
Single kidney transplantation from pediatric deceased donors in China: the outcomes and risk factors of graft survival
Highlight box
Key findings
• SKT from pediatric donors can achieve decent outcomes.
• Rejection is an independent risk factor of graft survival, especially for adolescent recipients.
• Child recipients may compromise early transplant outcomes due to vascular thrombosis, which may be related to small (DBW ≤11 kg) pediatric donors.
What is known and what is new?
• Pediatric deceased donors offer great potential for expanding the organ donor pool, but the transplant outcome and risk factors have not been well elucidated.
• This study demonstrated the safety, as well as the risk factors of the transplant outcome from pediatric deceased donors.
What is the implication, and what should change now?
• Our study revealed the risk factors of SKT from pediatric donors, and provided evidence that kidneys from pediatric donors can expand the donor pool.
Introduction
In recent years, the prevalence of end-stage renal disease (ESRD) has increased (1). Kidney transplantation is preferable to dialysis because of its superior long-term survival rates and better quality of life among recipients. However, an imbalance between donors and potential recipients has been aggravated by the rapid growth of ESRD patients on the waiting list, which has outpaced the collection of donor kidneys. Nonetheless, the donor pool had expanded due to an increase in the number of pediatric donors over the past few years (2-8). This expansion alleviates the conflict between the high demand among ESRD patients and the lack of donor organs. As there are currently no established criteria regarding the allocation of kidneys from pediatric donors, the practice and requirements differ in various countries.
According to the Organ Procurement and Transplantation Network (OPTN) and the Australia and New Zealand Organ Donation Registry (ANZOD), the proportion of pediatric donors has exceeded 10%, and the overall prognosis is also satisfactory (2,7-13). Moreover, studies indicated that the prognosis of kidneys from pediatric donors is comparable to or better than that of adult donors (14,15). Considering the numerous donations from pediatric donors, there is a pressing need to better utilize these kidneys. The allocation criteria for pediatric kidneys vary among different countries, and many centers give priority to pediatric recipients according to national policy (16-18). In China, regulations regarding organ sharing reflect the national priority of giving pediatric donor organs to children since August 2018. While there are challenges with preoperative matching, operation protocol, and long-term care after surgery, a few transplant centers have been exploring the rational utilization of pediatric donor kidneys. Many donor and recipient factors, such as donor type, pre-transplant weight status, donor-recipient size matching, blood type, hyperuricemia, hyper-filtration injury and primary disease have been found affecting renal allograft survival in different studies (19-23). However, risk factors of kidney transplantation from pediatric deceased donors have not been well explored. In the clinical setting, there are notable differences in donor-recipient size matching, transplant surgery, perioperative management, post-transplant complications and allograft survival when pediatric kidneys are transplanted to children or adult recipients. Therefore, survival analysis from the perspective of recipients age may provide specific information facilitating the improvement of transplant outcome of pediatric donor kidneys.
Compared with en bloc kidney transplantation (EBKT) from pediatric donors, single kidney transplantation (SKT) can better utilize the scarce donor pool. Moreover, the SKT surgical procedure for kidneys from pediatric donors is similar to that of adult donors, and it is more convenient to place only one kidney into the iliac fossa, especially in young child recipients. Therefore, SKT does not require a long training period. Studies have also reported that for surgery involving pediatric donors, the prognosis of SKT was comparable to that of EBKT (24-28). Kidneys from donors with low donor body weight (DBW) can be used for EBKT, but there are also centers performing SKT procedures, which provide more transplant opportunities for ESRD patients. Our center carried out SKT from pediatric donors in the early stages in China, and our experiences are worth summarizing. In this retrospective cohort study, we analyzed the 3-year curative effect of SKT and explored the associated risk factors of using pediatric donors in different recipient-age groups, aiming to improve the efficacy of SKT from pediatric donors. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-547/rc).
Methods
Study design
The research subjects were patients who received SKT from pediatric deceased donors at The First Affiliated Hospital of Sun Yat-sen University between January 2012 and March 2021. The inclusion criteria were as follows: (I) organ recipients who underwent allograft SKT at The First Affiliated Hospital of Sun Yat-sen University; (II) organ recipients who received donor kidneys from citizens after death; (III) organ donors aged <18 years at the time of donation; and (IV) patients who received regular follow-up after operation. The exclusion criteria were as follows: (I) organ recipients who underwent combined/multiple organ transplantation; (II) transplantation with incompatible ABO blood types; (III) organ recipients who received donor kidneys with definite kidney quality problems or constitutional problems; and (IV) recipients who did not meet the postoperative follow-up period of at least 3 months.
Recipients were grouped based on their age at the time of transplantation: child group (≤12 years), adolescent group (12–18 years), and adult group (≥18 years). Informed consent was obtained from patients or their legal guardians, and this study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (No. [2019]452). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and upheld the principles of the Declaration of Istanbul on Organ Trafficking and Transplant Tourism (29).
Surgical procedures and perioperative management
Kidney procurement from pediatric deceased donors primarily involved the combined recovery of the liver and kidneys, and simple en bloc resection of the kidneys (i.e., with no intention of liver harvesting). In this study, en bloc donor kidneys were split and trimmed into two single kidneys on the back table. All surgeries were performed according to the SKT procedure, as described previously (11,12).
Multiple approaches were utilized to reduce vasospasm and thrombosis. It is important to minimize stretching and isolation of the donor renal artery during the procurement and back-table surgery of the donor kidneys. Papaverine (3 mg/mL in saline) was injected into the artery before reperfusion and was pumped at 2 mL/h (1.2 mg/mL in saline) continuously for 3–5 days after the transplantation surgery. The renal hilum was infiltrated with lidocaine hydrochloride (20 mg/mL). Depending on the donor-kidney size, surgical drainage, urine properties, and coagulation function of recipients, low molecular weight heparin (LMWH; 50–100 IU/kg/d) was administered for anticoagulation for 3–5 days, followed by oral clopidogrel bisulfate (12.5–75 mg/day) thereafter. During perioperative management, systolic blood pressure was maintained at 120–130 mmHg for recipients whose donors were aged over 5 months or at 110–120 mmHg if the donor was aged less than 5 months.
All recipients received either anti-thymocyte globulin or anti-CD25 monoclonal antibody as induction therapy. The maintenance immunosuppressive regimen consisted of tacrolimus/cyclosporine, mycophenolic mofetil, or enteric-coated mycophenolic sodium with or without steroids.
Data collection
All recipients received regular outpatient visits or telephone follow-up in our study. Clinical data were collected from hospital records including baseline characteristics [donor type, donor age, body weight, height, kidney size, cause of death, warm ischemia time/cold ischemia time (WIT/CIT), recipient age, sex, body weight, height, type of dialysis, and the number of human leukocyte antigen (HLA) mismatches] and postoperative outcomes [patient survival, graft survival, graft function, primary non-function (PNF), delayed graft function (DGF), rejection, vascular and urinary complications, infection, and recurrence of primary diseases]. Graft survival was defined as re-transplantation, graft nephrectomy, return to dialysis irreversibly, or death with a functioning graft. DGF was defined as the need for dialysis within 1 week postoperatively. Biopsies were classified according to the Banff criteria by local pathologists. Graft function was evaluated by serum creatinine (SCr) and estimated glomerular filtration rate (eGFR). The eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation based on SCr (>16 years old) or Schwartz equation (≤16 years old) (30,31).
Statistical analysis
In the descriptive statistical analysis, if the quantitative index satisfied a normal distribution, it was expressed as the arithmetic mean and standard deviation (SD); otherwise, it was expressed as the median and interquartile range (IQR). Categorical variables were expressed as the number of cases, frequency number, and percentage. When comparing the baseline data and clinical outcomes, if it was a continuous variable, the analysis of variance (ANOVA) test (for normal distribution and homogeneity of variance) or Mann-Whitney rank sum test (for skewness distribution or homogeneity of variance if not satisfied) was performed. For categorical variables, the chi-square test or Fisher’s exact chi-square test was used. The Kaplan-Meier method was used to analyze the 1- and 3-year patient and graft survivals, and the log-rank test was used to compare whether there was a significant difference in survival probabilities between groups. The Cox proportional hazards model was used for univariate and multivariate analyses. The outcome variables included DBW, age at surgery, CIT, donor type, recipient gender, dialysis type, antithymocyte globulin induction, donor creatine, donor/recipient body weight ratio, donor/recipient body height ratio, HLA mismatch and rejection. Variables with a P value <0.20 in the univariate model were included in the multivariate model. All the tests were bilateral, and statistical significance was set at P<0.05. All statistical analyses were performed using SPSS for Windows (version 22.0, IBM Corp., Armonk, NY, USA) and ‘R’ language for Windows (version 4.1.0), which is a free software environment for statistical computing and graphics (https://www.r-project.org/).
Results
Population characteristics
This study involved 484 cases of pediatric-donor SKTs, including 229 pediatric recipients and 255 adult recipients. The characteristics of the donors and recipients are summarized in Tables 1,2, respectively. The donors were predominantly male (n=184, 59.7%) and included a high proportion of donations after brain death (n=213, 69.2%). The average ages of the donors and recipients were 7.15±5.83 and 26.24±17.58 years, respectively. The average body weights of the donors and recipients were 27.32±19.47 and 43.68±19.23 kg, respectively.
Table 1
| Recipient group | Overall | Child | Adolescent | Adult | P value |
|---|---|---|---|---|---|
| Donor number | 308 | 89 | 60 | 159 | |
| Age (years), mean (SD) | 7.15 (5.83) | 3.81 (4.88) | 5.68 (4.97) | 9.57 (5.54) | <0.001 |
| Male, n (%) | 184 (59.74) | 53 (59.55) | 36 (60.00) | 95 (59.75) | 1.000 |
| Body weight (kg), mean (SD) | 27.32 (19.47) | 17.82 (15.27) | 22.62 (15.61) | 34.87 (20.09) | <0.001 |
| Body height (cm), mean (SD) | 118.07 (37.43) | 97.78 (34.65) | 111.94 (33.40) | 133.12 (34.05) | <0.001 |
| Cause of death, n (%) | 0.017 | ||||
| Trauma | 130 (42.21) | 39 (43.82) | 16 (26.67) | 75 (47.17) | |
| Hypoxic-ischemic encephalopathy | 24 (7.79) | 8 (8.99) | 3 (5.00) | 13 (8.18) | |
| Cerebral hemorrhage | 22 (7.14) | 5 (5.62) | 2 (3.33) | 15 (9.43) | |
| CNS tumor | 18 (5.84) | 3 (3.37) | 5 (8.33) | 10 (6.29) | |
| Other | 114 (37.01) | 34 (38.20) | 34 (56.67) | 46 (28.93) | |
| Donor type, n (%) | 0.313 | ||||
| DBD | 213 (69.16) | 64 (71.91) | 45 (75.00) | 104 (65.41) | |
| DCD | 95 (30.84) | 25 (28.09) | 15 (25.00) | 55 (34.59) | |
| SCr pre-procurement, n (%) | 0.115 | ||||
| High | 91 (29.55) | 20 (22.47) | 16 (26.67) | 55 (34.59) | |
| Normal | 217 (70.45) | 69 (77.53) | 44 (73.33) | 104 (65.41) | |
| WIT (min), mean (SD) | 3.21 (4.93) | 3.26 (4.76) | 2.52 (4.99) | 3.45 (5.00) | 0.462 |
| CIT (hours), mean (SD) | 11.15 (5.88) | 11.76 (5.58) | 9.95 (3.93) | 11.29 (6.60) | 0.176 |
SD, standard deviation; CNS, central nervous system; DBD, donation after brain death; DCD, donation after cardiac death; SCr, serum creatinine; WIT, warm ischemia time; CIT, cold ischemia time.
Table 2
| Recipient group | Overall (n=484) | Child (n=143) | Adolescent (n=86) | Adult (n=255) | P value |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 26.24 (17.58) | 7.89 (2.84) | 14.47 (1.71) | 40.50 (11.91) | <0.001 |
| Male, n (%) | 264 (54.55) | 70 (48.95) | 44 (51.16) | 150 (58.82) | 0.130 |
| Body weight (kg), mean (SD) | 43.68 (19.23) | 20.75 (7.43) | 38.63 (9.91) | 58.24 (11.06) | <0.001 |
| Donor/recipient body weight ratio, mean (SD) | 0.65 (0.50) | 0.83 (0.70) | 0.55 (0.39) | 0.58 (0.34) | <0.001 |
| Body height (cm), mean (SD) | 147.95 (24.63) | 116.02 (18.59) | 151.62 (11.52) | 164.61 (7.67) | <0.001 |
| Pre-transplant dialysis, n (%) | |||||
| Hemodialysis | 349 (72.11) | 86 (60.14) | 58 (67.44) | 205 (80.39) | <0.001 |
| Peritoneal dialysis | 156 (32.23) | 63 (44.06) | 32 (37.21) | 61 (23.92) | 0.001 |
| Pre-emptive transplant | 53 (10.95) | 17 (11.89) | 11 (12.79) | 25 (9.80) | 0.680 |
| Waiting time since dialysis (days), mean (SD) | 635.38 (692.24) | 268.06 (248.33) | 345.50 (361.26) | 783.98 (763.38) | <0.001 |
| Re-transplant, n (%) | 23 (4.75) | 8 (5.59) | 5 (5.81) | 10 (3.92) | 0.661 |
| HLA-A, B, DR MM number, mean (SD) | 3.93 (1.18) | 3.81 (1.26) | 3.90 (1.03) | 4.07 (1.18) | 0.215 |
| Induction therapy, n (%) | <0.001 | ||||
| Lymphocyte depleting | 342 (70.66) | 78 (54.55) | 48 (55.81) | 216 (84.71) | |
| Basiliximab | 142 (29.34) | 65 (45.45) | 38 (44.19) | 39 (15.29) | |
| Maintenance regimen, n (%) | 0.718 | ||||
| Tac + MPA + Pred | 481 (99.38) | 142 (99.30) | 86 (100.00) | 253 (99.22) | |
| CsA + MPA + Pred | 3 (0.62) | 1 (0.70) | 0 (0.00) | 2 (0.78) | |
| Primary disease, n (%) | <0.001 | ||||
| Glomerulonephritis | 367 (75.83) | 79 (55.24) | 59 (68.60) | 229 (89.80) | |
| FSGS | 23 (4.75) | 13 (9.09) | 5 (5.81) | 5 (1.96) | |
| IgA nephropathy | 14 (2.89) | 2 (1.40) | 5 (5.81) | 7 (2.75) | |
| Other | 80 (16.53) | 49 (34.27) | 17 (19.77) | 14 (5.49) |
SD, standard deviation; HLA, human leukocyte antigen; MM, mismatch; Tac, tacrolimus; MPA, mycophenolic acid; Pred, prednisolone; CsA, cyclosporine; FSGS, focal segmental glomerular sclerosis; IgA, immunoglobulin A.
As shown in Table 1, there was a significant difference in donor age, DBW, and donor body height (DBH) between the groups (P<0.001), which were significantly higher in the adult group (P<0.001). There was no statistical difference between the adolescent group and the child group (P>0.05). DBW distribution suggested statistical differences between the recipient groups (P<0.001), while the DBW of the child group was the lowest (17.82±15.27 kg) (Table 1). The overall ratio of the donor-recipient body weight was 0.65±0.50, and the ratio of the child group (0.83±0.70) was higher than those of the adolescent (0.55±0.39; P<0.001) and adult (0.58±0.34; P<0.001) groups.
No significant differences were observed between the groups in terms of donor sex, donor type, donor SCr level, average CIT, recipient sex, preemptive kidney transplantation rate, maintenance immunosuppressive regimens, or HLA mismatch number.
Patient and graft survival
The median follow-up time was 26.7 (range, 9.1–109.6) months. The patient survival of this cohort is shown in Figure 1A. The 1-, 2-, and 3-year patient survival rates were 98.7%, 98.1%, and 96.8%, respectively. Ten patients died with a functioning graft during the follow-up period, 50% of whom died of pneumonia. One patient experienced graft loss due to graft rupture and subsequently died of coagulation disorder and severe hemorrhage. There was no significant survival difference among the three groups.
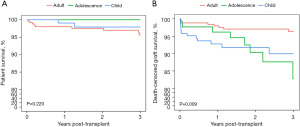
The death-censored graft survival (DCGS) is shown in Figure 1B. The 1-, 2-, and 3-year DCGS rates were 96.1%, 94.4%, and 92.7%, respectively. There was no statistical difference among the three groups (P=0.2). The 1-year DCGS of the child group was 92.8%, which was lower than the adult group (98.0%; P<0.05), but the difference was not statistically significant with that of the adolescent group (96.2%; P=0.379). A between-group statistical difference was observed in the 3-year DCGS (P=0.009). The 3-year DCGS of the adolescent group was 82.8%, which was lower than that of the adult group (96.3%; P=0.014), but no statistical differences were observed with that of the child group (90%; P=0.852).
The causes of graft loss included rejection (9/28, 32.14%), surgical-related complications (SRCs, including vascular thrombosis, artery stenosis, and peri-graft hematoma, 11/28, 39.29%), recurrent diseases (3/28, 10.71%), and other complications (5/28, 17.86%). The causes of graft loss in different recipient age groups are summarized in Table 3 and Figure 2. As demonstrated, 12 patients experienced graft loss in the child group, which was attributed to vascular thrombosis (6/12, 50%), rejections (3/12, 25%), recurrence of primary disease (2/12, 16.67%), and BK polyomavirus (BKV) infection (1/12, 8.33%). Eight recipients lost their graft function in the adolescent group, with rejections (4/8, 50%) being the main reason for the loss of function. Graft rupture due to graft bleeding (3/8, 37.5%) and recurrence of primary disease (1/8, 12.5%) also contributed to graft loss. For adult recipients, rejection (2/8, 25%), vascular thrombosis (1/8, 12.5%), graft rupture (1/8, 12.5%), and other complications (4/8, 50%) were the causes of graft loss.
Table 3
| Groups | Age (days) | Total | ||
|---|---|---|---|---|
| 0–30 | 31–360 | 361–1,080 | ||
| Rejection, n | 1 | 2 | 6 | 9 |
| Child | 0 | 1 | 2 | 3 |
| Adolescent | 0 | 1 | 3 | 4 |
| Adult | 1 | 0 | 1 | 2 |
| SRCs, n | 10 | 0 | 1 | 11 |
| Child | 6 | 0 | 0 | 6 |
| Adolescent | 2 | 0 | 1 | 3 |
| Adult | 2 | 0 | 0 | 2 |
| Recurrent disease, n | 0 | 2 | 1 | 3 |
| Child | 0 | 2 | 0 | 2 |
| Adolescent | 0 | 0 | 1 | 1 |
| Adult | 0 | 0 | 0 | 0 |
| Other, n | 0 | 3 | 2 | 5 |
| Child | 0 | 1 | 0 | 1 |
| Adolescent | 0 | 0 | 0 | 0 |
| Adult | 0 | 2 | 2 | 4 |
SRC, surgical-related complication.
Notably, the 1-month DCGS decreased markedly in the child group (95.8%), as six child recipients developed thrombosis within 1-month post-operatively, which led to graft failure (6/12, 50%) in the child group at that phase. However, the DCGS subsequently stabilized, and the 1-year (92.8%) and 1-month DCGS of the child group were relatively similar. Meanwhile, in the adolescent group, the 1-year DCGS was 96.2% but this rate dropped after 1 year. Further analysis showed that 60% (3/5) of the graft losses were caused by rejections at this phase, leading to a 3-year DCGS of 82.8% in the adolescent group, which was lower than that of the 1-year DCGS in the adolescent group.
Recovery of graft function
PNF was not observed postoperatively in any of the three groups. Seventy-two patients developed DGF and relied on dialysis for transition (72/484, 14.90%). Five of these patients experienced graft loss due to vascular thrombosis or renal rupture within 30 days postoperatively. Figure 3A indicated that the overall eGFR increased steadily after surgery. The eGFRs at 1, 6, and 12 months were 59.7±24.7, 74.8±23.5, and 80.0±24.5 mL/min/1.73 m2, respectively. For the next 2 years, the average eGFR maintained stability above 80 mL/min/1.73 m2, demonstrating a satisfactory graft function. The average eGFR of each group at 1-, 2-, and 3-year postoperatively is shown in Figure 3B. The eGFR of the child group at 1 year was higher than those of the adolescent and adult groups; however, the eGFR was similar between all groups at 3 years postoperatively.

Post-transplant complications
All postoperative complications are summarized in Table 4. The most common postoperative complication among the recipients was infection (168/484, 34.71%). Within 3 years post-transplant, pulmonary infection accounted for 17.36% of the total number of complications (84/484), including five deaths, and the incidence of urinary infections was up to 12.40%. Rejection affected 40 cases (8.26%) in total, and child (14/143, 9.79%) and adult (16/255, 6.27%) recipients had a relatively lower risk of rejection than adolescent recipients (10/86, 11.63%). Three adolescent recipients (3/4, 75%) developed graft loss resulting from rejection after 1 year postoperatively. Once rejection occurred, it appeared easier to develop graft loss in adolescent recipients (four graft losses from 10 rejections, 4/10, 40%) and child recipients (four graft losses from 14 rejections, 4/14, 28.57%) than in adult recipients (one graft losses from 16 rejections, 1/16, 6.25%).
Table 4
| Complications | Overall (n=484) | Child (n=143) | Adolescent (n=86) | Adult (n=255) | P value |
|---|---|---|---|---|---|
| PNF, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | – |
| DGF, n (%) | 72 (14.88) | 18 (12.59) | 16 (18.60) | 38 (14.90) | 0.464 |
| Infection, n (%) | |||||
| Pulmonary infection | 84 (17.36) | 31 (21.68) | 12 (13.95) | 41 (16.08) | 0.241 |
| Urinary infection | 60 (12.40) | 25 (17.48) | 11 (12.79) | 24 (9.41) | 0.064 |
| Other infection | 86 (17.77) | 36 (25.17) | 14 (16.28) | 36 (14.12) | 0.020 |
| Biopsy-proven rejection, n (%) | |||||
| ABMR | 10 (2.07) | 3 (2.10) | 3 (3.49) | 4 (1.57) | 0.557 |
| TCMR | 18 (3.72) | 7 (4.90) | 4 (4.65) | 7 (2.75) | 0.488 |
| Mixed-rejection | 12 (2.48) | 4 (2.80) | 3 (3.49) | 5 (1.96) | 0.703 |
| SRCs, n (%) | |||||
| Vascular thrombosis | 10 (2.07) | 7 (4.90) | 1 (1.16) | 2 (0.78) | 0.018 |
| Artery stenosis | 12 (2.48) | 4 (2.80) | 3 (3.49) | 5 (1.96) | 0.703 |
| Peri-graft hematoma | 13 (2.69) | 4 (2.80) | 4 (4.65) | 5 (1.96) | 0.409 |
| Ureteral stenosis, n (%) | 9 (1.86) | 5 (3.50) | 2 (2.33) | 2 (0.78) | 0.148 |
| Urinary leak, n (%) | 2 (0.41) | 1 (0.70) | 0 (0.00) | 1 (0.39) | 0.725 |
| Recurrence of primary disease, n (%) | 20 (4.13) | 8 (5.59) | 5 (5.81) | 7 (2.75) | 0.269 |
| Cause of death, n (%) | 0.541 | ||||
| Pneumonia | 6 (1.24) | 1 (0.70) | 0 (0.00) | 5 (1.96) | |
| Malignancy | 3 (0.62) | 1 (0.70) | 0 (0.00) | 2 (0.78) | |
| Others | 2 (0.41) | 0 (0.00) | 0 (0.00) | 2 (0.78) |
PNF, primary non-function; DGF, delayed graft function; ABMR, antibody mediated rejection; TCMR, T cell mediated rejection; SRC, surgical-related complication.
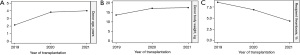
Regarding SRC, the child (13/143, 9.09%) and the adolescent (8/86, 9.30%) groups had higher incidences than the adult group (12/255, 4.71%). Among SRCs, the overall incidence of vascular thrombosis was low in our cohort (10/484, 2.07%), but all cases occurred within 1-month post-transplant and were more commonly observed in the child group (7/10, 70%). In addition, this may have led to graft loss at an early stage (within 1-month post-transplant) in the child group, as mentioned. For further analysis, the recipients were assigned to groups according to different DBW cut-off levels; according to the different rates of early thrombosis between the groups, 11 kg, as the highest value, was set as the cut-off value (Figure 4). Figure 5A shows that the thrombosis rate of child recipients was the highest among the three groups (4.9%), as child recipients possessed 54.55% kidneys from DBW ≤11 kg (Figure 5B). Also, the thrombosis rate was higher in recipients from DBW ≤11 kg (6/127, 4.72%) than those from DBW >11 kg (4/357, 1.12%) (Figure 5C). Figure 6 revealed an increasing trend of the DBW and donor age and a declining trend of thrombosis rate in recent years.


The recurrence of primary disease (20/484, 4.13%) and urological complications (11/484, 2.27%) were comparable between the groups (P>0.05). Five patients ultimately experienced graft loss, including four patients with recurrent focal segmental glomerular sclerosis (FSGS) and one patient with recurrent immunoglobulin A (IgA) nephropathy.
Graft survival risk factors
The recipient and donor risk factors were analyzed for overall DCGS, and the results are presented in Table 5. Univariate analysis revealed that DBW ≤11 kg [hazard ratio (HR) =3.08; 95% confidence interval (CI): 1.47–6.47; P=0.003], adolescent recipient group (HR =3.55; 95% CI: 1.33–9.50; P=0.012), child recipient group (HR =3.27; 95% CI: 1.33–8.02; P=0.010), and rejection (HR =4.88; 95% CI: 2.20–10.79; P<0.001) were risk factors for DCGS. In multivariate analysis, only rejection (HR =3.85; 95% CI: 1.71–8.66; P=0.001) was observed to be a significant risk factor for poor DCGS.
Table 5
| Variables | HR (95% CI, P) (univariable) | HR (95% CI, P) (multivariable) |
|---|---|---|
| DBW ≤11 kg | 3.08 (1.47–6.47, P=0.003) | 1.91 (0.82–4.45, P=0.137) |
| Adolescence recipient | 3.55 (1.33–9.50, P=0.012) | 2.46 (0.87–6.98, P=0.091) |
| Child recipient | 3.27 (1.33–8.02, P=0.010) | 2.09 (0.75–5.81, P=0.156) |
| CIT ≤10 hours | 0.63 (0.30–1.33, P=0.224) | – |
| DCD donor | 1.82 (0.87–3.84, P=0.114) | – |
| Male recipient | 0.61 (0.29–1.30, P=0.202) | – |
| Dialysis | 1.79 (0.42–7.54, P=0.429) | – |
| ATG induction | 0.54 (0.24–1.18, P=0.123) | – |
| Normal donor creatine | 0.85 (0.39–1.85, P=0.687) | – |
| Donor/recipient body weight ratio ≤0.5 | 1.13 (0.54–2.38, P=0.746) | – |
| Donor/recipient body height ratio ≤0.8 | 1.75 (0.79–3.87, P=0.166) | – |
| HLA MM ≤4 | 1.75 (0.58–5.31, P=0.324) | – |
| Rejection | 4.88 (2.20–10.79, P<0.001) | 3.85 (1.71–8.66, P=0.001) |
DCGS, death-censored graft survival; HR, hazard ratio; CI, confidence interval; DBW, donor body weight; CIT, cold ischemia time; DCD, donation after cardiac death; ATG, antithymocyte globulin; HLA, human leukocyte antigen; MM, mismatch.
Discussion
In this large-sample size cohort of SKT from pediatric deceased donors, we reported the SKT outcomes of pediatric donor kidneys, and more importantly discovered the specific risk factors of renal allograft survival among recipients of different ages. In this study, the overall 1-, 2-, and 3-year DCGS were 96.1%, 94.4%, and 92.7%, respectively. The graft survival was comparable or superior to the previously reported outcomes (25,26,32). The eGFR at 1 year after transplant was 80.0±24.5 mL/min/1.73 m2 and maintained stable thereafter during this study, indicating that satisfactory renal allograft function was obtained from the pediatric deceased donors. Survival analysis revealed rejection as a strong independent risk factor (HR =3.85) for the 3-year DCGS of pediatric donor kidneys. Moreover, a significant decrease in the DCGS 1 year after transplantation was observed in the adolescent group, which was mainly attributed to rejection (accounting for 60% of graft losses). We also identified vascular thrombosis as the main cause of graft loss in the very early phase (<30 days) after transplantation in the child group, and recipients who received kidneys from DBW ≤11 kg had a higher rate of thrombosis. These findings suggest that specific measures should be taken to further improve the transplant outcomes of pediatric donor kidneys by targeting risk factors for different recipient ages.
Rejection is increasingly recognized as an important influencing factor that can lead to poor post-transplant prognosis (33,34). In our multivariate model, rejection was observed to be the only risk factor for graft loss. Moreover, our research illustrated a rapid decline in the DCGS rate from 1- (96.2%) to 3-year (82.8%) post-transplant in the adolescent group. Rejection was the main cause of graft loss in this phase, leading to a relatively unsatisfactory long-term prognosis in the adolescent group. The underlying mechanisms of higher rejection incidences and the subsequent poor prognosis remain unclear. One of the chief culprits was non-adherence to medication. Two adolescent recipients in our cohort had once refused or forgot to take the immunosuppressants and developed rejection soon afterward, resulting in graft loss. Adolescents are a special population of kidney transplant recipients. They begin to assume responsibility for their healthcare and become less dependent on their families. It has been reported adolescent recipients after transplantation might have increased susceptibility to psychological illnesses and are prone to develop non-adherence to immunosuppressive medications, which might lead to rejection (35-38). In the adolescent group, 40% (4/10) of patients with rejection eventually progressed to graft loss, whereas this number was 28.57% (4/14) in the child group and 6.25% (1/16) in the adult group. This result indicates that rejection is more difficult to treat once it occurs or when it is diagnosed. Non-adherence-induced rejection often presents an acute and rapid process. Timely diagnosis and intervention are very important to obtain positive outcomes. Delayed detection and diagnosis can impair the responsiveness to anti-rejection therapy and lead to a poor prognosis. Therefore, particular attention should be paid to medication adherence during the age transition of adolescent recipients. Increasing the frequency of follow-up and developing better follow-up tools may also help to reduce rejection (39-41).
Thrombosis is common surgical complication after transplantation of pediatric donor kidneys and often leads to graft loss once it occurs (24,27,42,43). The vascular thrombosis rate in our cohort was 2.07%, which was relatively low. One of the reasons for this lower rate was the meticulous operation throughout the surgical procedure, which helped to reduce vascular stimulation intraoperatively and perioperatively, as lidocaine and papaverine favor vasospasm prevention (44). Additionally, the diameter of the vascular anastomosis can be enlarged using the aortic disc and inferior vena when performing SKT, and the risk of anastomotic stenosis and thrombosis can be significantly reduced (45). However, despite the preventative measures mentioned above, a higher rate of vascular thrombosis was observed in the child group than in the other two groups (4.9% vs. 1.16% vs. 0.78%, P=0.018). It is notable that the DCGS in the child group decreased markedly to 95.8% within 1 month after transplantation and stabilized at 92.8% thereafter. Vascular thrombosis was the main cause of early graft loss in this group. One explanation for this is that small pediatric recipients might be more prone to suffer thrombosis due to uremic coagulation disorders, as well as fine vessels, especially when the external iliac artery is used for anastomosis. Another more important reason may be the use of smaller pediatric donor kidneys in the child group. The DBW was considerably lower in the child group than in the other two groups (17.82±15.27 vs. 22.62±15.61 and 34.87±20.09 kg, P<0.001). DBW ≤11 kg was found to be a strong risk factor for DCGS (HR =3.08; P=0.003), while the proportion of DBW ≤11 kg was notably higher in the child group (54.55%) than in the other groups (29.07% and 9.41%) (Figure 5B). Further analysis revealed a recent trend toward a lower incidence of vascular thrombosis as DBW increased (Figure 6). These results suggest that increasing the body weight criteria of acceptable pediatric donors for single transplantation may facilitate the reduction of thrombosis and improve early graft survival to some extent, especially in younger pediatric recipients. This has significant implications for pediatric kidney transplant (PKT) in China, as most kidneys of PKT recipients are from pediatric donors. This study may provide insights into optimizing the allocation policy of pediatric donor kidneys.
This study has some limitations that should be noted. Firstly, this is a single-center retrospective study. Although our center is representative of the utilization of pediatric donor kidneys in China, a multicenter study is needed to validate our key findings. Also, the child group was inferior to the other two groups in terms of the early DCGS; however, this should not necessarily be interpreted as a denial of the allocation policy of pediatric kidneys to young children as the apparent bias of DBW among groups. Furthermore, owing to the lack of an adult donor cohort in this study, we could not compare the outcomes between pediatric and adult donors for pediatric recipients. Therefore, the long-term outcomes of pediatric donor kidneys for recipients of different age groups require further observation.
Conclusions
In this study, we explored the prognosis and safety of SKT in pediatric donors. Recipients of different ages might have varying risk factors at different post-transplant phases. Rejection was the only lasting obstacle in our multivariate model and was particularly risky for adolescent recipients at 1–3 years post-transplant. Moreover, child recipients may compromise their postoperative outcomes for thrombosis at an early stage, which may be related to the lower DBW. Hence, paying more attention to medication adherence for adolescent recipients in the early-middle phase after surgery and using kidneys from DBW >11 kg could achieve a better prognosis. Overall, SKT from pediatric donors could achieve satisfactory outcomes. Our study revealed the risk factors of SKT from pediatric donors and provided evidence that kidneys from pediatric donors can expand the donor pool.
Acknowledgments
Funding: The research was supported by grants from the Key Scientific and Technological Program of Guangzhou City (No. 201803040011), the Science and Technology Planning Project of Guangdong Province, China (No. 2015B020226002), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010884), the National Natural Science Foundation of China (Nos. 81870511, 82170770), the Guangdong Provincial Key Laboratory on Organ Donation and Transplant Immunology (Nos. 2017B030314018, 2020B1212060026), and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation, No. 2020A0505020003).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-547/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-547/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-547/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from patients or their legal guardians, and this study was approved by the Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (No. [2019]452). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and upheld the principles of the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johansen KL, Chertow GM, Foley RN, et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2021;77:A7-A8. [Crossref] [PubMed]
- Damji S, Callaghan CJ, Loukopoulos I, et al. Utilisation of small paediatric donor kidneys for transplantation. Pediatr Nephrol 2019;34:1717-26. [Crossref] [PubMed]
- Sui M, Zhao W, Zhu Y, et al. Increasing deceased donor transplants among pediatric candidates under the revised allocation strategy in China. Pediatr Transplant 2020;24:e13829. [Crossref] [PubMed]
- Suneja M, Kuppachi S, Katz D, et al. Small Split Pediatric Kidneys to Expand the Donor Pool: An Analysis of Scientific Registry of Transplant Recipients (SRTR) Data. Transplantation 2019;103:2549-57. [Crossref] [PubMed]
- Liu L, Zhang H, Fu Q, et al. Current status of pediatric kidney transplantation in China: data analysis of Chinese Scientific Registry of Kidney Transplantation. Chin Med J (Engl) 2014;127:506-10. [PubMed]
- Peng J, Dai H, Zhang H, et al. Comparison of Outcomes of Kidney Transplantation From Extremely Low Body Weight </=5kg Versus Larger Body Weight Pediatric Donors. Front Immunol 2021;12:738749. [Crossref] [PubMed]
- Zhu L, Fu C, Chen S, et al. Successful Single-kidney Transplantation in Adult Recipients Using Pediatric Donors Aged 8 to 36 Months: Comparable Outcomes With Those Using Pediatric Donors Aged >3 Years. Transplantation 2019;103:2388-96. [Crossref] [PubMed]
- Li JF, Liu J, Guo T, et al. Kidney transplantation from pediatric donors in a single Chinese center. Cell Biochem Biophys 2014;70:1713-7. [Crossref] [PubMed]
- Hart A, Lentine KL, Smith JM, et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am J Transplant 2021;21:21-137. [Crossref] [PubMed]
- Registry AaNZOD. ANZOD 2021 Annual Report. Available online: https://www.anzdata.org.au/wp-content/uploads/2021/08/s05_kidney_2021_v0.5_20210802.pdf (Accessed Oct. 2021).
- Su X, Shang W, Liu L, et al. Transplantation of a single kidney from pediatric donors less than 10 kg to children with poor access to transplantation: a two-year outcome analysis. BMC Nephrol 2020;21:250. [Crossref] [PubMed]
- Chen C, Su X, Wu C, et al. Successful single kidney transplantation from pediatric donors less than or equal to 10 kg to adult recipient: a retrospective cohort study. Transl Pediatr 2021;10:1618-29. [Crossref] [PubMed]
- Mitrou N, Aquil S, Dion M, et al. Transplantation of pediatric renal allografts from donors less than 10 kg. Am J Transplant 2018;18:2689-94. [Crossref] [PubMed]
- Moudgil A, Martz K, Stablein DM, et al. Good outcome of kidney transplants in recipients of young donors: a NAPRTCS data analysis. Pediatr Transplant 2011;15:167-71. [PubMed]
- Pape L, Hoppe J, Becker T, et al. Superior long-term graft function and better growth of grafts in children receiving kidneys from paediatric compared with adult donors. Nephrol Dial Transplant 2006;21:2596-600. [Crossref] [PubMed]
- Agarwal S, Oak N, Siddique J, et al. Changes in pediatric renal transplantation after implementation of the revised deceased donor kidney allocation policy. Am J Transplant 2009;9:1237-42. [Crossref] [PubMed]
- Harambat J, van Stralen KJ, Schaefer F, et al. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transplant 2013;13:2066-74. [Crossref] [PubMed]
- Pape L, Ahlenstiel T, Kanzelmeyer NK. Consequences of the change in Eurotransplant allocation system on kidney allocation in children. Clin Transplant 2013;27:650-1. [Crossref] [PubMed]
- Ehren R, Habbig S, Krupka K, et al. Prevalence and potential relevance of hyperuricemia in pediatric kidney transplant recipients-a CERTAIN registry analysis. Pediatr Transplant 2022;26:e14265. [Crossref] [PubMed]
- Kaur K, Jun D, Grodstein E, et al. Outcomes of underweight, overweight, and obese pediatric kidney transplant recipients. Pediatr Nephrol 2018;33:2353-62. [Crossref] [PubMed]
- Shapiro R, Sarwal MM. Pediatric kidney transplantation. Pediatr Clin North Am 2010;57:393-400. table of contents. [Crossref] [PubMed]
- Giuliani S, Gamba PG, Chokshi NK, et al. The effect of donor/recipient body surface area ratio on outcomes in pediatric kidney transplantation. Pediatr Transplant 2009;13:290-9. [Crossref] [PubMed]
- Shen Q, Fang X, Man X, et al. Pediatric kidney transplantation in China: an analysis from the IPNA Global Kidney Replacement Therapy Registry. Pediatr Nephrol 2021;36:685-92. [Crossref] [PubMed]
- Mohanka R, Basu A, Shapiro R, et al. Single versus en bloc kidney transplantation from pediatric donors less than or equal to 15 kg. Transplantation 2008;86:264-8. [Crossref] [PubMed]
- Al-Shraideh Y, Farooq U, El-Hennawy H, et al. Single vs dual (en bloc) kidney transplants from donors </= 5 years of age: A single center experience. World J Transplant 2016;6:239-48. [Crossref] [PubMed]
- Yaffe HC, Friedmann P, Kayler LK. Very small pediatric donor kidney transplantation in pediatric recipients. Pediatr Transplant 2017; [Crossref] [PubMed]
- Borboroglu PG, Foster CE 3rd, Philosophe B, et al. Solitary renal allografts from pediatric cadaver donors less than 2 years of age transplanted into adult recipients. Transplantation 2004;77:698-702. [Crossref] [PubMed]
- Sui M, Zhao W, Chen Y, et al. Optimizing the utilization of kidneys from small pediatric deceased donors under 15 kg by choosing pediatric recipients. Pediatr Transplant 2016;20:39-43. [Crossref] [PubMed]
- The Declaration of Istanbul on Organ Trafficking and Transplant Tourism. (2018 Edition). Transplantation 2019;103:218-9. [PubMed]
- Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629-37. [Crossref] [PubMed]
- Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937-44. [Crossref] [PubMed]
- Hoyer DP, Dittmann S, Buscher A, et al. Kidney transplantation with allografts from infant donors-Small organs, big value. Pediatr Transplant 2020;24:e13794. [Crossref] [PubMed]
- Kim M, Martin ST, Townsend KR, et al. Antibody-mediated rejection in kidney transplantation: a review of pathophysiology, diagnosis, and treatment options. Pharmacotherapy 2014;34:733-44. [Crossref] [PubMed]
- Ciancio G, Gaynor JJ, Guerra G, et al. Antibody-mediated rejection implies a poor prognosis in kidney transplantation: Results from a single center. Clin Transplant 2018;32:e13392. [Crossref] [PubMed]
- Zelikovsky N, Dobson T, Norman J. Medication beliefs and perceived barriers in adolescent renal transplant patients and their parents. Pediatr Nephrol 2011;26:953-9. [Crossref] [PubMed]
- Sundaram SS, Landgraf JM, Neighbors K, et al. Adolescent health-related quality of life following liver and kidney transplantation. Am J Transplant 2007;7:982-9. [Crossref] [PubMed]
- Rianthavorn P, Ettenger RB. Medication non-adherence in the adolescent renal transplant recipient: a clinician's viewpoint. Pediatr Transplant 2005;9:398-407. [Crossref] [PubMed]
- Kaboré R, Couchoud C, Macher MA, et al. Age-Dependent Risk of Graft Failure in Young Kidney Transplant Recipients. Transplantation 2017;101:1327-35. [Crossref] [PubMed]
- Steinberg EA, Moss M, Buchanan CL, et al. Adherence in pediatric kidney transplant recipients: solutions for the system. Pediatr Nephrol 2018;33:361-72. [Crossref] [PubMed]
- Myaskovsky L, Jesse MT, Kuntz K, et al. Report from the American Society of Transplantation Psychosocial Community of Practice Adherence Task Force: Real-world options for promoting adherence in adult recipients. Clin Transplant 2018;32:e13353. [Crossref] [PubMed]
- Zhao L, Yan J, Yang GL, et al. A Study on Adherence to Follow-up, Quality of Life, and Associated Factors Among Renal Transplant Recipients in China. Transplant Proc 2017;49:1285-90. [Crossref] [PubMed]
- Sureshkumar KK, Reddy CS, Nghiem DD, et al. Superiority of pediatric en bloc renal allografts over living donor kidneys: a long-term functional study. Transplantation 2006;82:348-53. [Crossref] [PubMed]
- Nghiem DD. En bloc transplantation of kidneys from donors weighing less than 15 kg. into adult recipients. J Urol 1991;145:14-6. [Crossref] [PubMed]
- Wang HY, Li J, Liu LS, et al. En bloc kidney transplantation from infant donors younger than 10 months into pediatric recipients. Pediatr Transplant 2017; [Crossref] [PubMed]
- Sharma A, Ramanathan R, Behnke M, et al. Single pediatric kidney transplantation in adult recipients: comparable outcomes with standard-criteria deceased-donor kidney transplantation. Transplantation 2013;95:1354-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



