
ADA2 deficiency (DADA2) misdiagnosed as systemic onset juvenile idiopathic arthritis in a child carrying a novel compound heterozygous ADA2 mutation: a case report
Introduction
Deficiency of adenosine deaminase 2 (DADA2), first described in 2014 by two separate groups (1,2), is a monogenic autoinflammatory disorder caused by autosomal recessive bi-allelic loss-of-function mutation in adenosine deaminase 2 (ADA2). DADA2 is characterized by various systemic vascular and inflammatory manifestations that affect multiple organs. In the early reported cases, patients with DADA2 mainly presented with early-onset polyarteritis nodosa-like manifestations (3,4). As the number of reported patients has increased, the clinical spectrum of DADA2 has expanded considerably and is highly variable, making DADA2 difficult to differentiate from other inflammatory diseases. Here, we describe the case of a 3-year-old boy who had been misdiagnosed with systemic onset juvenile idiopathic arthritis (soJIA) for nearly 2 years before he was definitively diagnosed with DADA2. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-261/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
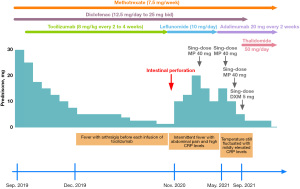
A previously healthy 3-year-old boy was admitted to Tianjin Children’s Hospital with a recurrent fever for more than 20 days in July 2019. He had been experiencing 2–3 temperature spikes per day with a maximum temperature of 39.5 ℃. Physical examination revealed mild enlargement of the cervical lymph nodes. No other unpleasant or abnormal symptoms, such as rash, headache, abdominal pain, arthritis, or arthralgia, or physical observations were noted. He was an only child; his family history was negative, and no consanguinity was reported in the family. Blood tests revealed a hemoglobin count of 110 g/L, a white blood cell count of 12.66×109/L—of which 72% were neutrophils and 22% were lymphocytes, and a platelet count of 577×109/L. The C-reactive protein (CRP) concentration was 88.5 mg/L, and the erythrocyte sedimentation rate (ESR) was 33 mm/h. The routine biochemistry and immunology tests were normal (Tables S1-S3). His fever did not improve after intravenous immunoglobulin and continuous antibiotic therapy for 28 days. His parents took him to another hospital, where the patient was diagnosed with soJIA on account of his persistent fever lasting more than 2 weeks and enlarged lymph nodes without clear etiologies, which fulfilled the revised (Edmonton 2001) International League of Associations for Rheumatology (ILRA) classification criteria for soJIA (5). Subsequently, he was treated with prednisone, methotrexate, and diclofenac. Although his temperature decreased to normal with concomitant reductions in CRP and ESR after the treatment (Figure 1), he developed another fever during the tapering course of prednisone. Tocilizumab was administered in September 2019; however, the patient developed severe asymptomatic hypertension after the first. This was attributed to a severe drug reaction, and he was treated with a combination of antihypertensive therapy. His temperature was normal, with asymptomatic mildly elevated blood pressure for several months. The results of a renal Doppler ultrasound, routine urine testing, and serum biochemical assays were all normal (Table S3). The patient returned to Tianjin Children’s Hospital for the convenience of treatment. Nevertheless, when prednisone was tapered to 5 mg/day, fever with arthralgia and positive CRP levels occurred before each infusion of tocilizumab and resolved following the treatment (Figure 1). Owing to persistent hypertension, which is usually not seen in soJIA, and the flaring of symptoms when prednisone was tapered, large and medium-sized arteritis were suspected. Whole-body computed tomography (CT) angiography was performed in March 2020. However, no vascular abnormalities were observed. Head magnetic resonance imaging (MRI) or head magnetic resonance angiography (MRA) were not performed owing to the lack of neurological symptoms including stroke.
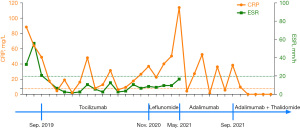
In November 2020, the patient developed acute and severe abdominal pain accompanied by nausea and vomiting. Physical examination revealed typical signs of peritonitis, and abdominal radiography and CT revealed an intestinal perforation. The patient recovered well after the intestinal repair. The intestinal perforation was suspected to be due to tocilizumab treatment; therefore, tocilizumab was discontinued, and leflunomide was added. Over the following 6 months, the patient experienced 3 episodes of intermittent fever with abdominal pain and high CRP levels that did not respond to anti-infective therapy but were resolved after the prescription of diclofenac or an extra dose of methylprednisolone (Figure 1). The clinical characteristics of the patient did not conform to a typical case of soJIA, and he did not respond well to therapy.
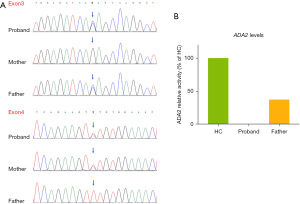
Hence, in May 2021, we reassessed the diagnosis and performed whole-exome sequencing for suspected monogenic inherited autoinflammatory disorders. Simultaneously, leflunomide was replaced by a tumor necrosis factor-alpha (TNF-α) inhibitor (adalimumab); methotrexate and diclofenac were continued (Figure 2). About 1 month later, the gene sequence analysis revealed a novel compound heterozygous mutation in ADA2, c.737 G>C (p. Arg246Thr) located in exon 3 and c.827 T>C (p. Phe276Ser) located in exon 4 (Figure 3A). To further identify the functional consequence of the ADA2 mutation, blood samples from the patient and his father were sent to Professor Qing Zhou at the Life Science Institute, Zhejiang University, China, who first described DADA2 in 2014. ADA2 enzyme activity was almost completely lost in the patient, and a marked reduction was observed in his father, who was a carrier (Figure 3B). A blood sample was obtained from the mother for gene sequence analysis, but a second sample could not be obtained for ADA2 enzyme activity testing. After DADA2 was diagnosed, the use of TNF-α inhibitor was continued. His blood pressure normalized, but his temperature still fluctuated slightly (Figure 1). Therefore, thalidomide was added in September 2021, after which he has had a stable condition (Figure 1).
Discussion
In this case report, we describe a patient who initially presented with a recurrent fever, mildly enlarged lymph nodes, and increased acute phase reactants, all of which were nonspecific symptoms. After examination, tumors and other common diseases were ruled out, and autoinflammatory disorders such as soJIA were considered. However, intractable hypertension, which is not a clinical feature of soJIA, was observed during treatment. This finding suggested that, rather than classifying the occurrence as an adverse drug reaction, the diagnosis should be reconsidered. Monogenic inherited autoinflammatory disorders were not suspected until the patient developed an intestinal perforation with ensuing recurrent abdominal pain that coincided with a fever.
DADA2 has broad manifestations that mainly depend on the affected arteries of the respective organ or system. Hypertension is a common clinical phenomenon that is either nephrogenic in nature or independent of renal pathology (6-9). Gastrointestinal symptoms, including recurrent abdominal pain and intestinal perforation, have also been reported in several case series (9-11). Hypertension and gastrointestinal symptoms are all vasculopathy-related manifestations. Angiography in some patients has revealed stenosis and/or aneurysms of abdominal arteries, particularly the mesenteric, celiac, and renal arteries, and the intestinal histopathology has revealed necrotizing vasculitis (1,2,12). However, the CT angiography examination of our patient did not reveal any aneurysm or arterial stenosis, possibly because the vessels involved in DADA2 were small, and CT and MRA are not always sensitive enough to identify such affected vessels. In addition, our patient did not exhibit other frequent clinical features of DADA2, such as livedo reticularis, ischemic lacunar stroke, renal lesions, or immunological and hematologic manifestations (Table S1).
With an increasing number of confirmed cases and a highly variable clinical presentation, genotype-phenotype correlations have become a concern. Lee et al. (13) reported that most vasculitis-associated ADA2 mutations are missense variants. Conversely, more variable mutation types, including indels, are found in hematologic manifestations, and mutations associated with all phenotypes are distributed throughout the gene without a preferential domain. G47R is the most common mutation in patients with vasculitis but not in patients with hematologic involvement, whereas R169Q and H112Q can be found in both phenotypes (13). Most patients with DADA2 presenting with diverse hematologic abnormalities do not exhibit features of vasculitis but have recurrent infections and hepatosplenomegaly, which are absent in vasculitis-predominant patients (13,14). Our patient, who carried a novel compound heterozygous mutation (R246T, F276S) in ADA2, presented with vasculitis without hematologic manifestations. Although the association between the genotype and phenotype of this mutation locus remains unclear, owing to the lack of cases with the same mutation, it can be assumed that this mutated locus is associated with vasculitis. Because most vasculitis phenotypes harbor missense mutations, they usually display more residual ADA2 activity compared with that of the hematologic phenotypes (13). However, some patients with vasculitis have undetectable ADA2 activity, which was the case in our patient. Further follow-up is necessary to determine whether this novel compound heterozygous mutation is associated with other phenotypes.
As an inflammatory disorder, DADA2 usually responds well to high steroid doses. However, steroid dependence is often observed, as was in the case of our patient. DADA2 patients mostly display poor or unsustained clinical responsiveness to immunosuppressive treatments. Tocilizumab is effective for controlling fever and inflammatory markers and has been used successfully in a patient with a Castleman disease-like presentation of DADA2 (15). However, it is ineffective in preventing ischemic or hemorrhagic events (16,17). Our patient developed an intestinal perforation after tocilizumab treatment. TNF-α inhibitor therapy is a mainstay treatment, is highly efficacious for patients with a vasculitis-predominant phenotype, and can prevent strokes (18,19). However, the patient’s health insurance did not cover the TNF-α inhibitor therapy, and his family’s financial situation was insufficient to support frequent dosing. In addition, thalidomide can selectively inhibit TNF-α production (20). Thalidomide achieved complete remission in a small number of patients in an Italian cohort study (4). Therefore, thalidomide may be an effective and less expensive therapeutic strategy in some patients with DADA2. Our patient had mild disease flares after adalimumab treatment and remained stable after combination therapy with thalidomide. However, DADA2 patients with severe hematologic and immunologic phenotypes do not respond to treatment with TNF-α inhibitors and require hematopoietic stem cell transplantation (14,21). Gene therapy may be an effective strategy for addressing the challenges of transplant donor shortage and posttransplant rejection. Fresh-frozen plasma can be used as an alternative treatment; however, the rapid clearance of exogenous ADA2 makes this approach less feasible (18). Recombinant pegylated ADA2, targeting adenosine in an enzyme activity-dependent manner, may be a novel treatment in the future (22).
DADA2 is mainly characterized by chronic or recurrent systemic inflammation with elevated acute phase reactants, which are common features of autoinflammatory disorders that render early diagnosis difficult when there is a lack of features suggestive of ADA2 mutation. However, when a patient develops unexplained manifestations or does not respond well to treatment, the diagnosis should be re-evaluated. Persistent hypertension and subsequent intestinal perforation were both important diagnostic clues for this patient, suggesting systemic vasculitis. Moreover, monogenic inherited diseases, including DADA2, should be considered for early-onset vasculitis, which is the most common phenotype of DADA2. Early identification and treatment will result in a significant improvement in disease outcomes.
Acknowledgments
We thank Professor Qing Zhou from Zhejiang University for her assistance with the diagnosis. We would like to thank Editor Sharoni from Editage for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-261/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-261/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-261/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient’s legal guardians for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 2014;370:911-20. [Crossref] [PubMed]
- Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 2014;370:921-31. [Crossref] [PubMed]
- Keer N, Hershfield M, Caskey T, et al. Novel compound heterozygous variants in CECR1 gene associated with childhood onset polyarteritis nodosa and deficiency of ADA2. Rheumatology (Oxford) 2016;55:1145-7. [Crossref] [PubMed]
- Caorsi R, Penco F, Grossi A, et al. ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Ann Rheum Dis 2017;76:1648-56. [Crossref] [PubMed]
- Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390-2.
- Tanatar A, Karadağ ŞG, Sözeri B, et al. ADA2 Deficiency: Case Series of Five Patients with Varying Phenotypes. J Clin Immunol 2020;40:253-8. [Crossref] [PubMed]
- Nanthapisal S, Murphy C, Omoyinmi E, et al. Deficiency of Adenosine Deaminase Type 2: A Description of Phenotype and Genotype in Fifteen Cases. Arthritis Rheumatol 2016;68:2314-22. [Crossref] [PubMed]
- Caorsi R, Penco F, Schena F, et al. Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatr Rheumatol Online J 2016;14:51. [Crossref] [PubMed]
- Batu ED, Karadag O, Taskiran EZ, et al. A Case Series of Adenosine Deaminase 2-deficient Patients Emphasizing Treatment and Genotype-phenotype Correlations. J Rheumatol 2015;42:1532-4. [Crossref] [PubMed]
- Sahin S, Adrovic A, Barut K, et al. Clinical, imaging and genotypical features of three deceased and five surviving cases with ADA2 deficiency. Rheumatol Int 2018;38:129-36. [Crossref] [PubMed]
- Gibson KM, Morishita KA, Dancey P, et al. Identification of Novel Adenosine Deaminase 2 Gene Variants and Varied Clinical Phenotype in Pediatric Vasculitis. Arthritis Rheumatol 2019;71:1747-55. [Crossref] [PubMed]
- Pimpale Chavan P, Ramadoss D, Khan A, et al. Deficiency of Adenosine Deaminase 2 (DADA2): One Disease, Several Faces. Indian J Pediatr 2021;88:828-30. [Crossref] [PubMed]
- Lee PY, Kellner ES, Huang Y, et al. Genotype and functional correlates of disease phenotype in deficiency of adenosine deaminase 2 (DADA2). J Allergy Clin Immunol 2020;145:1664-72.e10. [Crossref] [PubMed]
- Pinto B, Deo P, Sharma S, et al. Expanding spectrum of DADA2: a review of phenotypes, genetics, pathogenesis and treatment. Clin Rheumatol 2021;40:3883-96. [Crossref] [PubMed]
- Van Eyck L, Liston A, Wouters C. Mutant ADA2 in vasculopathies. N Engl J Med 2014;371:480. [Crossref] [PubMed]
- Liu L, Wang W, Wang Y, et al. A Chinese DADA2 patient: report of two novel mutations and successful HSCT. Immunogenetics 2019;71:299-305. [Crossref] [PubMed]
- Lee PY, Huang Y, Zhou Q, et al. Disrupted N-linked glycosylation as a disease mechanism in deficiency of ADA2. J Allergy Clin Immunol 2018;142:1363-5.e8. [Crossref] [PubMed]
- Ombrello AK, Qin J, Hoffmann PM, et al. Treatment Strategies for Deficiency of Adenosine Deaminase 2. N Engl J Med 2019;380:1582-4. [Crossref] [PubMed]
- Cooray S, Omyinmi E, Hong Y, et al. Anti-tumour necrosis factor treatment for the prevention of ischaemic events in patients with deficiency of adenosine deaminase 2 (DADA2). Rheumatology (Oxford) 2021;60:4373-8. [Crossref] [PubMed]
- Sampaio EP, Sarno EN, Galilly R, et al. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 1991;173:699-703. [Crossref] [PubMed]
- Hashem H, Bucciol G, Ozen S, et al. Hematopoietic Cell Transplantation Cures Adenosine Deaminase 2 Deficiency: Report on 30 Patients. J Clin Immunol 2021;41:1633-47. [Crossref] [PubMed]
- Wang L, Londono LM, Cowell J, et al. Targeting Adenosine with Adenosine Deaminase 2 to Inhibit Growth of Solid Tumors. Cancer Res 2021;81:3319-32. [Crossref] [PubMed]


