
Interleukin-6 serum levels are independently associated with severe adenovirus pneumonia in children: a cross-sectional study
Highlight box
Key findings
• Interleukin-6 serum levels are independently associated with SAP in children.
What is known and what is new?
• IL-6 may be related with risk of SAP.
• The nonlinear relationship between IL-6 and SAP also been determined.
What is the implication, and what should change now?
• Early control of Il-6 has positive significance for the prevention of SAP.
Introduction
Human adenovirus (HAdV) infections have been increasing in incidence in China in recent years (1). HAdV is a common cause of upper and lower respiratory tract infections (2), particularly in children under the age of 5, and accounts for approximately 5–10% of acute lower respiratory tract infections (3). Clinically, one-third of children with adenovirus pneumonia progress to severe disease (4). Severe adenovirus pneumonia (SAP) is characterized by acute onset, severe illness, rapid progression, multisystem complications, and a poor prognosis; thus, it represents a major medical issue and imposes a heavy burden on patients, their families, and broader society (5).
Interleukin-6 (IL-6), discovered in 1986 (6), is a pleiotropic cytokine which, in addition to immune responses, is also involved in inflammation, hematopoiesis, bone metabolism, embryonic development, and other fundamental processes (7). IL-6 has been implicated in several complex chronic inflammatory diseases. Research suggests that the expression of serum inflammatory markers, such as C-reactive protein and lactate dehydrogenase enzyme, may be elevated in patients with SAP compared to those with mild infection (8). In children with SAP, high expression of inflammatory markers has been observed such as IL-6 (9). IL-6 expression is also significantly associated with exacerbations in SAP (8). In particular, whether there is a nonlinear relationship between the two is worth exploring. However, little direct proof of the independent association between IL-6 and SAP in children has been published, which calls for further clarification and research.
Several recent studies have evaluated risk factors for exacerbations in children with adenoviral pneumonia using statistical models (9-12). However, the results of these studies may be unreliable due to the potential effect of allocation bias of factors among patients with SAP. Although the clinical role of IL-6 has been partly discussed, the impact of confounding factors has been pervasively neglected in most of the current research on this topic. Therefore, further research to determine the confounder-adjusted association between IL-6 and SAP is called for. Moreover, the nonlinear relationship between IL-6 and SAP also needs to be measured, which have not been seen in previous studies. This will be more helpful in guiding clinical practice and has significant clinical value.
To address these research needs, the current study aimed to examine the independent association between IL-6 serum levels and SAP in children after adjustment for confounders. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-585/rc).
Methods
Study design and participants
A retrospective hospital-based cross-sectional study of patients who presented to Tianjin Children’s Hospital between January 2019 and December 2019 was conducted. Inclusion criteria were established in accordance with the guidelines for the diagnosis and treatment of adenovirus pneumonia in children (2019 version) (13). The diagnosis of adenovirus pneumonia was based on evidence of the presence of adenovirus infection at admission. This study was approved by the ethics board of Tianjin Children’s Hospital (No. L2021-21), and the requirement to obtain individual consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patients who showed positive results on the following (positive results on one of the following) were recruited for this study: (I) an antigen test for viruses from a nasal swab; (II) analyze the genetic code of adenoviruses from nasopharyngeal swabs; (III) Test for serum IgM antibodies specific to adenovirus; and (IV) next-generation sequencing of bronchoalveolar lavage fluid by adenovirus and metagenomic analysis.
The following patients were excluded from the study: (I) patients whose course of illness lasted for 2 weeks after hospital admission (II) patients who developed nosocomial infection; (III) children with severe allergic conditions; and (IV) patients who died during hospitalization or who were automatically discharged from the hospital for various reasons. Figure 1 illustrates the study flowchart.
Study variables
The primary independent variable was the serum level of IL-6, which was measured using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA; Multiskan Ascent fully automated ELISA) (ThermoFisher, China). Patients’ IL-6 values were categorized into quintiles, with the first quintile (Q1) representing the lowest values and the fifth quintile (Q5) representing the highest values. The serum levels of IL-6 were divided as follows: ≤9.86 (Q1), 9.87–20.82 (Q2), 20.83–33.82 (Q3), 33.83–51.52 (Q4), and ≥51.53 (Q5).
Response variable
The primary outcome measure was the occurrence of SAP. The definitive diagnostic criteria for severe pneumonia, which were developed based on the guidelines for the diagnosis and treatment of community-acquired pneumonia (CAP) in China (14), were as follows: (I) poor health in general; (II) daily of diet refusals; (III) unclear consciousness; (IV) a high frequency breathing) [infants (28 days – 1 year), >70 breaths per minute; older children (>1 year), >50 breaths per minute]; (V) signs include difficulty breathing and cyanosis; (VI) the symptoms of dyspnea include moaning, three concave signs, and nasal flaring; (VII) an infiltration of multiple lobes or at least two-thirds of the lungs; (VIII) an effusion of the pleura; (IX) saturation of percutaneous oxygen ≤0.92; and (X) extrapulmonary problems (cerebral abscess, meningitis, pericarditis, osteomyelitis, endocarditis, arthritis, hemolytic uremic syndrome, sepsis, etc.).
The patients were divided into two groups: those who developed SAP [1] and those who developed non-SAP [0].
Controlling for confounders
A standardized data collection form was used to extract data from patients’ medical records, including sex, age, maximum temperature, and duration of fever. Detailed clinical and imaging data were acquired upon admission for all participants. Real-time quantitative PCR was performed to detect HAdV DNA in patients’ respiratory secretions after admission, using a kit obtained from Shenzhen Precon Biotechnology Co. An enzyme rate assay was performed to measure the following biochemical parameters by using Roche COBAS 8000 C701 fully automated biochemical analysis module instrument, Germany): alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, and lactate dehydrogenase. A double-antibody sandwich ELISA (Multiskan Ascent fully automated ELISA) was conducted to measure serum levels of IL-6. All laboratory tests were performed in the laboratory department of Tianjin Children’s Hospital.
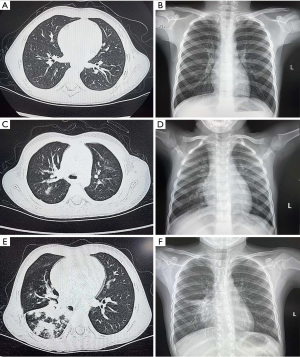
The patients’ preoperative imaging data were analyzed in detail. Radiographic parameters were measured using chest X-ray (Siemens, DXR656, Germany) and thoracic computed tomography (Siemens Force CT, 120 kv 220 mA, Germany). The severity of lung inflammation and lung plaque assays was demonstrated (Figure 2).
Data management and statistical analysis
The EpiData software (The EpiData Association, Odense, Denmark, version 3.1) was used to collect data before their transfer to R (4.1.0, R Foundation for Statistical Computing, Vienna, Austria) for statistical analysis. The median (interquartile range) of continuous variables is expressed, while absolute frequencies and percentages are expressed for categorical variables. Continuous and categorical data were analyzed using the Kruskal-Wallis test and Fisher’s exact chi-square test, respectively. For further analysis, the presence of SAP was taken as a response variable and IL-6 expression as an independent variable. To examine the association between IL-6 level and the presence of SAP, multivariable logistic regression analysis was conducted with adjustment for potential confounding factors. Regression analysis was conducted according to the method described (15). The threshold effect of IL-6 level was examined using a two-piecewise regression model. A trial method was used to determine the threshold level of IL-6 at which the relationship between IL-6 and SAP began to change and became significant. Trial inflection points were moved along a predefined interval in order to identify the inflection points that showed the maximum model likelihood. A two side P value <0.05 were considered to be significant.
Based on the empirical estimation of the sample size estimation, the sample was defined as having at least 10 outcome events for each variable (EPV). This means that there were at least 10 outcome events for each estimated parameter. The sample size in this study has met the basic principles of EPV.
Results
Baseline and demographic characteristics
In total, 542 patients met the inclusion criteria and were eligible for enrolment (Figure 1), of whom 223 (41.1%) were male and 319 (58.9%) were female. The median IL-6 level for the entire study population was 27.085 [interquartile range (IQR): 12.6, 46.76] pg/mL. The median IL-6 level was 28.33 (IQR: 13.32, 47.55) for the male patients and 26.65 (IQR: 12.36, 45.76) pg/mL for the female patients. The overall incidence of SAP was 42.6% (213 of 542). Baseline characteristic and variables for the descriptive statistics according to severe adenovirus pneumonia (SAP) in children (Table 1). The SAP incidence rates in Q1–5 were 29.4%, 34.3%, 41.3%, 50.9%, and 57.4%, respectively. The baseline characteristics of the study population according to subgroups based on IL-6 expression are shown in Table 2.
Table 1
| Variable | SAP =0 (N=311, 57.4%) | SAP =1 (N=231, 42.6%) | P value |
|---|---|---|---|
| IL-6 (pg/mL), median (IQR) | 21.91 (10.19, 41.08) | 34.48 (17.36, 53.94) | <0.01 |
| Sex, n (%) | 0.54 | ||
| Male | 124 (39.9) | 99 (42.9) | |
| Female | 187 (60.1) | 132 (57.1) | |
| Age (years), median (IQR) | 3.00 (2.00, 5.00) | 3.00 (2.00, 6.00) | 0.96 |
| Weight (kg), median (IQR) | 3.35 (3.00, 3.60) | 3.25 (3.00, 3.60) | 0.09 |
| Peak fever (℃), median (IQR) | 39.60 (39.20, 40.00) | 39.90 (39.50, 40.10) | <0.01 |
| WBC (×109·L−1), median (IQR) | 9.14 (6.39, 12.90) | 9.44 (7.16, 13.47) | 0.14 |
| Neutrophils (%), median (IQR) | 57.00 (38.60, 68.70) | 63.00 (49.20, 73.90) | <0.01 |
| Lymphocytes (%), median (IQR) | 32.80 (21.30, 47.00) | 27.80 (18.90, 40.80) | <0.01 |
| Monocytes (%), median (IQR) | 8.30 (6.60, 10.70) | 7.60 (5.90, 9.80) | <0.01 |
| HGB (U/g), median (IQR) | 122.00 (115.00, 130.00) | 122.00 (114.00, 129.00) | 0.39 |
| PLT (U/L), median (IQR) | 256.00 (214.00, 319.00) | 281.00 (222.00, 350.00) | 0.03 |
| CRP (mg·L−1), median (IQR) | 13.80 (4.30, 29.60) | 13.30 (3.70, 35.30) | 0.89 |
| ALB (U/L), median (IQR) | 42.50 (40.50, 44.90) | 42.20 (39.90, 44.50) | 0.07 |
| ALT (U/L), median (IQR) | 13.00 (10.00, 16.00) | 14.00 (11.00, 19.00) | <0.01 |
| γ-GT (U/L), median (IQR) | 10.00 (8.00, 12.00) | 10.00 (8.00, 13.00) | 0.02 |
| PCT (U/L), median (IQR) | 0.18 (0.08, 0.43) | 0.21 (0.08, 0.60) | 0.36 |
| AST (U/L), median (IQR) | 33.00 (26.00, 43.00) | 38.56 (29.00, 52.00) | <0.01 |
| BUN (U/L), median (IQR) | 2.80 (2.20, 3.40) | 2.90 (2.30, 3.40) | 0.51 |
| CRE (U/L), median (IQR) | 27.00 (22.00, 32.00) | 26.00 (21.00, 31.00) | 0.29 |
| CK (U/L), median (IQR) | 87.00 (63.00, 128.00) | 94.00 (65.00, 146.00) | 0.16 |
| CK-MB (U/L), median (IQR) | 4.00 (4.00, 7.00) | 4.00 (4.00, 7.00) | 0.52 |
| La (U/L), median (IQR) | 2.60 (2.14, 3.20) | 2.67 (2.17, 3.35) | 0.39 |
| LDH (U/L), median (IQR) | 349.00 (283.00, 427.00) | 405.00 (317.00, 560.00) | <0.01 |
| Bilateral lung, n (%) | 0.04 | ||
| No | 52 (16.7) | 24 (10.4) | |
| Yes | 259 (83.3) | 207 (89.6) | |
| Lung marking, n (%) | 0.21 | ||
| No | 49 (15.8) | 27 (11.7) | |
| Yes | 262 (84.2) | 204 (88.3) | |
| Lung patch, n (%) | <0.01 | ||
| No | 205 (65.9) | 97 (42.0) | |
| Yes | 106 (34.1) | 134 (58.0) | |
| Pulmonary inflammation, n (%) | <0.01 | ||
| No | 303 (97.4) | 200 (86.6) | |
| Yes | 8 (2.6) | 31 (13.4) |
SAP, severe adenovirus pneumonia; IL-6, interleukin-6; IQR, interquartile range; WBC, white blood cells; PLT, platelet count; HGB, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; γ-GT, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; CRE, serum creatinine; CK, creatine kinase; CK-MB, creatine kinase-MB; CRP, C-reactive protein; PCT, procalcitonin; LDH, lactate dehydrogenase; La, blood lactate.
Table 2
| Variable | Q1 (N=109) | Q2 (N=108) | Q3 (N=109) | Q4 (N=108) | Q5 (N=108) | P value |
|---|---|---|---|---|---|---|
| Sex, n (%) | 0.75 | |||||
| Male | 41 (37.6) | 47 (43.5) | 41 (37.6) | 48 (44.4) | 46 (42.6) | |
| Female | 68 (62.4) | 61 (56.5) | 68 (62.4) | 60 (55.6) | 62 (57.4) | |
| Age (years), median (IQR) | 3.00 (1.00, 5.00) | 3.00 (2.00, 5.00) | 3.00 (2.00, 6.00) | 4.00 (3.00, 7.00) | 3.00 (2.00, 5.00) | <0.01 |
| Weight (kg), median (IQR) | 3.35 (3.10, 3.65) | 3.31 (3.00, 3.57) | 3.30 (3.00, 3.65) | 3.35 (3.00, 3.70) | 3.20 (2.97, 3.50) | 0.35 |
| Peak fever (℃), median (IQR) | 39.20 (38.70, 39.60) | 39.50 (39.20, 40.00) | 39.70 (39.30, 40.00) | 39.90 (39.50, 40.15) | 40.00 (39.60, 40.20) | <0.01 |
| WBC (×109·L−1), median (IQR) | 7.33 (5.85, 9.79) | 8.95 (6.55, 11.68) | 9.37 (6.98, 12.88) | 11.11 (7.56, 14.29) | 11.00 (7.41, 15.23) | <0.01 |
| Neutrophils (%), median (IQR) | 37.70 (30.60, 58.00) | 60.45 (47.20, 65.70) | 61.90 (48.50, 69.30) | 64.25 (49.95, 75.00) | 65.20 (55.22, 75.85) | <0.01 |
| Leukocytes (%), median (IQR) | 49.00 (31.80, 58.10) | 30.00 (23.95, 41.60) | 28.40 (21.70, 40.60) | 25.25 (17.05, 37.65) | 24.50 (15.75, 35.75) | <0.01 |
| Monocytes (%), median (IQR) | 8.00 (6.10, 10.50) | 8.15 (6.95, 10.10) | 8.00 (6.80, 10.50) | 8.00 (5.90, 10.40) | 8.00 (6.00, 10.10) | 0.51 |
| HGB (U/g), median (IQR) | 122.00 (115.00, 130.00) |
123.00 (117.00, 129.50) |
122.00 (114.00, 129.00) |
122.50 (114.50, 131.00) |
121.00 (112.00, 127.00) |
0.19 |
| PLT (U/L), median (IQR) | 299.00 (238.00, 365.00) |
271.50 (224.50, 307.00) |
256.00 (204.00, 335.00) |
255.50 (215.50, 318.00) |
262.50 (208.50, 327.50) |
0.02 |
| CRP (mg·L−1), median (IQR) | 2.90 (2.50, 11.80) | 8.80 (3.80, 19.00) | 12.90 (4.90, 24.60) | 20.55 (7.85, 49.50) | 30.10 (15.40, 61.40) | <0.01 |
| ALB (U/L), median (IQR) | 43.40 (41.80, 46.00) | 42.85 (41.20, 44.80) | 42.50 (40.50, 44.10) | 41.40 (39.75, 44.00) | 41.10 (38.60, 43.65) | <0.01 |
| ALT (U/L), median (IQR) | 15.00 (11.00, 19.00) | 12.00 (10.00, 17.00) | 13.00 (11.00, 16.00) | 12.50 (10.00, 17.00) | 13.00 (10.00, 17.00) | 0.05 |
| γ-GT (U/L), median (IQR) | 10.00 (7.00, 12.00) | 10.00 (8.00, 13.00) | 10.00 (8.00, 12.13) | 10.00 (8.00, 12.00) | 10.00 (8.00, 12.13) | 0.78 |
| PCT (U/L), median (IQR) | 0.07 (0.05, 0.17) | 0.13 (0.06, 0.37) | 0.22 (0.10, 0.47) | 0.22 (0.12, 0.51) | 0.47 (0.22, 1.02) | <0.01 |
| AST (U/L), median (IQR) | 38.00 (28.00, 47.00) | 34.50 (26.00, 46.00) | 34.00 (28.00, 44.00) | 34.00 (26.50, 47.00) | 35.00 (28.00, 46.00) | 0.71 |
| BUN (U/L), median (IQR) | 2.80 (2.20, 3.50) | 2.90 (2.45, 3.40) | 2.70 (2.20, 3.40) | 2.80 (2.40, 3.40) | 2.80 (2.30, 3.35) | 0.76 |
| CRE (U/L), median (IQR) | 24.00 (18.00, 29.00) | 27.00 (23.00, 31.00) | 26.00 (21.00, 32.00) | 28.00 (22.00, 33.00) | 27.00 (21.00, 32.50) | <0.01 |
| CK (U/L), median (IQR) | 94.00 (70.00, 139.00) |
84.00 (64.50, 133.00) |
89.00 (66.00, 136.00) |
96.00 (63.00, 138.71) |
87.00 (59.00, 155.50) |
0.88 |
| CK-MB (U/L), median (IQR) | 7.00 (4.00, 11.00) | 4.00 (4.00, 6.00) | 4.00 (4.00, 6.50) | 4.00 (4.00, 6.00) | 4.00 (3.00, 6.00) | <0.01 |
| La (U/L), median (IQR) | 2.78 (2.24, 3.67) | 2.41 (1.98, 3.09) | 2.62 (1.96, 3.09) | 2.70 (2.22, 3.12) | 2.63 (2.24, 3.41) | 0.04 |
| LDH (U/L), median (IQR) | 345.00 (282.00, 454.00) |
340.50 (289.50, 422.50) |
379.00 (308.00, 477.00) |
363.00 (304.00, 468.00) |
386.00 (321.50, 495.50) |
<0.01 |
| Bilateral lung, n (%) | 0.12 | |||||
| No | 10 (9.2) | 11 (10.2) | 22 (20.2) | 18 (16.7) | 15 (13.9) | |
| Yes | 99 (90.8) | 97 (89.8) | 87 (79.8) | 90 (83.3) | 93 (86.1) | |
| Lung marking, n (%) | 0.02 | |||||
| No | 10 (9.2) | 11 (10.2) | 25 (22.9) | 18 (16.7) | 12 (11.1) | |
| Yes | 99 (90.8) | 97 (89.8) | 84 (77.1) | 90 (83.3) | 96 (88.9) | |
| Lung patch, n (%) | 0.18 | |||||
| No | 63 (57.8) | 54 (50.0) | 70 (64.2) | 54 (50.0) | 61 (56.5) | |
| Yes | 46 (42.2) | 54 (50.0) | 39 (35.8) | 54 (50.0) | 47 (43.5) | |
| Pulmonary inflammation, n (%) | 0.74 | |||||
| No | 99 (90.8) | 103 (95.4) | 100 (91.7) | 100 (92.6) | 101 (93.5) | |
| Yes | 10 (9.2) | 5 (4.6) | 9 (8.3) | 8 (7.4) | 7 (6.5) | |
IQR, interquartile range; WBC, white blood cells; PLT, platelet count; HGB, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALB, albumin; γ-GT, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; CRE, serum creatinine; CK, creatine kinase; CK-MB, creatine kinase-MB; CRP, C-reactive protein; PCT, procalcitonin; LDH, lactate dehydrogenase; IL-6, interleukin-6; La, blood lactate.
Outcome of multivariable statistical
The multivariable-adjusted odds ratios (ORs) for SAP per SD (standard deviation) increment in IL-6 were as follows: 1.70 [95% confidence interval (CI): 1.30–2.23] in the crude model; 1.86 (95% CI: 1.34–2.51) when adjusted for sex, age, weight, and radiographic variables; and 1.66 (95% CI: 1.14–2.41) with additional adjustment for other confounders (Table 3). In the crude model, when the highest quintile (Q5) was compared with the lowest quintile (Q1), IL-6 was significantly associated with SAP (OR, 3.24; 95% CI: 1.85–5.69; P for trend <0.001) (Table 3). In analyses adjusted for sex, age, weight, and radiographic variables, the adjusted OR for SAP was 4.11 (95% CI: 2.22–7.61) in individuals with IL-6 in the highest quintile compared with those in the lowest quintile (P for trend <0.001) (Table 3). After additional adjustment for other confounders, the IL-6 level remained significantly associated with SAP (OR, 2.85; 95% CI: 1. 32–6.14; P for trend =0.002) (Table 3).
Table 3
| IL-6 (pg/mL) | No. of patients | No. of SAP | OR (95% CI) | ||
|---|---|---|---|---|---|
| Model 1d | Model 2e | Model 3f | |||
| IL-6 (per SD)c | 542 | 231 | 1.70 (1.30–2.23) | 1.86 (1.34–2.51) | 1.66 (1.14–2.41) |
| IL-6 (quintile) | |||||
| ≤9.86 (Q1) | 109 | 32 | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) |
| 9.87–20.82 (Q2) | 108 | 37 | 1.25 (0.71, 2.22) | 1.31 (0.71, 2.43) | 1.17 (0.59, 2.35) |
| 20.83–33.82 (Q3) | 109 | 45 | 1.69 (0.97, 2.97) | 2.09 (1.13, 3.88) | 1.79 (0.88, 3.63) |
| 33.83–51.52 (Q4) | 108 | 55 | 2.50 (1.43, 4.37) | 2.91 (1.57, 5.38) | 2.31 (1.12, 4.76) |
| ≥51.53 (Q5) | 108 | 62 | 3.24 (1.85, 5.69) | 4.11 (2.22, 7.61) | 2.85 (1.32, 6.14) |
| P for trendb | – | – | <0.001 | <0.001 | 0.002 |
a, multivariable logistic regression analysis was used to sequentially adjust for covariates; b, P for trend: P for linear trend was calculated by modeling the median of the IL-6 for each quintile as a continuous variable; c, continuous variables; d, crude model; e, adjusted for sex, age, weight, and radiographic variable (bilateral lung marking thickening, lung patch shadowing, pulmonary inflammation). Adjusted ORs (95% CIs) (all such values); f, additional adjustment for peak fever, white blood cells, neutrophils, lymphocytes, monocytes, hemoglobin, platelet count, C-reactive protein, albumin, alanine aminotransferase, γ-glutamyl transpeptidase, procalcitonin, aspartate aminotransferase, blood urea nitrogen, serum creatinine, creatine kinase, creatine kinase-MB, blood lactate, and lactate dehydrogenase. IL-6, interleukin-6; SD, standard deviation; OR, odds ratio; CI, confidence interval.
After adjustment for factors potentially related to SAP, a nonlinear relationship was observed between IL-6 level and SAP (Figure 3). The risk of SAP increased as the level of IL-6 increased up to 40.78 pg/mL (adjusted OR, 1.029; 95% CI: 1.008–1.051; P=0.007); however, above this point, the level of IL-6 was no longer associated with the risk of SAP (OR, 1.003; 95% CI: 0.996–1.010; P=0.384) (Table 4).

Table 4
| Inflection point of IL-6 | OR (95% CI) | P value |
|---|---|---|
| ≤40.78 | 1.029 (1.008–1.051) | 0.007 |
| >40.78 | 1.003 (0.996–1.010) | 0.384 |
a, adjusted for sex, age, weight, and radiographic variable (bilateral lung marking thickening, lung patch shadowing, pulmonary inflammation), peak fever, white blood cells, neutrophils, leukocytes, monocytes, hemoglobin, platelet count, C-reactive protein, albumin, alanine aminotransferase, γ-glutamyl transpeptidase, procalcitonin, aspartate aminotransferase, blood urea nitrogen, serum creatinine, creatine kinase, creatine kinase-MB, blood lactate, and lactate dehydrogenase. Adjusted OR (95% CI) (all such values). IL-6, interleukin-6; OR, odds ratio; 95% CI, 95% confidence interval.
Discussion
HAdV is a proinflammatory virus that can trigger the release of high levels of inflammatory cytokines and chemokines in children and disease severity differs depending on their concentration levels (16). One study has been conducted to investigate the incidence of HAdV pneumonia in children, and acute respiratory disease has been shown to be the most common clinical manifestation of HAdV infection (17). Our analyses found that IL-6 was significantly and positively associated with SAP independent of several risk factors. These results help to clarify the relationship between IL-6 and the incidence of SAP, with an increased level of IL-6 indicating a poor prognosis in patients with severe disease. Previous study has reported an association between SAP and aspartate aminotransferase, lactate dehydrogenase, and procalcitonin (17).
HAdV invades lung tissue cells, causing cell lysis and necrosis. Recognition of injured lung tissue cells by immune cells promotes the secretion of several cytokines, inducing a series of immune inflammatory responses (18). Toll-like receptor 9 (TLR9), which is expressed on the surface of immune cells, recognizes the viral DNA released by infected cells after lysis (19), transduces signals through the myeloid molecular factor 88-dependent pathway, and activates protein-1 through the nuclear factor-κ B-activated or mitogen-activated protein kinase pathways (20). Finally, it induces the release of the proinflammatory cytokines IL-1, IL-6, IL-8, and tumor necrosis factor-α, and many chemokines, which stimulates a number of immune cells to migrate to the lungs, causing a storm of inflammatory factors (21).
Univariate analysis showed that pulmonary lobe involvement was a risk factor for SAP, suggesting that wide-ranging lesions and severe pulmonary function damage are fundamental factors that lead to critical illness. Current evidence shows that several imaging features contribute to the poor prognosis of SAP (22). Therefore, we additionally adjusted for sex, weight, and radiographic variables, such as bilateral lung marking thickening, lung patch shadowing, and pulmonary inflammation. When the top quintile was compared to the bottom quintile, the risk of SAP was increased by 2.85 times (OR, 2.85; 95% CI: 1.32–6.14). After adjustment for confounding factors, the independent effect of IL-6 expression on the occurrence of SAP was quantified. These results indicate that IL-6 may independently increase the risk of SAP development. Our study implied that the effect of IL-6 expression on the risk of SAP in children was weaker with multivariable adjustment for clinical and imaging features than without OR adjustment [3.24; IQR (1.85, 5.69)]. This finding indicates that multifactorial adjustments played a role in controlling for the confounding factors. After adjustment for other inflammatory factors, our multivariate regression analysis revealed that IL-6 was the only independent risk factor for SAP, suggesting that an adenovirus-induced inflammatory storm plays a decisive role in the occurrence of this disease. This result is consistent with the previous study (23).
In this study, we also observed a nonlinear association observed between IL-6 and SAP. The data further showed that the risk of SAP increased as IL-6 levels increased up to 40.78 pg/mL. We explored the nonlinear relationship between the IL-6 levels and occurrence of SAP. It is indicated that early intervention with IL-6 has a certain significance in predicting the occurrence of SAP.
Conclusions
Our study indicated that the serum level of IL-6 is independently associated with the risk of SAP in children. A nonlinear association was also observed between IL-6 and SAP. Therefore, clinicians should be concerned about IL-6 levels in children.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-585/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-585/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-585/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics board of Tianjin Children’s Hospital (No. L2021-21), and the requirement to obtain individual consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu PQ, Zeng SQ, Yin GQ, et al. Clinical manifestations and risk factors of adenovirus respiratory infection in hospitalized children in Guangzhou, China during the 2011-2014 period. Medicine (Baltimore) 2020;99:e18584. [Crossref] [PubMed]
- Castro-Rodriguez JA, Daszenies C, Garcia M, et al. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: a 5-year follow-up. Pediatr Pulmonol 2006;41:947-53. [Crossref] [PubMed]
- Tabain I, Ljubin-Sternak S, Cepin-Bogović J, et al. Adenovirus respiratory infections in hospitalized children: clinical findings in relation to species and serotypes. Pediatr Infect Dis J 2012;31:680-4. [Crossref] [PubMed]
- Lu MP, Ma LY, Zheng Q, et al. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J Pediatr 2013;9:346-9. [Crossref] [PubMed]
- Xie L, Zhang B, Zhou J, et al. Human adenovirus load in respiratory tract secretions are predictors for disease severity in children with human adenovirus pneumonia. Virol J 2018;15:123. [Crossref] [PubMed]
- Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986;324:73-6. [Crossref] [PubMed]
- Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 2021;33:127-48. [Crossref] [PubMed]
- Sun J, Xiao Y, Zhang M, et al. Serum Inflammatory Markers in Patients with Adenovirus Respiratory Infection. Med Sci Monit 2018;24:3848-55. [Crossref] [PubMed]
- Huang H, Chen Y, Ma LY, et al. Analysis of the clinical features and the risk factors of severe adenovirus pneumonia in children. Zhonghua Er Ke Za Zhi 2021;59:14-9. [Crossref] [PubMed]
- Li J, Wei J, Xu Z, et al. Cytokine/Chemokine Expression Is Closely Associated Disease Severity of Human Adenovirus Infections in Immunocompetent Adults and Predicts Disease Progression. Front Immunol 2021;12:691879. [Crossref] [PubMed]
- Gu J, Su QQ, Zuo TT, et al. Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection 2021;49:1-13. [Crossref] [PubMed]
- Zhong L, Lin J, Dai J. Risk factors for the development of bronchiolitis obliterans in children with severe adenovirus pneumonia: A retrospective study with dose-response analysis. J Med Virol 2020; Epub ahead of print. [Crossref]
- Guideline for diagnosis and treatment of adenovirus pneumonia in children (2019 version). Chinese Journal of Clinical Infectious Diseases 2019;12:161-6.
- Subspecialty Group of Respiratory Diseases, The Society of Pediatrics; Chinese Medical Association The Editorial Board, Chinese Journal of Pediatrics. Guidelines for management of community acquired pneumonia in children(the revised edition of 2013) (II). Zhonghua Er Ke Za Zhi 2013;51:856-62.
- Qiao F, Li L, Zhang J, et al. Operation time is independent associated with serious postoperative symptom in patients with mandibular third molar removal. Ann Palliat Med 2021;10:4080-9. [Crossref] [PubMed]
- Lim JU, Choi JY, Jeong HJ, et al. Comparison of clinical characteristics and inflammatory cytokines between hypoxemic and non-hypoxemic human adenovirus 55 pneumonia. J Thorac Dis 2020;12:4044. [Crossref] [PubMed]
- Huang M, Luo R, Fu Z. Risk factors for poor prognosis in children with severe adenovirus pneumonia. Zhongguo Dang Dai Er Ke Za Zhi 2017;19:159-62. [Crossref] [PubMed]
- Hoşnut FÖ, Ozçay F, Malbora B, et al. Severe adenovirus infection associated with hemophagocytic lymphohistiocytosis. Turk J Haematol 2014;31:103-5. [Crossref] [PubMed]
- Xu N, Chen P, Wang Y. Evaluation of Risk Factors for Exacerbations in Children with Adenoviral Pneumonia. Biomed Res Int 2020;2020:4878635. [Crossref] [PubMed]
- Sun B, He H, Wang Z, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care 2014;18:456. [Crossref] [PubMed]
- Yoon JS, Kim HH, Lee Y, et al. Cytokine induction by respiratory syncytial virus and adenovirus in bronchial epithelial cells. Pediatr Pulmonol 2007;42:277-82. [Crossref] [PubMed]
- Radke JR, Cook JL. Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis 2018;31:251-6. [Crossref] [PubMed]
- Jin L, Liu EM, Xie XH, et al. Comparative analysis of clinical features and risk factors of severe pneumonia development in pediatric patients hospitalized with seasonal influenza or swine-origin influenza infection. Adv Clin Exp Med 2020;29:971-7. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)



