Immunotherapy for the treatment of pediatric brain tumors: a narrative review
Introduction
Pediatric central nervous system (CNS) tumors are now the most common cause of pediatric solid cancer in patients aged 0–14 and the leading cause of cancer mortality in the most recent Central Brain Tumor Registry of the United States (CBTRUS) data from 2014–2018 (1). Long term overall survival rates for pediatric CNS tumors approach 70%. However, there remains a portion of tumor types in which survival outcomes remain dismal despite advances in treatment as well as improved understanding in the molecular make-up and genetic drivers of tumors. Pediatric malignant gliomas have a median survival of 20 months from diagnosis with median survival of recurrent malignant glioma showing only minimal improvement over the past thirty years from 4 months (before 2006) to 8 months (after 2006) (2,3). Survival rates of medulloblastoma, the most common malignant pediatric brain tumor, are near 70% with use of adjuvant chemoradiation, but recurrent medulloblastoma has a 1-year survival rate of 40% and 5-year survival rate under 20% with no standard of care therapy (4-6). Despite advances in molecular analysis and understanding of tumor drivers, diffuse midline gliomas have a median survival of 12 months from diagnosis which has not improved appreciably in decades (7,8).
Cancer immunotherapy is a growing field that offers a novel approach to tumor types that have not responded historically to standard chemoradiation. There has been a rapid expansion in FDA-approved immunotherapies across all cancer types. The majority of approved treatments have focused on immune checkpoint inhibition including PDL-1 and CTLA-4 inhibition (9). However, CNS tumors have not benefitted from checkpoint inhibition to the degree of other non-CNS solid malignancies. CNS tumors exist in a unique environment including the presence of the blood brain barrier, have immune evasion techniques of the tumor environment, and most tumors have a low mutational burden, all of which has limited the efficacy of these immunotherapies (10,11). Research into varying realms of immunotherapy including adoptive transfer of engineered immune cells, tumor specific vaccines, cytokine modulation, oncolytic viruses and others is ongoing to target CNS tumors (9,12). The majority of immunotherapy clinical research has focused on adult glioblastoma. Using similar platforms of immunotherapy as previous adult studies, research focusing on immunotherapy specific to pediatric CNS tumors and their unique targets continues to expand. The goal of this narrative review is to report and summarize the completed pediatric immunotherapy clinical trials for primary CNS tumors. We present the following article in accordance with Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-86/rc).
Methods
The databases MEDLINE via PubMed, Embase via Elsevier, and Scopus via Elsevier were searched from inception to October 14, 2021. The authors, including a medical librarian (SK), composed the search utilizing a combination of keywords and subject headings to represent topics of pediatrics, brain tumors, and immunotherapies. All search strategies are available in Appendix 1. Non-human studies were removed, when possible, but no other restrictions were used. Search results were compiled in EndNote (Clarivate, Philadelphia) and imported into Covidence (Melbourne) for screening (SK). Additional studies were identified by reviewing references and review articles.
Inclusion criteria included the following: completed clinical trials or retrospective institutional reviews that evaluated a defined immunotherapy intervention; patient population that was at least half pediatric patients diagnosed by 18 years; newly diagnosed or recurrent primary CNS tumors as defined by the WHO 2021 brain tumor classification; reported results of at least one primary outcome including safety, feasibility, immune response, or survival. Exclusion criteria included non-human studies; abstracts without full article available; studies not available in English. Studies were then grouped for analysis and discussion based on the type of immunotherapy intervention explored. Two reviewers reviewed the search results and completed a manual full text review (CS and EH). Studies were marked for inclusion or exclusion with exclusion reason independently based on the pre-defined criteria (see Table 1). In instances of disagreement, a third reviewer (EMT) was utilized independently and resolved any disputes. Data extraction included author, publication date, immunotherapy intervention used, patient demographics including age and histology of brain tumor, and individual primary outcomes identified in each study including safety, immune response, and progression and/or overall survival. When available, median survival was obtained and reported in months. Data was extracted by two reviewers independently. Given the large heterogeneity in results collected and reported within each study, descriptive analysis of each study’s primary and secondary outcomes were compiled and reported.
Table 1
| Items | Specification |
|---|---|
| Date of search | October 14, 2021 |
| Databases and other sources searched | MEDLINE via PubMed, Embase via Elsevier, and Scopus via Elsevier |
| Search terms used | Containing clear pediatric content evidenced by pediatric keywords, and brain tumor keywords, and immunotherapy keywords appearing in the journal title, article title, or MESH terms. Resulting publications were individually inspected for misattributed/aberrant keyword results |
| Timeframe | Inception to October 14, 2021 |
| Inclusion and exclusion criteria | Inclusion criteria included the following: completed clinical trials or retrospective institutional reviews that evaluated a defined immunotherapy intervention; patient population that was at least half pediatric patients diagnosed by 18 years; newly diagnosed or recurrent primary central nervous system tumors as defined by the WHO 2021 brain tumor classification; reported results of at least one primary outcome including safety, feasibility, immune response, or survival. Exclusion criteria included non-human studies; abstracts without full article available; studies not available in English |
| Selection process | Two reviewers reviewed the search results and completed a manual full text review (CS and EH). Studies were marked for inclusion or exclusion with exclusion reason independently based on the pre-defined criteria. In instances of disagreement, a third reviewer (EMT) was utilized independently and resolved any disputes |
Results
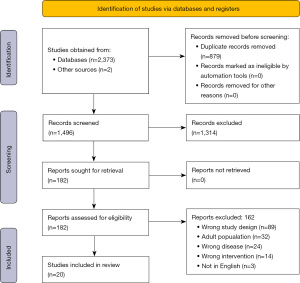
Search results returned 1,494 articles and screening titles and abstracts resulted in 180 articles for full text review (Figure 1) (13). Of the 180 articles, 160 were excluded and 18 were included for analysis. Another two articles (14,15) were ultimately included after review of references and inclusion of newly published articles for a total of 20 included articles (Table 2). For the 20 articles, primary reported outcomes varied significantly and included safety and feasibility, variety of measures of immune response to intervention, and progression free and overall survival and are included as a descriptive analysis of results. Eleven articles reported median overall survival and these results are reported in Table 2.
Table 2
| Type of immunotherapy | Authors/publication date | Trial type | Tumor type(s) | No. of patients | Specific immunotherapy | Grade 3–4 toxicities | Summary of findings |
|---|---|---|---|---|---|---|---|
| CAR T-cell | Vitanza et al. [2021] | Phase I | Recurrent pediatric CNS HER2+ tumors | 3 | HER2 CAR T-cell intraventricular vs. intratumoral infusion | Headache/fever with infusions, tolerable | No dose limiting toxicities |
| Clinical and laboratory evidence of local CNS immune activation | |||||||
| Autologous vaccines | Ardon et al. [2010] | Prospective | 33 HGG | 45 | Autologous DC loaded with tumor lysate injection | No grade 3 or 4 toxicities | 7 HGG, 3 ATRT, 1 ependymoma patients alive at follow-up |
| 5 MB/PNET | All patients with MB/PNET died | ||||||
| 4 EPN | HGG and ATRT response more favorably to vaccination than MB/PNET and ependymoma | ||||||
| 3 ATRT | Median OS: 13.5 months | ||||||
| Caruso et al. [2004] | Prospective | 4 HGG | 7 | Tumor RNA pulsed DC at recurrence | No grade 3 or 4 toxicities | No evidence of immune response post vaccines | |
| 3 EPN | Clinical improvement in 3/7 patients | ||||||
| DC RNA vaccines are safe and feasible for use in children | |||||||
| Benitez-Ribas [2018] | Prospective, phase Ib | DIPG | 9 | Allogenic Cell line lysate (previous H3K27M tumors) Q2 weeks 5 then 3-month boosters | Osteomyelitis | ADCV preparation is feasible and safe | |
| DIPG specific immune responses were detected in primary blood mononuclear cells and CSF | |||||||
| Lasky et al. [2013] | Prospective, pilot | 5 GBM | 3 | Tumor lysate pulsed DC | No grade 3 or 4 toxicities | Limited toxicity | |
| 2 AA | DC vaccine is tolerable and feasible with pediatric patients with HGG with some limitations | ||||||
| Peptide vaccines | Pollack et al. [2014] | Prospective | 20 BSG | 26 | Glioma associated antigen peptides (Eph2, IL-13Ra2, survivin) with Montanide-ISA-51 | No grade 3 or 4 toxicities | 13/21 had positive immune response to at least one GAA epitope |
| 6 non-brainstem HGG | No dose-limiting toxicities | ||||||
| Median survival 12.7 for BSG, 25.1 for HGG | |||||||
| Preliminary evidence of immunologic and clinical response | |||||||
| Pollack et al. [2016] | Prospective | 12 recurrent HGG | 12 | Glioma associated antigen peptides (Eph2, IL-13Ra2, survivin) with Montanide-ISA-51 | No grade 3 or 4 toxicities | 9/10 had positive immune response to at least one GAA | |
| No dose-limiting toxicities | |||||||
| Median PFS 4.1 months, 6-month PFS and OS of 33% and 73% respectively | |||||||
| Preliminary evidence of immunologic and clinical response | |||||||
| Median OS: 12.9 months | |||||||
| Pollack et al. [2016] | Prospective | 9 PA | 14 | Glioma associated antigen peptides (Eph2, IL-13Ra2, survivin) with Montanide-ISA-51 | Urticaria | 12/12 with positive immune response to at least one GAA | |
| 2 PMA | No dose-limiting toxicities | ||||||
| 2 DIG | Median PFS 9.0 months, 6-month PFS 85%, 12-month PFS 42% | ||||||
| 1 OPT/NF | Preliminary evidence of immunologic and clinical response | ||||||
| Oncolytic virus | Friedman et al. [2021] | Phase I | 12 recurrent, supratentorial HGG | 12 | HSV-1 G207 given intratumorally with or w/o radiation | No grade 3 or 4 toxicities | 4/11 alive at 18 months |
| No viral shedding detected | |||||||
| Responses observed in 11 patients | |||||||
| 4 patients with tissue after with substantial immune infiltrate increase | |||||||
| G207 converted immunologically “cold tumors to “hot | |||||||
| Median OS: 12.2 months | |||||||
| Kieran et al. [2019] | Phase I | 6 GBM | 8 | AdV-tk (adenoviral vector expressing the herpes simplex virus tk gene) intratumorally at surgery followed by 14 days of valtrex | Headache | 3 patients alive >24 months | |
| 1 AA | Median survival 8.9 months for dose level 1 | ||||||
| 1 EPN | Median survival 25 months for dose level 2 | ||||||
| GMCI can be safely used with radiation with or without temozolomide in pediatric patients | |||||||
| Immunomodulatory | Fangusaro et al. [2021] | Phase I | 4 AA | 29 | Oral pomalidomide capsule | Diarrhea, thrombocytopenia, lung infection, neutropenia | Maximum tolerated dose of 2.6 mg/m2 |
| 3 Astrocytoma | Immune correlates demonstrating a serum response | ||||||
| 3 EPN | Initiation of a subsequent phase 2 study | ||||||
| 6 GBM | |||||||
| 2 MB | |||||||
| 1 Meningioma | |||||||
| 2 ODG | |||||||
| 1 PA | |||||||
| 1 PPT | |||||||
| 2 SPNT | |||||||
| Fangusaro et al. [2021] | Phase II | 22 HGG | 52 | Oral pomalidomide capsule | 2 HGGs achieved objective response or long-term stable disease | ||
| 11 DIPG | No objective response or LTSD in medulloblastoma, or DIPG patients | ||||||
| 9 EPN | Treatment with POM monotherapy did not meet primary measure of success | ||||||
| 10 MB | Median OS (months): | ||||||
| HGG: 5.1 | |||||||
| DIPG: 3.8 | |||||||
| Ependymoma: 12.0 | |||||||
| Medulloblastoma:11.6 | |||||||
| Fried et al. [2018] | Phase I | DIPG | 9 | Intravenous pidilizumab | Neutropenia, HTN | 4 patient died within 1 year of diagnosis | |
| 3 patients died within 2 years of diagnosis | |||||||
| Median event free survival 9.3 months | |||||||
| MDV9300 is a safe and may be effective for children with DIPG | |||||||
| Median OS: 15.6 months | |||||||
| Shevtsov et al. [2014] | Pilot study | 2 GBM | 12 | Heat shock protein 70 resection cavity infusion | Headache | 1 patient with complete response | |
| 3 AA | Shift towards Th1 cytokines from Th2 | ||||||
| 2 Astrocytoma | Reduced T-regulatory cell levels | ||||||
| 2 AE | Feasibility of intratumoral delivery | ||||||
| 1 PNET | |||||||
| 1 CPC | |||||||
| 1 lymphoma | |||||||
| Cacciotti et al. [2020] | Retrospective single-institution | 2 DIPG | 11 | Intravenous checkpoint inhibitor infusion (ipilimumab/nivolumab, pembrolizumab, nivolumab) | Transaminitis, rash, mucositis, DM1, infection, nausea | Median duration of treatment 6.1 months | |
| 5 HGG | 3 partial response, 7 stable, 1 progressive disease | ||||||
| 1 EPN | Durable response noted in 2 patients | ||||||
| 1 CRPG | Immune checkpoint inhibitors are well tolerated in a wide range pediatric brain tumors | ||||||
| 1 HGNET | |||||||
| 1 NGGCT | |||||||
| Gorsi et al. [2019] | Retrospective | 5 HGG | 10 | Nivolumab | No grade 3 or 4 toxicities | 3 patients showed partial response at primary tumor, with metastatic progression | |
| 1 LGG | Use of immune checkpoint inhibitors should be limited to those with elevated PD-1 expression and TMB | ||||||
| 1 Pineoblastoma | Median OS (months): | ||||||
| 1 Embryonal | PD1+: 13.7 | ||||||
| 1 EPN | PD1−: 4.2 | ||||||
| 1 MB | |||||||
| Other | Khatua et al. [2020] | Phase I, dose escalation study | 5 MB | 9 | Intraventricular autologous NK cells | Headache, fever, seizure | 4 out 5 patients with progressive disease radiographically |
| 4 EPN | At higher doses, NK cells increase in the CSF during treatment | ||||||
| Intraventricular infusion of autologous NK cells is safe and feasible | |||||||
| Peres et al. [2008] | Pilot | 1 EPN | 3 | Tumor lysate vaccine, collection of T-cells, exvivo expansion, and reinfusion | No grade 3 or 4 toxicities | Well tolerated in all patients | |
| 2 HGG | All patients with tumor specific immune response durable at 16, 23, and 48 months | ||||||
| Packer et al. [1996] | Prospective | DIPG | 32 | Intravenous beta interferon infusion | Skin toxicity neutropenia, hepatotoxicity, renal toxicity, neurotoxicity | Some patients hepatic and hematologic toxicity requiring modification | |
| 1 patient death during maintenance therapy | |||||||
| 30/32 patients with progressive disease | |||||||
| Median time to progression 5 months | |||||||
| Median OS: 9 months | |||||||
| Van Gool et al. [2020] | Retrospective | DIPG | 41 | Combination therapy: NDV, modulated electrohyperthermia, autologous DCs vaccination | No grade 3 or 4 toxicities | Multimodal immunotherapy is feasible | |
| No major toxicities | |||||||
| A Th1 shift was associated with longer survival | |||||||
| Median OS: 9.1 months |
CNS, central nervous system; HGG, high grade glioma; MB, medulloblastoma; PNET, primitive neuro-ectodermal tumor; EPN, ependymoma; ATRT, atypical teratoid-rhabdoid tumour; DC, dendritic cell; OS, overall survival; DIPG, diffuse intrinsic pontine glioma; ADCV, autologous dendritic cell vaccine; CSF, cerebrospinal fluid; GBM, glioblastoma multiforme; AA, anaplastic astrocytoma; BSG, brainstem glioma; GAA, glioma-associated antigens; PFS, progression free survival; PA, pilocytic astrocytoma; PMA, pilomyxoid astrocytoma; DIG, desmoplastic infantile gangliogliomas; OPT, optic pathway tumor; NF, neurofibromatosis; GMCI, gene-mediated cytotoxic immunotherapy; ODG, oligodendroglioma; PPT, pineal parenchymal tumor; SPNT, Supratentorial primitive neuroectodermal tumor; LTSD, long-term stable disease; POM, pomalidomide; HTN, hypertension; AE, anaplastic ependymoma; CPC, choroid plexus carcinoma; CRPG, craniopharyngioma; HGNET, high grade neuroepithelial tumor; NGGCT, non germinoamatous germ cell tumor; LGG, low grade glioma; TMB, tumor mutational burden; NDV, Newcastle disease virus.
Autologous dendritic cells (DC)
DC are the most potent antigen presenting cells and are highly effective at generating an immune response and activating naïve T-cells (16,17). DC vaccines in cancer immunotherapy aim to recognize tumor specific antigens and ultimately result in the removal of cancer cells as well as producing long term immunity (16). This approach has become popular in neuro-oncology, and in response to positive outcomes in adults using autologous dendritic cells with various tumor targeting agents, dendritic cell therapy has been explored in pediatric CNS tumors. Four published pediatric specific studies utilized autologous dendritic cells pulsed with variations of tumor lysate or RNA from surgical resections. There are four phase I trials including Caruso et al. 7 patients, Ardon et al. 45 patients, Lasky et al. 3 patients, and Benitez-Ribas et al. 9 patients to evaluate the feasibility and safety of autologous dendritic cells vaccines in the pediatric CNS tumor population (18-21). Diagnostic inclusions varied but were limited to patients with no further standard of care treatment options and tumor types with historically poor outcomes including recurrent high grade glioma (HGG) 37 patients, medulloblastoma 5 patients, and ependymoma 7 patients, as well as newly diagnosed diffuse intrinsic pontine glioma (DIPG) 9 patients. Overall, patients tolerated the treatment well with no serious adverse events in the 64 patients across all four studies. Due to small patient numbers and a primary focus on safety and feasibility, the reports of outcomes including survival rates and immune response varied and the authors did not draw conclusions on the efficacy of the interventions. In the largest study of 45 patients receiving autologous dendritic cells pulsed with tumor cell lysate, Ardon et al. reported that 6/33 HGGs, 0/5 medulloblastoma, and 1/4 ependymoma patients were long term survivors over 24 months with a median overall survival of 13.5 months (18). Caruso et al. reported 0/7 showed positive immune response after receiving vaccines with concern about the function of the adoptive immune system in these previously heavily treatment patients (21). Benitez-Ribas et al., however, showed that all 9 of newly diagnosed DIPG patients showed a positive immune response following vaccines (19). Ultimately, published pediatric studies of autologous dendritic cells loaded with tumor lysate/RNA have demonstrated safety and feasibility in a total of 64 pediatric patients but have yet to establish improvement in progression and overall survival in large, randomized trials or compared to historical controls. Other primary outcomes including the evaluation of the immune response to the vaccines were mixed with one study showing a positive immune response and a second showing no immune response which requires further investigation.
Oncolytic viruses/viral immunotherapy
Oncolytic viruses have been shown to be a promising treatment in cancer immunotherapy with the first and only FDA approved oncolytic virus for advanced melanoma using modified herpes virus (22). The use of oncolytic viral therapy for CNS tumors is enticing given the multiple mechanisms in which the engineered viral therapy can affect malignant tumor cells and the tumor microenvironment. This includes engineering to preferentially infect tumor cells causing direct tumor lysis and cell death. This lysis, in turn, leads to the release of tumor associated antigens that can recruit and stimulate the immune system to clear the tumor cells as well as promote lymphocytes to the tumor microenvironment, potentially increasing the response to other immunotherapies (23,24). Oncolytic therapy in CNS tumors remains challenging due to the nature of the blood brain barrier and ensuring the adequate exposure and delivery to the tumor itself. Genetically engineered viruses including herpes simplex virus (HSV), adenovirus, poliovirus, and measles viruses are all being evaluated in cancer immunotherapy (24-26). Pediatric CNS tumors, particularly HGGs and recurrent embryonal tumors have been the initial targets of two completed early phase studies published that evaluate the safety and feasibility of intratumoral delivery of genetically engineered oncolytic viruses. Friedman et al. published the results of 12 recurrent HGGs who received a direct infusion of the modified HSV-1 G207 into the recurrent tumor tissue. Descriptive characteristics include: 50% female and age range (7 to 18 years). There were no grade 3 or 4 toxicities. Median overall survival was 12.2 months with four alive at greater than 18 months (27). Kieran et al. published an early phase trial utilizing adenovirus genetically engineered with the tk-HSV gene in eight patients. Considered a gene-mediated cytotoxic immunotherapy, this was a done in eight newly diagnosed patients undergoing second look surgery followed by 14 days of valacyclovir. Descriptive characteristics include: age range (7–17 years). This was shown to be overall by safe (one grade 3 reaction for headache) with a median survival of 9 months for dose level 1 and 25 months for dose level 2 (28). Overall, both studies demonstrate oncolytic therapy as preliminarily safe with only one case of grade 3 headache. Next steps include a larger multi-institutional clinical trial currently under progress.
Chimeric antigen receptor (CAR) T-cells
Modified T-cells expressing CARs were first engineered over twenty years ago and decades of preclinical and clinical studies with multiple generations of CARs have resulted in FDA approval and standard of care use in refractory hematologic malignancies targeting the CD19 B-cell antigen with remarkable responses (29-31). CAR T-cells are programmed to target tumor specific antigens and ultimately result in immune activation and cytotoxic lysis of cancer cells (29,32). Clinical use in solid tumors, however, has been shown to be more challenging and largely refractory to T-cell therapies up until this point. These challenges in CNS tumors include the heterogeneity in tumors and the specific antigens they express which potentially makes single agent targets less effective. In addition, the immunosuppressive nature of the tumor microenvironment limits the efficacy and survival of the CAR T-cells (32,33). Despite the challenges of solid tumor CAR T-cells, CAR therapy is showing to be a promising treatment in CNS tumors. Currently, there is only one published study focusing on CAR T-cells in pediatric type CNS tumors which is an interim analysis of an early phase I study of CAR T-cell vaccine feasibility, safety, and tolerability. Vitanza et al., reported the results of the first three patients (all over 18 but diagnosed as pediatric patients) with refractory human epidermal growth factor receptor 2 (HER2)+ CNS tumors. Descriptive characteristics include: 1 female, 2 males, age range (19 to 26 years). The CAR T-cells were delivered intratumorally/intraventrically with no unexpected toxicities. Reported toxicities were manageable with headache and fever with infusion and no dose-limiting toxicity. Secondary objective assessing CAR T-cell distribution and disease response demonstrated positive cytokine levels in the cerebrospinal fluid (CSF) of patients consistent with immune. activation. Overall, this small sample demonstrated the feasibility of producing HER2-specific CAR T-cells that are well tolerated and mediate a localized immune response. The study is ongoing with dose escalation among pediatric HER2+ CNS tumors (14).
Peptide vaccines
Peptide cancer vaccines represent an exciting form of treatment for pediatric brain tumors. They are designed to induce a specific immune response against antigens that are expressed by tumor cells. Additionally, peptide-based vaccination has demonstrated the ability to elicit a strong biologic response. Several previous studies have evaluated peptide-based vaccine approaches for both pediatric and adult tumors, which have demonstrated safety and feasibility (34-46). Three published pediatric specific studies utilizing peptide-based vaccination in the treatment of recurrent low-grade gliomas, recurrent HGGs and newly diagnosed malignant brainstem and non-brainstem gliomas respectively, were completed by Pollack et al. between 2014 to 2016 (47-49). All three of these studies followed a similar vaccination schedule and targeted the same three peptide epitopes that have been observed to be highly expressed in pediatric gliomas: IL-13Ra2, EphA2, and survivin (50). All three studies focused on the primary endpoints of vaccine safety and T-cell responses against vaccine targeted glioma-associated antigens (GAA) epitopes via enzyme-linked immunosorbent spot analysis (ELISPOT).
The first study published in 2014, focused on patients with newly diagnosed malignant brainstem and non-brainstem gliomas. Twenty-six patients were included, 12 patients with newly diagnosed brainstem or HGG treated with radiation and concurrent chemotherapy and 14 patients with newly diagnosed brainstem glioma treated with irradiation. Descriptive characteristics include: 50% female, age range (2.2 to 17.9 years). There were no dose related toxicities reported nor grade 3 or higher systemic toxicities. Preliminary clinical outcome data assessed radiographically with magnetic resonance imaging (MRI) demonstrated 19 patients with stable disease, 2 patients with a partial response, one patient had a minor response, 2 patients had a prolonged disease-free status following surgery, and 2 patients had progressive disease through the first two vaccine courses. Overall median survival was 13.3 months from diagnosis, 12.7 months among patients with brainstem glioma, and 25.5 months among patients with HGG. ELISPOT analysis showed GAA responses in 13 patients including 10 to IL-13Ra2, 11 to EphA2, and 3 to survivin.
Pollack et al. then published their next study focused on pediatric patients with recurrent low-grade gliomas (48). This study included 14 patients, 2 patients were excluded one patient for grade 3 urticaria and one patient due to progressive disease. Descriptive characteristics include: 50% female, age range (1.9 to 19.0 years). Aside from grade 3 urticaria, no other regimen limiting toxicities were observed. Preliminary clinical outcome data was assessed radiographically with MRI one child had asymptomatic pseudoprogression noted at 6 weeks after starting the treatment regimen, followed by dramatic tumor regression >75% shrinkage. Three children had sustained partial responses lasting greater than 10-, 31- and 45-month respectively, and one patient had a transient response. Median progression-free survival was 9.9 months and overall survival was 100% with an average follow-up of 42 months. ELISPOT analysis showed GAA responses in all patients including 3 to IL-13Ra2, 11 to EphA2, and 3 to survivin.
Finally, in 2016 Pollack et al. published their most recent peptide study focused on pediatric patients with recurrent HGGs (47). This study included 12 patients, 6 with glioblastoma, 5 with anaplastic astrocytoma and one patient with malignant gliomatosis cerebri. Descriptive characteristics include: 50% female, age range (2.3 to 23.3 years). There we no reported dose-limiting toxicities reported or grade 3 or high toxicities. Preliminary clinical outcome data was assessed radiographically with MRI, one child had symptomatic pseudoprogression response to treatment, 1 child had a partial response. Median progression-free survival from the start of vaccination was 4.1 months and median overall survival was 12.9 months. At 6 months, progression-free survival was 33% and overall survival was 73%. ELISPOT analysis showed GAA responses in 9 patients including 4 to IL-13Ra2, 9 to EphA2, and 3 to survivin.
Overall, the three published studies utilizing peptide vaccination in the treatment of pediatric brain tumors have demonstrated safety with only one case of grade 3 urticaria and no dose limiting toxicities. Additionally, these studies also demonstrated feasibility in eliciting an immunologic response several patients showed evidence of T-cell responses against vaccine targeted GAA epitopes on ELIPSOT analysis. Thus, while these data are promising, larger studies are needed to further assess the benefits of this strategy through a multi-institutional setting.
Immunomodulation
Pomalidomide
Pomalidomide is an FDA approved agent with a variety of antitumor properties including immunomodulatory and antiangiogenic features that aid in T-cell and NK cell immunity. In comparison to a similar precursor immunomodulatory agent, lenalidomide, in pre-clinical testing it has been demonstrated to have a potency 500–2,000 times stronger at T-cell stimulation and proliferation (51,52). Two studies have been published by Fangusaro et al. including a phase I and phase II utilizing pomalidomide to treat pediatric brain tumors. The phase I study utilized pomalidomide as an immunomodulatory agent in children with recurrent, progressive, and refractory CNS tumors (53). The primary aim of this study was to establish a maximum tolerated dose for a subsequent phase II study. This study included 29 children, 25 of which were evaluated for dose-limiting toxicity. Four patients were excluded total, one due to insufficient labs to monitor for toxicity, and three due to progressive disease. Patient characteristics include: 55% female, age range (5.4 to 20.8 years), 8 patients with astrocytoma, 11 different brain tumor types (Table 2). Five dose-limiting toxicities were observed, 4 at dose level-3 at 3.4 mg/m2 that included diarrhea, thrombocytopenia, lung infection and neutropenia. One patient experienced dose-limiting toxicity at dose level-2 at 2.6 mg/m2. The most common adverse events observed lymphopenia, leukopenia, neutropenia, anemia, fatigue, and headaches. The median number of treatment courses for all patients was 2, 12-month progression free survival was 3.5% and 12-month overall survival was 28.1%. Twenty-four patients also had immune correlates collected primarily for exploratory and descriptive purposes. At all dose levels, all patients experienced an increase in GZMB+ T-cells, Tregs, and NKp46 NK levels at days 15–21 when compared to baseline consistent with immunomodulation.
In the subsequent phase II study, children and young adults with recurrent or progressive primary brain tumors received pomalidomide 2.6 mg/m2 on days 1–21 of a 28-day cycle based on the findings from the mentioned phase I study (15). This study included 53 enrolled patients with 46 patients evaluable for treatment response: 19 with HGG, 9 with DIPG, 9 with ependymoma, and 9 with medulloblastoma. Descriptive characteristics include: 36.5% female, age range (4 to 18 years). Of the patients a few of the listed reasons included screening failure, and one patient never receiving treatment. The primary endpoint if this study was either objective response or long-term stable disease both assessed radiographically via standard MRI. The rate of objective response or long-term stable disease was 10.5% for HGG and 11.1% for ependymoma. Progression-free survival analysis for HGG, DIPG, ependymoma and medulloblastoma revealed 89.5%, 100%, 100% and 88.9% respectively. Overall survival rates demonstrated 63.2%, 77.8%, 55.6% and 44.4% respectively for HGG, DIPG, ependymoma and medulloblastoma. In terms of overall safety, for HGG, DIPG, ependymoma, and medulloblastoma, 63.6%, 45.5%, 77.8% and 80.0% experienced a pomalidomide related adverse event. Ten patients died during treatment due to progressive disease and 1 patient died due to adverse event due sepsis.
Ultimately, the results of these two studies of pomalidomide failed to demonstrate a clinically meaningful level of efficacy as a monotherapy regimen in pediatric patients with brain tumors, though the grade 3 and 4 toxicities were consistent with previous studies. While the phase II sample size was small, this study overall reinforces the need for further evaluation of tumor resistance against pomalidomide.
IgG monoclonal antibody pidilizumab
Pidilizumab is an immunomodulatory agent that is an IgG1 monoclonal antibody (54). While the specific mechanism remains uncertain, it has been demonstrated to enhance endogenous antitumor immunoreactivity. Activity response has been associated with increases in the rate of infiltrative CD4 T-cells, and CD4/CD8 central memory cells (55). One study published in 2018 by Fried et al. published a study utilized pidilizumab, in children with DIPG (54). This study included 9 enrolled children with DIPG. Descriptive characteristics include: age range (3 to 18 years). The primary objective of this study was assessment of efficacy and toxicity, secondary objectives included event-free survival and overall survival. Reported common side effects of treatment included fatigue, neutropenia (grade 1–3), loss of appetite, and lymphopenia. Overall survival at 6 and 12 months was 100% and 55% respectively. Four patients survived less than one-year, 3 of which had a Karnofsky score below 60 at diagnosis. The median event-free survival was 9.3 months and median overall survival was 15.6 months. Overall, as this is one of the first studies investigate feasibility and toxicity of immune modulating antibodies in pediatric brain tumors, the authors demonstrated both feasibility and safety with a low toxicity profile. However, further studies are needed to confirm these preliminary findings.
PD-1 and CTLA-4 immune checkpoint blockade
Immune checkpoint inhibitors (ICIs) are a class of drugs that promote T-cell mediated response towards tumor cells and block co-inhibitory signaling pathways (56). The primary targets of checkpoint inhibition are to block the interaction between programed cell death 1 (PD-1) and the programmed cell death ligand 1 (PD-L1) or inhibition of cytotoxic T lymphocyte antigen 4 (CTLA-4). The blockage of the PD-1/PD-L1 interaction activates T-cells and aides in prevention of immune tolerance. CTLA-4 inhibition prevents T-cell inhibition and aides the activation of effector T-cells. Ipilimumab targets CTLA-4, while nivolumab and pembrolizumab target PD-1 (56-59). These agents have demonstrated efficacy in adult cancers and have been approved in the treatment of non-small cell lung cancer, renal cell carcinoma and others. Two published studies utilized immune checkpoint inhibition through PD-1 and or CTLA-4 blockade. Cacciotti et al. utilized immune checkpoint inhibition through PD-1 and CTLA-4 blockade for the treatment of recurrent or refractory CNS tumors of various types (60). The aim of this study was to describe a single institutional experience utilizing ICIs in the treatment of pediatric patients and adolescents with recurrent and or refractory primary CNS tumors. This study was a retrospective chart review of pediatric patients with recurrent or refractory CNS tumors treated with either ipilimumab, nivolumab and or pembrolizumab. Eleven patients were included in this study with a median age of 13.9 years (range 4.1 to 20.7 years). Eight patients had recurrent disease while 3 had refractory disease. Nine patients were treated with combination ICI therapy (ipilimumab/nivolumab), 1 patient received monotherapy with nivolumab, and one patient received monotherapy with pembrolizumab. Seven patients discontinued treatment due to disease progression, 2 patients discontinued treatment after experienced significant adverse events (colitis and transaminitis) and 2 patients remained on therapy at the time of data collection. Three patients experienced partial response, 7 patients remained stable, and 1 patient experienced progressive disease. Three patients eventually died to disease progression while the remaining 8 survived at the time of collection. Ten patients developed toxicity of any grade during therapy including rash, colitis, mucositis, type 1 diabetes, fatigue, and infection.
Gorsi et al. utilized immunomodulation via immune checkpoint inhibition through the PD-1 inhibitor nivolumab (61). The aim of this study was to describe a single-institution experience utilizing nivolumab in the treatment of pediatric patients with recurrent or refractory pediatric brain tumors. This study was a retrospective chart review. The primary outcomes reported included both adverse events and toxicities, tumor mutation burden, survival and clinical response assessed radiographically by MRI. A total of 10 patients were included in this study had received and failed multiple standard therapies for their specific disease before nivolumab treatment initiation. Five patients had HGG, one patient with low-grade glioma, one patient with pineoblastoma, one patient with CNS embryonal tumor, one patient with ependymoma, and one patient with medulloblastoma. Descriptive characteristics include: 40.0% female, age range (1.5 to 17 years). Grade 2 toxicities were observed without any dose limiting toxicities. Reported adverse events included leukopenia, transaminitis, hyperglycemia, hypoalbuminemia, pancreatitis, anemia, nausea, and vomiting. Tumor mutation burden, assessed by the total number of somatic mutations, was to intermediate (median 1.3, range 0 to 6.3). Nine patients had radiographic disease progression, with a median time to progression of 5.5 weeks. Three patients showed a partial response to treatment at the primary tumor site, of which 2 patients developed progression of metastatic disease. Median survival for PD-L1 positive patients was 13.7 versus 4.2 weeks for PD-L1 negative patients (P=0.08). Overall, the results of this study demonstrate overall safety in the administration of nivolumab. While these early findings, the data suggests that future trials should consider stratification of pediatric patients based on tumor subtype and PD-L1 expression status.
Heat shock protein based immunotherapy
Recent evidence has highlighted potential antitumoral activity by heat shock protein 70 (Hsp70), a molecular chaperone. It has been shown that Hsp70 has the ability to deliver tumor associated peptide to antigen-presenting cells which can subsequently present these antigens via major histocompatibility class I to CD8+ T-lymphocytes to induce the formation of a tumor-specific immune response (62). Shevtsov et al. published a study utilizing immunomodulation via recombinant Hsp70 in the treatment of malignant brain tumors in pediatric patients (63). The aim of this study was to explore safety and feasibility in patients with brain tumors and to assess for evidence of clinical and immunologic response. This study enrolled 12 patients. Descriptive characteristics include: 42% female, age range (4 to 13 years). Intratumoral infusions of Hsp70 were well tolerated except in 3 patients who experienced grade 3 adverse effect headaches. Additional adverse effects included fever and vomiting. There were no treatment related hematologic, hepatic, or renal toxicities. Clinical response was evaluated radiographically via MRI, nine patients showed no change following the last injection of Hsp70, one patient showed a complete response, one patient showed a partial response, and one patient showed disease progression. One patient showed no signs of the contrast enhancing lesion 4 weeks after the final administration of Hsp70. Immunologic response was observed by increase in the relative number of T-lymphocytes following Hsp70 injection in all 12 patients. The authors also noted a shift in T-helper cell response with an increased number of cytokines mediated from type 1 T-helper cells cytokines from type 2 T-helper cytokines in peripheral blood. Additionally, there was a significant decrease in the relative number of regulatory T-cells in all 12 patients 8.1% to 5.7%. Hsp70 did not increase the relative levels of natural killer cell populations (CD3, CD16 and CD56) as there was a non-significant increase from 5.4% to 8.2%. Overall, Hsp70 is demonstrated to be feasible and safe with a low toxicity profile. However, while immunomodulation was observed via changes in T-cell mediated activity, further studies via randomized controlled trial are necessary to further understand anti-tumoral effects of Hsp70.
Beta-interferon based immunotherapy
Recombinant beta-interferon is a genetically altered interferon that has been shown to have antitumor effects in both adult and pediatric malignant gliomas and is thought to have synergist effects when combined with radiation (64-67). In 1996 Packer et al. published a study evaluating the benefit of recombinant beta-interferon with hyper fractionated radiotherapy in patients with newly diagnosed diffuse intrinsic brainstem gliomas (68). The aim of this study was to determine the safety, toxicity of treatment, survival and response assessed radiographically via MRI to the combined therapy. A total 32 pediatric patients were enrolled. Descriptive characteristics include: 50% female, age range (2 to 17 years). There were 24 cases of grade 3 or 4 toxicity in 13 patients resulting in discontinuation in 6 and dose reduction in 7. Radiographically, among the 32 patients treated, 2 patients had a complete response, 5 had a partial response, 4 with a minor response, 15 with stable disease and 6 with progressive disease at completion of radiotherapy and interferon. The median time to progression from study entry was 5 months, and median time to death was 9 months. Overall, beta-interferon did demonstrate grade 3 and 4 toxicity including one case of severe neurotoxicity, though it was well tolerated overall. However, this study demonstrated little evidence of clinical efficacy and other immunomodulatory therapies should be considered alternatively.
Other pediatric immunotherapy studies
Adoptive transfer of ex-vivo expanded T-cells
In 2008 Peres et al. reported the results of three pediatric patients with recurrent brain tumors who received high-dose chemotherapy followed by adoptive immunotherapy via transfer of ex-vivo expanded T-cells with a primary goal of establishing safety and secondary outcomes of immune response and survival (69). Prior studies have demonstrated autologous tumor cell transfer is both safe and efficacious in patients with glioblastoma multiforme where marked T-lymphocyte activation correlated with delay tumor recurrence and increase patient survival (70-72). The primary outcome of this study was to assess the induction of an immune response against pediatric brain tumors. Secondary outcomes assessed for tumor recurrence via serial MR. Three patients were enrolled including two HGGs and one ependymoma. Descriptive characteristics include: age range (0.4 to 17 years). There were no serious adverse reactions related to the adoptive immunotherapy with no autoimmune phenomena observed. Two patients had major clinical and radiographic responses and were alive at 18 months and three years post diagnosis. Additionally, all three patients demonstrated tumor specific response on neuroimaging. Overall, this study demonstrated good tolerance and promising results in survival though the sample size is limited.
Multimodal therapy
In 2020, Van Gool et al. published a retrospective study focused on the utilization of a multimodal therapy consisting of Newcastle disease virus, modulatory hyperthermia, and autologous dendritic cell vaccination as part of an individualized combinatorial approach in the treatment of pediatric patients with DIPG (73). A retrospective analysis was conducted among patients with a primary diagnosis of DIPG who were actively treated with immunotherapy as an individualized treatment approach at the Immun-Onkologisches Zentrum Köln (IOZK). The primary goal of this study. Was to assess the feasibility, immune response, and overall survival of this multimodal approach. Immune response was monitored via PanTum detect tests used to monitor mRNA expression level of PDL-1. Forty-one patients from 16 countries were included in this study and received this multimodal immunotherapy at IOZK. These 41 patients consisted of three groups, (Group 1) patients who received immunotherapy prior to radiotherapy or chemotherapy, (Group 2) patients who received immunotherapy in conjunction with the first-line treatment provided by their local oncologist, and (Group 3) treated with immunotherapy upon disease progression. Descriptive characteristics include: 32% female, age range (2 to 19 years). No major toxicities were reported. Disease progression was difficult to assess in this study due to patient heterogeneity and thus for this retrospective study, progression free survival was defined as the moment a new treatment strategy was implemented by a local oncology center. Among Group 1 and Group 2 patients, progression-free survival at 6-month was 90.9%. Among Group 3 patients, progression-free survival at 6-month was 53.8% (P=0.13). The median overall survival for Group 1 and Group 2 patients was 14.4 months with a 1-year overall survival rate of 64.3%. The median overall survival for Group 3 patients was 9.1 months (P=0.057). Additionally, there was a shift in immune response towards type 1 T-helper mediated response in PanTum Detect tests. Overall, this study reported safety with no major toxicities and feasibility of multimodal therapy and immune response monitoring via PanTum Detect testing.
Ex-vivo expanded autologous natural killer cells
Studies have demonstrated safety of administration of natural killer cells into adult brain tumors and autologous NK cells have been demonstrated to have high-functionality against medulloblastoma cells in-vitro (74-76). One phase I study published in 2020 by Khatua et al. utilized intraventricular infusions of ex-vivo expanded autologous natural killer cells in pediatric patients with recurrent medulloblastoma and ependymoma (77). The primary aim of this study was to explore dose-escalation, toxicity, and feasibility of NK cell production. Secondary outcomes included descriptive analysis of clinical and radiologic responses. Nine patients were enrolled and received the therapy up to 3 infusions weekly with escalating doses up to 3 cycles. Descriptive characteristics include: 33% female, age range (8 to 18 years). There were no dose limiting toxicities or severe adverse outcomes. MRI imaging was used to assess for response. Eleven patients demonstrated progressive disease after infusions except one patient who was stable at one month at study follow-up. One patient had transient response to treatment after 5 infusions before eventual disease progression. Additionally, NK cells were found to be increased in the CSF during treatment with repetitive infusions with a mean of 11.6-fold increase. Overall, this study demonstrated a low toxicity profile and efficacy of intratumoral NK infusion. A follow-up study is in development to attempt NK cell delivery at longer intervals for more cycles.
Implications for future research
Immunotherapy offers the potential to improve treatment outcomes for pediatric patients with brain tumors. In this review, we have highlighted published clinical trials utilizing immunotherapies to treat primary brain tumors in pediatric and adolescent patients. These studies represent a diverse set of immunotherapy approaches that include CAR T-cell therapy, autologous vaccines, peptide vaccines, oncolytic viruses/viral immunotherapy, and immunomodulation.
Autologous dendritic vaccines are currently undergoing phase II study to further evaluate the efficacy of the use of tumor lysate loaded dendritic cells vaccines in HGGs in children and adults (NCT01213407). In addition, due to the power of dendritic cells in activating the immune system, they remain a focus of cancer immunotherapy and pediatric CNS tumor treatment with other tumor specific antigens. Trials investigating dendritic cells with other tumor specific targets are underway including ctyomegalovirus (CMV) viral peptide in HGG (NCT03615404), stem cell loaded dendritic cells in recurrent HGG or medulloblastoma (NCT01171469), DIPG tumor neoantigens in newly diagnosed DIPG (NCT03914768), Wilms’ tumor-1 antigen mRNA loaded DCs (NCT04911621), and total tumor RNA loaded DCs in recurrent medulloblastoma (NCT01326104), newly diagnosed DIPG (NCT04837547, NCT03396575), and HGG (NCT03334305).
Oncolytic viruses for pediatric CNS tumors present an exciting advancement in cancer immunotherapy and early phase trials of direct intratumoral delivery show overall safety and feasibility as well as early encouraging survival benefit. Phase II studies are underway to better understand the efficacy and ongoing safety monitoring. In addition, there are ongoing trials evaluating other oncolytic viral therapy including a phase I study with adenovirus (DNX2401) in newly diagnosed DIPG (NCT03178032), measles virus (MV-NIS) in recurrent medulloblastoma and atypical rhabdoid/teratoid tumor (NCT02962167), reovirus in recurrent high-grade tumors (NCT02444546), and poliovirus (PVSRIPO) in recurrent HGG (NCT03043391).
CAR T-cells are continuing to be evaluated in both adult and pediatric CNS tumors. Similar adult glioblastoma targeted CAR T-cell studies with targets including IL13a2, HER2, and epidermal growth factor receptor (EGFR)vIII have shown safety and feasibility as well as positive anti-tumor activity as well as some patients with radiographic response on MRI in some patients (78-80). CAR T-cell investigations with varying target antigens common in pediatric high-grade tumors are underway to continue to evaluate the safety, ideal dose and schedule, and ultimate efficacy. Current investigations include CAR T-cells targeting IL13a2 (NCT04510051), GD2 (NCT04196413, NCT04099797), HER2 (NCT03500991, NCT02442297), EGFR (NCT03638167), and B7H3 (NCT04185038). The early interim results of the first pediatric focused CAR T-cell therapy demonstrates the feasibility and early safety data with ongoing studies evaluating a multitude of different target antigen CARs in pediatric CNS tumors.
Lastly, there are currently several ongoing clinical trials utilizing peptide vaccines in the treatment of pediatric brain tumors. NCT03299309 (PRiME) is a phase I clinical trial using PEP-CMV, a peptide vaccine that contains a long synthetic peptide, in the treatment of recurrent medulloblastoma and malignant glioma. NCT01795313 is an ongoing phase I clinical trial studying the use of HLA-A2 restricted peptides in combination with imiquimod in the treatment of recurrent ependymoma. NCT04749641 is a currently ongoing clinical phase I clinical trial the use of peptide vaccination targeting the H3.3.K27M neoantigen peptide in pediatric patients with DIPG.
To date, many of these published studies were phase I and pilot studies focused primarily in establishing safety, maximum dose-tolerance, toxicity, and efficacy in utilizing these therapeutics in pediatric patients. However, as additional trials continue to develop, novel immunotherapies may help in delivering more specific and targeted therapy directed at tumor-specific features.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-86/rc).
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-86/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-86/coif). EMT serves as an unpaid editorial board member of Translational Pediatrics from August 2021 to July 2023. EMT reports funding sources of his lab or clinical trials from Pediatric Brain Tumor Foundation, Musella Foundation, Department of Defense CA171067, R01 FD007283-01; he serves as a scientific advisor in Oncoheroes Biosciences; and also serves of the DSMB in University of Alabama Birmingham for NCT03911388. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ostrom QT, Cioffi G, Waite K, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol 2021;23:iii1-iii105. [Crossref] [PubMed]
- Kline C, Felton E, Allen IE, et al. Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol 2018;137:103-10. [Crossref] [PubMed]
- Lam S, Lin Y, Zinn P, et al. Patient and treatment factors associated with survival among pediatric glioblastoma patients: A Surveillance, Epidemiology, and End Results study. J Clin Neurosci 2018;47:285-93. [Crossref] [PubMed]
- Sabel M, Fleischhack G, Tippelt S, et al. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol 2016;129:515-24. [Crossref] [PubMed]
- Sun YL, Liu JJ. Survival of children with recurrent medulloblastoma undergoing sequential therapy: an analysis of 101 cases. Zhongguo Dang Dai Er Ke Za Zhi 2021;23:164-8. [Crossref] [PubMed]
- Johnston DL, Keene D, Strother D, et al. Survival Following Tumor Recurrence in Children With Medulloblastoma. J Pediatr Hematol Oncol 2018;40:e159-63. [Crossref] [PubMed]
- Vuong HG, Le HT, Ngo TNM, et al. H3K27M-mutant diffuse midline gliomas should be further molecularly stratified: an integrated analysis of 669 patients. J Neurooncol 2021;155:225-34. [Crossref] [PubMed]
- Wang Y, Feng LL, Ji PG, et al. Clinical Features and Molecular Markers on Diffuse Midline Gliomas With H3K27M Mutations: A 43 Cases Retrospective Cohort Study. Front Oncol 2020;10:602553. [Crossref] [PubMed]
- Fecci PE, Sampson JH. The current state of immunotherapy for gliomas: an eye toward the future. J Neurosurg 2019;131:657-66. [Crossref] [PubMed]
- Reardon DA, Brandes AA, Omuro A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1003-10. [Crossref] [PubMed]
- Reardon DA, Kim TM, Frenel JS, et al. Treatment with pembrolizumab in programmed death ligand 1-positive recurrent glioblastoma: Results from the multicohort phase 1 KEYNOTE-028 trial. Cancer 2021;127:1620-9. [Crossref] [PubMed]
- Ratnam NM, Gilbert MR, Giles AJ. Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro Oncol 2019;21:37-46. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021;74:790-9. [Crossref] [PubMed]
- Vitanza NA, Johnson AJ, Wilson AL, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med 2021;27:1544-52. [Crossref] [PubMed]
- Fangusaro J, Cefalo MG, Garré ML, et al. Phase 2 Study of Pomalidomide (CC-4047) Monotherapy for Children and Young Adults With Recurrent or Progressive Primary Brain Tumors. Front Oncol 2021;11:660892. [Crossref] [PubMed]
- Constantino J, Gomes C, Falcão A, et al. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res 2017;65:798-810. [Crossref] [PubMed]
- Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 2005;175:1373-81. [Crossref] [PubMed]
- Ardon H, De Vleeschouwer S, Van Calenbergh F, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer 2010;54:519-25. [Crossref] [PubMed]
- Benitez-Ribas D, Cabezón R, Flórez-Grau G, et al. Immune Response Generated With the Administration of Autologous Dendritic Cells Pulsed With an Allogenic Tumoral Cell-Lines Lysate in Patients With Newly Diagnosed Diffuse Intrinsic Pontine Glioma. Front Oncol 2018;8:127. [Crossref] [PubMed]
- Lasky JL 3rd, Panosyan EH, Plant A, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res 2013;33:2047-56.
- Caruso DA, Orme LM, Neale AM, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol 2004;6:236-46. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Zeng J, Li X, Sander M, et al. Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas. Front Immunol 2021;12:721830. [Crossref] [PubMed]
- Hamid O, Hoffner B, Gasal E, et al. Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother 2017;66:1249-64. [Crossref] [PubMed]
- Foster JB, Madsen PJ, Hegde M, et al. Immunotherapy for pediatric brain tumors: past and present. Neuro Oncol 2019;21:1226-38. [Crossref] [PubMed]
- Landi DB, Thompson EM, Ashley DM. Immunotherapy for pediatric brain tumors. Neuroimmunology and Neuroinflammation 2018;5:29.
- Friedman GK, Johnston JM, Bag AK, et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med 2021;384:1613-22. [Crossref] [PubMed]
- Kieran MW, Goumnerova L, Manley P, et al. Phase I study of gene-mediated cytotoxic immunotherapy with AdV-tk as adjuvant to surgery and radiation for pediatric malignant glioma and recurrent ependymoma. Neuro Oncol 2019;21:537-46. [Crossref] [PubMed]
- Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024-8. [Crossref] [PubMed]
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439-48. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Feldman L, Brown C, Badie B. Chimeric Antigen Receptor (CAR) T Cell Therapy for Glioblastoma. Neuromolecular Med 2022;24:35-40. [Crossref] [PubMed]
- Almåsbak H, Aarvak T, Vemuri MC. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res 2016;2016:5474602. [Crossref] [PubMed]
- Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 2011;29:330-6. [Crossref] [PubMed]
- Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010;28:4722-9. [Crossref] [PubMed]
- Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 2008;68:5955-64. [Crossref] [PubMed]
- Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med 2007;5:67. [Crossref] [PubMed]
- Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 2005;11:4160-7. [Crossref] [PubMed]
- Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 2005;11:5515-25. [Crossref] [PubMed]
- Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 2004;64:4973-9. [Crossref] [PubMed]
- Okada H, Pollack IF. Cytokine gene therapy for malignant glioma. Expert Opin Biol Ther 2004;4:1609-20. [Crossref] [PubMed]
- Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother 2004;27:452-9. [Crossref] [PubMed]
- Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer 2003;89:1172-9. [Crossref] [PubMed]
- Okada H, Lieberman FS, Edington HD, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol 2003;64:13-20. [Crossref] [PubMed]
- Okada H, Pollack IF, Lieberman F, et al. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther 2001;12:575-95. [Crossref] [PubMed]
- Okada H, Pollack IF, Lotze MT, et al. Gene therapy of malignant gliomas: a phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum Gene Ther 2000;11:637-53. [Crossref] [PubMed]
- Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study. J Neurooncol 2016;130:517-27. [Crossref] [PubMed]
- Pollack IF, Jakacki RI, Butterfield LH, et al. Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas. Neuro Oncol 2016;18:1157-68. [Crossref] [PubMed]
- Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol 2014;32:2050-8. [Crossref] [PubMed]
- Okada H, Low KL, Kohanbash G, et al. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol 2008;88:245-50. [Crossref] [PubMed]
- Li S, Gill N, Lentzsch S. Recent advances of IMiDs in cancer therapy. Curr Opin Oncol 2010;22:579-85. [Crossref] [PubMed]
- Görgün G, Calabrese E, Soydan E, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood 2010;116:3227-37. [Crossref] [PubMed]
- Fangusaro J, Mitchell DA, Kocak M, et al. Phase 1 study of pomalidomide in children with recurrent, refractory, and progressive central nervous system tumors: A Pediatric Brain Tumor Consortium trial. Pediatr Blood Cancer 2021;68:e28756. [Crossref] [PubMed]
- Fried I, Lossos A, Ben Ami T, et al. Preliminary results of immune modulating antibody MDV9300 (pidilizumab) treatment in children with diffuse intrinsic pontine glioma. J Neurooncol 2018;136:189-95. [Crossref] [PubMed]
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Wang SS, Bandopadhayay P, Jenkins MR. Towards Immunotherapy for Pediatric Brain Tumors. Trends Immunol 2019;40:748-61. [Crossref] [PubMed]
- Teng F, Meng X, Kong L, et al. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: A systematic review. Cancer Lett 2018;414:166-73. [Crossref] [PubMed]
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904-11. [Crossref] [PubMed]
- Cacciotti C, Choi J, Alexandrescu S, et al. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol 2020;149:113-22. [Crossref] [PubMed]
- Gorsi HS, Malicki DM, Barsan V, et al. Nivolumab in the Treatment of Recurrent or Refractory Pediatric Brain Tumors: A Single Institutional Experience. J Pediatr Hematol Oncol 2019;41:e235-41. [Crossref] [PubMed]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 2005;35:2518-27. [Crossref] [PubMed]
- Shevtsov MA, Kim AV, Samochernych KA, et al. Pilot study of intratumoral injection of recombinant heat shock protein 70 in the treatment of malignant brain tumors in children. Onco Targets Ther 2014;7:1071-81. [Crossref] [PubMed]
- Yung WK, Prados M, Levin VA, et al. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol 1991;9:1945-9. [Crossref] [PubMed]
- Allen J, Packer R, Bleyer A, et al. Recombinant interferon beta: a phase I-II trial in children with recurrent brain tumors. J Clin Oncol 1991;9:783-8. [Crossref] [PubMed]
- Chang AY, Keng PC. Potentiation of radiation cytotoxicity by recombinant interferons, a phenomenon associated with increased blockage at the G2-M phase of the cell cycle. Cancer Res 1987;47:4338-41.
- Gould MN, Kakria RC, Olson S, et al. Radiosensitization of human bronchogenic carcinoma cells by interferon beta. J Interferon Res 1984;4:123-8. [Crossref] [PubMed]
- Packer RJ, Prados M, Phillips P, et al. Treatment of children with newly diagnosed brain stem gliomas with intravenous recombinant beta-interferon and hyperfractionated radiation therapy: a childrens cancer group phase I/II study. Cancer 1996;77:2150-6. [Crossref] [PubMed]
- Peres E, Wood GW, Poulik J, et al. High-dose chemotherapy and adoptive immunotherapy in the treatment of recurrent pediatric brain tumors. Neuropediatrics 2008;39:151-6. [Crossref] [PubMed]
- Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science 2004;305:200-5. [Crossref] [PubMed]
- Perek D, Perek-Polnik M. Brain tumors in children. Przegl Lek 2003;60:27-34.
- Gabrilovich DI, Ishida T, Nadaf S, et al. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 1999;5:2963-70.
- Van Gool SW, Makalowski J, Bonner ER, et al. Addition of Multimodal Immunotherapy to Combination Treatment Strategies for Children with DIPG: A Single Institution Experience. Medicines (Basel) 2020;7:29. [Crossref] [PubMed]
- Ishikawa E, Tsuboi K, Saijo K, et al. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res 2004;24:1861-71.
- Pérez-Martínez A, Fernández L, Díaz MA. The therapeutic potential of natural killer cells to target medulloblastoma. Expert Rev Anticancer Ther 2016;16:573-6. [Crossref] [PubMed]
- Fernández L, Portugal R, Valentín J, et al. In vitro Natural Killer Cell Immunotherapy for Medulloblastoma. Front Oncol 2013;3:94. [Crossref] [PubMed]
- Khatua S, Cooper LJN, Sandberg DI, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol 2020;22:1214-25. [Crossref] [PubMed]
- Brown CE, Badie B, Barish ME, et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res 2015;21:4062-72. [Crossref] [PubMed]
- Ahmed N, Brawley V, Hegde M, et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol 2017;3:1094-101. [Crossref] [PubMed]
- O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 2017;9:eaaa0984. [Crossref] [PubMed]