- DeVore GR, Satou G, Sklansky M. Abnormal Fetal Findings Associated With a Global Sphericity Index of the 4-Chamber View Below the 5th Centile. J Ultrasound Med 2017;36:2309-18. [Crossref] [PubMed]
- Vijayalakshmi IB, Yavagal ST, Prabhudev N. Role of echocardiography in assessing the mechanism and effect of ramipril on functional mitral regurgitation in dilated cardiomyopathy. Echocardiography 2005;22:289-95. [Crossref] [PubMed]
- Savu O, Jurcuţ R, Giuşcă S, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012;5:289-97. [Crossref] [PubMed]
- Na L, Liu L, Zhu L, et al. Correlation of ultrasound three-dimensional speckle tracking imaging combined with cardiac magnetic resonance imaging-gadolinium delayed enhancement sequence on left ventricular global systolic function and myocardial fibrosis in adults with hypertrophic cardiomyopathy. Chinese Journal of Ultrasound Imaging 2020;29:6-12.
- Luo Y, Xiao F, Long C, et al. Evaluation of the sphericity index of the fetal heart during middle and late pregnancy using fetalHQ. J Matern Fetal Neonatal Med 2021; Epub ahead of print. [Crossref] [PubMed]
- Shao Q. The clinical significance of color Doppler ultrasonography in the diagnosis of fetal blood flow changes in pregnant women with gestational hypertension. Chinese Journal of Family Planning 2019;27:394-395, 399.
- Liu Y, Wang F, Du M, et al. Effects of gestational hypertension on fetal cardiac Tei index during the third trimester. Chinese Journal of Misdiagnosis 2011;11:4326.
- Wang S, Wang X, Hou Z. Ultrasound multi-parameter assessment of the effects of pregnancy-induced hypertension on fetal cardiac function. China Modern Medical Journal 2021;31:28-33.
- Shen Y, Tan F, Yang J, et al. A preliminary study on fetal cardiac morphology and systolic function of normal and anemic pregnant women by fetal heart quantification technology. Transl Pediatr 2022;11:1336-45. [Crossref] [PubMed]
- Li T, Han J, Han Y, et al. Hemodynamic analysis of fetuses with premature contraction or closure of the ductus arteriosus by conventional fetal echocardiography combined with fetal cardiac quantitative technology. Chinese Journal of Ultrasound Imaging 2021;30:213-8.
- Wang D, Liu C, Liu X, et al. Evaluation of prenatal changes in fetal cardiac morphology and function in maternal diabetes mellitus using a novel fetal speckle-tracking analysis: a prospective cohort study. Cardiovasc Ultrasound 2021;19:25. [Crossref] [PubMed]
- Cruz-Lemini M, Crispi F, Valenzuela-Alcaraz B, et al. A fetal cardiovascular score to predict infant hypertension and arterial remodeling in intrauterine growth restriction. Am J Obstet Gynecol 2014;210:552.e1-e22. [Crossref] [PubMed]
- Li W, Zhao B, Pan M, et al. Preliminary study on the 24-segment spherical index of fetal ventricle in middle and late pregnancy by quantitative fetal heart analysis technique. Chinese Journal of Ultrasound Imaging 2020;29:586-91.
- Li Y, Wang H, Tan F, et al. Preliminary study on evaluating fetal heart shape using fetal heart quantitative analysis technology. Chinese Journal of Ultrasound Medicine 2021;37:1032-5.


