
Prevalence of ground glass nodules in preschool children: a cross-sectional study
Introduction
The concept of ground-glass nodules (GGNs) was proposed with the advent of high-resolution computed tomography (CT). It is not only a new concept in imaging, but also a new clinical problem. With the broad application of thin-slice CT, GGNs, the incidence of which is increasing, have attracted widespread attention (1,2) in the Asian population. A Korean screening study (3) reported GGN lesions in 2.7% of participants.
In 1993, Remy-Jardin (4) systematically defined ground-glass opacity (GGO) for the first time. According to their definition, GGO must meet the following four standards: (I) ground-glass shadow with increased density; (II) the pulmonary blood vessels and pulmonary bronchi underneath are not blocked; (III) a high-resolution CT imaging result; and (IV) there is a wider window width setting [window width: 1,500–2,000 Hounsfield unit (HU), window level: −500 to 700 HU]. According to the Fleischner Society, GGN is an imaging feature characterized by increased density of ground glass and the ability to simultaneously show underlying pulmonary vascular and bronchial shadows (5). For known studies: most pure GGNs and mixed GGNs regressed or disappeared within 3 months, which suggested their inflammatory nature (6), the NELSON study showed that approximately 63% of GGN disappeared after 3 months of follow-up (7), and that persistent GGN often heralds malignant lesions. Ye reported that 92.6% of persistent GGN were finally confirmed to be malignant (8). Most reports have predominantly involved adults, with few teenagers or children. Although there is an increasing number of young patients aged 20–30, it is not known when the development of GGN occurs (9). In recent years, more cases of children with lung adenocarcinoma have been reported (8,10). Matsuguma et al. (11) and Kobayashi et al. (12) reported that 41% and 29% of mixed GGNs, respectively, showed significant growth. The similar Korean study was also reported. Chang et al. reported that 12% of pure GGNs increased significantly (13). This leads to some small nodules, which are ignored. If atypical adenomatous hyperplasia is regarded as a precancerous lesion of lung cancer, more than 90% of persistent GGNs are lesions related to lung cancer, especially lung adenocarcinoma (14). We aimed to analyze the GGN among preschool children, especially the incidence of persistent GGN and whether GGN have occurred in this age group. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-465/rc).
Methods
The data of all preschoolers who underwent CT examination in the Children’s Hospital of Zhejiang University School of Medicine from 2013 to 2020 were collected. The majority of children who have CT scans are reviewed for pneumonia, leukemia, and tumors. There were 13,361 cases in the basic data. These data were filtered according to the following exclusion criteria: (I) severe artifacts; (II) data with identical names to the original data; and (III) patients without follow-up records (≥3 months). After this process, 704 patients were selected (Table 1), age at diagnosis (year): 3.15±1.91, female: 310 (44.0%), male: 394 (56.0%), follow-up interval (month): 7±6, leukemia: 177 (25.1%), pneumonia: 59 (8.4%), lung cyst: 14 (2.0%), space-occupying lesions outside the lungs: 130 (18.5%), foreign body in respiratory tract: 12 (1.7%), fever: 105 (14.9%), other blood disorders: 57 (8.09%), other diseases: 104 (14.8%), no obvious abnormality: 148 (21.0%). The following inclusion criteria were applied: must have undergone thin-slice CT (≤1.25 mm) at the first and last follow-up. A total of 311 patients were finally selected (Table 2). Age at diagnosis (year): 3.56±1.84, female: 147 (47.3%), male: 164 (52.7%), follow-up interval (month): 6.90±4.74, leukemia: 99 (31.8%), pneumonia: 21 (6.8%), lung cyst: 8 (2.6%), space-occupying lesions outside the lungs: 69 (22.9%), foreign body in respiratory tract: 6 (1.9%), fever: 46 (14.8%), other blood disorders: 34 (10.9%), other diseases: 30 (9.6%), no obvious abnormality: 54 (17.4%). Next, 2 thoracic radiologists with 5 years of experience in chest CT image interpretation independently evaluated these images. The images were read with the following lung window settings: window width, 1,500 HU; window level, −600 HU, and with the following mediastinal window settings: window width, 250 HU; window level, 40 HU. A GGO was defined as an area of hazy attenuation within the lungs such that vessel edges and bronchi were preserved. Increasing attenuation in the lung that masked underlying structures was defined as solid attenuation, the CT value of macroscopically assessed GGO was approximately −700 to −300 HU (15). Based on this imaging standard, image interpretation, if there was a difference between the reading of the two radiologists, another senior chief imaging radiologist reinterpreted the image, and their judgment comprised the final result (Figure 1). Sample size estimation: the use of routinely collected data allows large cross-sectional studies to be made at little or no expense. This experiment is not of between-group designs, thus, the sample size cannot be determined by indicators such as the power of a statistical test. Given that other studies have screened multiple cases of GGNs based on a sample size of 6,406 (3), we believe it is reasonable that we performed thin-slice CT to screen for GGN in 331 of 13,361 children. In addition, we used several years of all pediatric CT data from our hospital, which are routinely collected to allow for a large cross-sectional study.
Table 1
| Variables | No. of patients (%) (n=704) |
|---|---|
| Age at diagnosis (years) | 3.15±1.91 |
| Gender | |
| Female | 310 (44.0) |
| Male | 394 (56.0) |
| Follow-up interval (months) | 7±6 |
| Past medical history | |
| Leukemia | 177 (25.1) |
| Pneumonia | 59 (8.4) |
| Lung cyst | 14 (2.0) |
| Space-occupying lesions outside the lungs | 130 (18.5) |
| Foreign body in respiratory tract | 12 (1.7) |
| Fever | 105 (14.9) |
| Other blood disorders | 57 (8.09) |
| Other diseases | 104 (14.8) |
| No obvious abnormality | 148 (21.0) |
Values are expressed as mean ± standard deviation or number (percentage).
Table 2
| Variables | No. of patients (%) (n=311) |
|---|---|
| Age at diagnosis (years) | 3.56±1.84 |
| Gender | |
| Female | 147 (47.3) |
| Male | 164 (52.7) |
| Follow-up interval (months) | 6.90±4.74 |
| Past medical history | |
| Leukemia | 99 (31.8) |
| Pneumonia | 21 (6.8) |
| Lung cyst | 8 (2.6) |
| Space-occupying lesions outside the lungs | 69 (22.9) |
| Foreign body in respiratory tract | 6 (1.9) |
| Fever | 46 (14.8) |
| Other blood disorders | 34 (10.9) |
| Other diseases | 30 (9.6) |
| No obvious abnormality | 54 (17.4) |
Values are expressed as mean ± standard deviation or number (percentage).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, China ethics committee (No. 2022-IRB-073) and individual consent for this retrospective analysis was waived.
Statistical analysis
As we modified, this article involves a cross-sectional study designed to show the GGN prevalence in children, rather than a controlled trial or longitudinal study, which precludes statistical methods such as hypothesis testing or regression models. Therefore, it is not applicable to include a subsection to describe statistical methods that do not exist.
Results
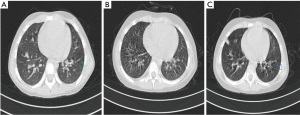
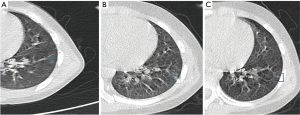
Among the images of 311 selected patients which were interpreted by two thoracic radiologists and a senior chief imaging radiologist, only 1 patient was screened out. The results showed that between 2013 and 2020, the incidence of GGNs that could be basically determined in the Children’s Hospital of Zhejiang University School of Medicine was 0.32%. Therefore, the probability of occurrence was interpreted as very low. We found that our exclusion condition that the patient must do thin-layer CT at the first assessment and last follow-up led to the exclusion of some patients who had undergone long-term follow-up and thin-layer CT, but did not receive thin-layer CT at either their first or last appointment. Subsequently, we relaxed the restrictions appropriately; there were 3 patients who did not fully meet the inclusion requirements but had typical radiographic pulmonary GGNs. The follow-up CT scans of 2 cases of GGNs with long-term follow-up are displayed in Figure 2 and Figure 3. Figure 4 displays the image of a patient with more than 3 months of follow-up for whom the nodule in the left lower lobe had not disappeared; because the initial scan was a thick layer CT, it had been excluded from the formal process (Figure 4). The next 2 cases had undergone follow-up for more than 3 months. Although the report pointed out that the nodules had not disappeared, partial imaging films could not be read (Figure 5).


Discussion
In this study, we found that the incidence of GGNs in preschool children is very rare. In the discovery of early lung cancer, most patients are found to have GGNs on physical examination (16), but this group of people are predominantly adults over 18 years old. With the advent of high-resolution CT, more tiny GGNs are being detected (17). In other fields such as thyroid, more and more positive results are being detected with more advanced auxiliary examinations. A study has also shown that not only adults have a high positive rate, but children also have a high incidence of disease (18). However, there have been very few such studies in the pulmonary field. This study is the first to systematically study the incidence of lung GGNs in preschool children. Preschool children’s lungs are developing and their immune systems are improving, so we selected children of this age group (19,20), and we hypothesized that if children of this age group have a high incidence of GGNs in the lungs, that the GGNs exist at a very young age, only we do not pay attention to them (21,22). The final data showed a low incidence of pulmonary GGNs in preschoolers, which is different from other tumors that tend arise at a younger age (23,24). The data showed that most of the children were presenting at the hospital due to pneumonia, and that the GGNs were incidentally detected (25). There are currently no guidelines for pulmonary GGNs in children, and it remains unclear what size a pulmonary nodule should be to require follow up or treatment. There are less evident data for children in this field, and protocols have been inferred from adult guidelines (26). Basically, the diagnosis and treatment of lung adenocarcinoma in children refer to adult lung cancer guidelines. The Fleischner Society indicates for solitary partial solid nodules less than 6 mm in diameter, it is recommended to follow up for 3–6 months, followed by at least an annual check for the subsequent 5 years, according to the association (27). Until now, GGNs in children have not been defined, and most people think of them as inflammation; anti-inflammatory medications are usually given, most of them are not followed up. Conversely, revisits might be too frequent, as often as every 10 days for 2 months, which poses a very high radiation risk to children’s bodies (28). However, some clinicians do not pay attention to lung shadows and do not conduct follow-up and review, which increases the risk of early lung cancer later in life (29), and also leads to some missing data. These inconsistencies are due to the lack of consensus on the diagnosis, follow-up, and treatment of GGNs in children and preschool children.
Conclusions
This study is the first big data study to investigate the incidence of GGNs in preschool children. According to the results of the data analysis, the incidence of pulmonary GGNs in this age group is indeed not high. Due to the strict screening conditions and the lack of long-term follow-up, the nature of pulmonary GGNs cannot be determined. The film quality of preschool children is particularly poor, which greatly increases the difficulty of manual reading; there may also be some misdiagnosis. The actual incidence is probably higher than the theoretical one, but not related to congenital diseases. In any case, the results suggest that the incidence of pulmonary GGNs in preschool children is not of concern. However, there is still a gap in the field of GGN treatment and treatment of GGNs in children. More data or multicenter studies are needed to bridge this gap.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-465/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-465/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-465/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, China ethics committee (No. 2022-IRB-073) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Bueno J, Landeras L, Chung JH. Updated Fleischner Society Guidelines for Managing Incidental Pulmonary Nodules: Common Questions and Challenging Scenarios. Radiographics 2018;38:1337-50. [Crossref] [PubMed]
- Chong S, Lee KS, Chung MJ, et al. Lung cancer screening with low-dose helical CT in Korea: experiences at the Samsung Medical Center. J Korean Med Sci 2005;20:402-8. [Crossref] [PubMed]
- Remy-Jardin M, Remy J, Giraud F, et al. Computed tomography assessment of ground-glass opacity: semiology and significance. J Thorac Imaging 1993;8:249-64. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Oh JY, Kwon SY. Clinical significance of a solitary ground-glass opacity (GGO) lesion of the lung detected by chest CT . Lung Cancer 2007;55:67-73. [Crossref] [PubMed]
- Scholten ET, de Jong PA, de Hoop B, et al. Towards a close computed tomography monitoring approach for screen detected subsolid pulmonary nodules? Eur Respir J 2015;45:765-73. [Crossref] [PubMed]
- Ye T, Deng L, Xiang J, et al. Predictors of Pathologic Tumor Invasion and Prognosis for Ground Glass Opacity Featured Lung Adenocarcinoma. Ann Thorac Surg 2018;106:1682-90. [Crossref] [PubMed]
- Wang Y, Zhang Z, Wang H, et al. Segmentation of the Clustered Cells with Optimized Boundary Detection in Negative Phase Contrast Images. PLoS One 2015;10:e0130178. [Crossref] [PubMed]
- Koh J, Jung E, Jang SJ, et al. Case of mucinous adenocarcinoma of the lung associated with congenital pulmonary airway malformation in a neonate. Korean J Pediatr 2018;61:30-4. [Crossref] [PubMed]
- Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013;143:436-43. [Crossref] [PubMed]
- Kobayashi Y, Fukui T, Ito S, et al. How long should small lung lesions of ground-glass opacity be followed? J Thorac Oncol 2013;8:309-14. [Crossref] [PubMed]
- Chang B, Hwang JH, Choi YH, et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest 2013;143:172-8. [Crossref] [PubMed]
- Cho S, Yang H, Kim K, et al. Pathology and prognosis of persistent stable pure ground-glass opacity nodules after surgical resection. Ann Thorac Surg 2013;96:1190-5. [Crossref] [PubMed]
- Ozuno NT, Akamatsu H, Takahashi H, et al. Quantitative diagnosis of connective tissue disease-associated interstitial pneumonia using thoracic computed tomography images. Clin Rheumatol 2015;34:2113-8. [Crossref] [PubMed]
- Miyoshi T, Aokage K, Wakabayashi M, et al. Prospective evaluation of watchful waiting for early-stage lung cancer with ground-glass opacity: a single-arm confirmatory multicenter study: Japan Clinical Oncology Group study JCOG1906 (EVERGREEN study). Jpn J Clin Oncol 2021;51:1330-3. [Crossref] [PubMed]
- Gao JW, Rizzo S, Ma LH, et al. Pulmonary ground-glass opacity: computed tomography features, histopathology and molecular pathology. Transl Lung Cancer Res 2017;6:68-75. [Crossref] [PubMed]
- Vaccarella S, Lortet-Tieulent J, Colombet M, et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: a population-based study. Lancet Diabetes Endocrinol 2021;9:144-52. [Crossref] [PubMed]
- Shenker RC, Santayana G. What Are the Options for the Treatment of Stuttering in Preschool Children? Semin Speech Lang 2018;39:313-23. [Crossref] [PubMed]
- Fern LA, Birch R, Whelan J, et al. Why can’t we improve the timeliness of cancer diagnosis in children, teenagers, and young adults? BMJ 2013;347:f6493. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol 2015;26:156-61. [Crossref] [PubMed]
- Lee CT. What do we know about ground-glass opacity nodules in the lung? Transl Lung Cancer Res 2015;4:656-9. [PubMed]
- Takano T. Natural history of thyroid cancer Review. Endocr J 2017;64:237-44. [Crossref] [PubMed]
- Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: the increasing incidence of thyroid cancer. Endocr Pract 2015;21:686-96. [Crossref] [PubMed]
- de Cordova XF, Wang H, Mehrad M, et al. Mucinous Adenocarcinoma With Intrapulmonary Metastasis Harboring KRAS and GNAS Mutations Arising in Congenital Pulmonary Airway Malformation. Am J Clin Pathol 2021;156:313-9. [Crossref] [PubMed]
- Mullen CJR, Barr RD, Franco EL. Timeliness of diagnosis and treatment: the challenge of childhood cancers. Br J Cancer 2021;125:1612-20. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Salerno S, Laghi A, Cantone MC, et al. Overdiagnosis and overimaging: an ethical issue for radiological protection. Radiol Med 2019;124:714-20. [Crossref] [PubMed]
- Mullen CJR, Barr RD, Franco EL. Correction to: Timeliness of diagnosis and treatment: the challenge of childhood cancers. Br J Cancer 2021;125:1178. [Crossref] [PubMed]
(English Language Editor: J. Jones)



