
Clinical performance evaluation of serum amyloid A module of Mindray BC-7500CS automated hematology analyzer
Highlight box
Key findings
• In this study, we validated the performance of the BC-7500CS SAA module with a reportable range of SAA up to nearly 2000 mg/mL.
What is known and what is new?
• Due to the hook effect, extremely high concentrations of analytes may lead to erroneous low results when using immunoscattering turbidimetry or immunoturbidimetry, reducing the accuracy of the assay.
• The Mindray BC-7500CS automated hematology analyzer addresses the hook effect by identifying reaction curve characteristics and flagging abnormalities. SAA-D function can detect specimens with high SAA concentration accurately.
What is the implication, and what should change now?
• The SAA-D high-value function can significantly improve the efficiency of medical testing departments, reduce errors caused by manual operations, and reduce biological risks.
Introduction
Serum amyloid A (SAA) is a class of lipid-binding protein in plasma and is mainly secreted by hepatocytes, adipocytes, and tumor cells (1,2). SAA is a family of polymorphic proteins encoded by polymorphic genes, and as precursors of tissue amyloid A, they are acute-phase proteins that can be used for auxiliary diagnosis of multiple diseases, with high levels found in bacterial and viral infections, atherosclerosis, coronary heart disease, acute transplant rejection, neoplasms, and other diseases. SAA is also viewed as a biomarker for evaluation of therapeutic effects and follow-up analysis of prognosis (3), and it is highly expressed in several malignancies, including esophageal squamous cell carcinoma, ovarian cancer, lung cancer, gastric cancer, breast cancer, and hepatocellular carcinoma (4-6). Moreover, as a typical positive acute-phase protein, SAA can promote the recirculation of cholesterol and is implicated in retinol transport, innate immune modulation, and pathological responses to various diseases during their acute phases (7-9). SAA is of great value in the auxiliary diagnosis of infectious diseases, the risk prediction of cardiovascular diseases, and the evaluation of the curative effect and prognosis of tumor patients, and thus it has been extensively utilized in clinical practice.
SAA is mainly detected by means of latex-enhanced rate nephelometry (LERN), dot immuno-gold filtration assay (DIGFA), and time-resolved fluorescence microsphere immunochromatographic assay. The Mindray BC-7500CS automated hematology analyzer utilizes LERN for SAA detection. Specifically, lyse for hematology analyzer is mixed with the blood cell specimens, thus removing the interference of blood cell particles on LERN. SAA reagent is then mixed with the specimen processed by the hemolytic agent, and the antibody-labeled latex microspheres in the latex reagent agglutinate with the SAA, raising the turbidity of the solution, after which the SAA concentration is measured by LERN. The measured SAA concentration is then substituted into a formula to obtain the SAA concentration in the whole-blood specimens.
In SAA detection, the hook effect may occur during the antigen–antibody reaction. The condition of antibody/antigen excess is referred to as the prozone/postzone phenomenon. While using immunonephelometry or immunoturbidimetry, an extremely high concentration of the analyte may cause falsely low results, lowering the accuracy of detection. The Mindray BC-7500CS automated hematology analyzer addresses the hook effect by identifying reaction curve characteristics and flagging abnormalities. That is, according to the kinetic characteristics of the antigen–antibody reaction, the A value of the immune complex generated at a specific time point in the reaction curve is monitored, and the ratio of the A value between different times of the reaction is then calculated and compared with the preset parameter, thus identifying and flagging the hook effect automatically. On this basis, SAA autodilution (SAA-D) function can detect specimens with high SAA concentration accurately.
In this study, we evaluated the analysis performance of the Mindray BC-7500CS for SAA and compared it with the Upper Ottoman-1000 automated hematology analyzer for clinical performance. In addition, we verified the function of the SAA-D function. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-661/rc).
Methods
This study involved a clinical evaluation of in vitro diagnostics. Leftover specimens, whose laboratory results had been reported by the hospital, were tested on the evaluation instrument, avoiding violating the privacy and interests of the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics board of the Children’s Hospital of Nanjing Medical University (CH) (No. 202008096-1). Due to the study’s retrospective nature, the requirement to obtain signed informed consent from the patients was waived. Data are not available due to ethical restrictions.
Specimen source
In total, 764 venous whole-blood specimens anticoagulated with EDTA-K2 were collected from CH outpatients and inpatients between May 2022 and July 2022. The volume of each sample was not less than 2 mL. As per the Clinical and Laboratory Standards Institute (CLSI) H04-A6 (8,9) standard, samples with hemolysis and coagulation were not included in the assessment. Specimens were placed at room temperature (18–25 ℃) and tested within 8 hours of collection.
Instruments and reagents
The instruments and reagents used in this study included the Mindray BC-7500CS and its supporting calibrator, control material, and reagent (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China); the Upper Ottoman-1000 (Shanghai Upper Bio-Tech Pharma Co., Ltd., Shanghai, China); and an SAA testing control instrument that has been marketed and widely applied clinically. The instruments were calibrated in a valid manner with good daily quality control monitoring, and all reagents had not expired.
Verification of basic performance
Background (limit of blank)
Background was tested by adding 2 mL diluent to clean, anticoagulant-free blank tubes each day. The sample was run under whole blood CDR (complete blood cell count, white blood cell differential count, and reticulocyte count) + CRP (C-reactive protein) + SAA mode consecutively 10 times for three days. The analyzer background results were obtained using the nonparametric method referred to in CLSI EP17-A2.
Repeatability
At least 20 SAA samples were selected to cover the repeatability range (5–350 mg/L) as best as possible. After thorough mixing, the measurement was repeated 10 times in the CDR + CRP + SAA mode of BC-7500CS, and the mean value, standard deviation (SD), and coefficient of variation (CV) of each group were calculated. Ten tests were completed within 30 minutes. The repeatability was examined to determine whether it was consistent with the manufacturer’s claim (SAA: 0–10 mg/L, SD ≤1.0; SAA: 10.01–350 mg/L, CV ≤8%).
Precision
After the instrument was turned on and the performance was confirmed stable, the SAA control materials of normal and pathological concentration were consecutively tested twice in the corresponding quality control procedures as the first run of test results on that day. After 2 hours, the SAA control materials of normal and pathological concentration were tested twice consecutively as the second run of test results on that day. Test data of the 2 runs (testing twice per run) per day and testing for at least 20 consecutive days were required. Data were calculated with reference to the method in CLSI EP05-A2.
Linearity
The linearity of different concentrations was prepared by mixing SAA high-value linear quality control solution with DS diluent, covering the entire linear range segment (5.00–350.00) as far as possible. The entire linear range segment needed to be configured with no less than 9 concentration points. The distribution of concentration points did not need to be isometric but should cover the main medical decision level points of each parameter as far as possible. After the configuration was completed, measurements were performed sequentially and linearity statistics were performed as per CLSI EP6-A2.
Reportable range
A whole-blood specimen with a high SAA concentration of approximately 2,000 mg/L was selected, and the pure analyte was added, if necessary, followed by calculation of the theoretical value. The whole-blood specimen with a high SAA concentration was diluted with the hematology analyzer diluent at a ratio of 5:1 (6-fold dilution). The diluted specimen was measured 3 times, and the average value was taken. The original concentration was calculated according to the following formula, and the relative deviation between the original concentration and the theoretical concentration was calculated.
where:
C: original concentration;
C’: measured concentration after dilution;
Fh: conversion factor for hematocrit (HCT; the HCT value here refers to that of the predilution blood specimen).
Intermode comparison
No fewer than 50 fresh venous whole-blood specimens were selected and tested once in the whole-blood mode and the micro-whole-blood mode of the Mindray BC-7500CS, with an interval of no more than 2 hours between the 2 test modes.
Interference study
Interfering substances quantitatively evaluated in this study included bilirubin (BIL), endogenous lipid (triglycerides, TG), vitamin C (VitC), rheumatoid factor (RF), human anti-mouse antibodies (HAMA), and hemoglobin (HGB). First, stock solutions of the interfering substances were used to prepare 5 test groups of different (high and low) concentrations of the interfering substances according CLSI EP07-A2. The interfering substance samples in the 5 test groups were then tested in the whole-blood SAA module for a total of 3 rounds in the order of groups 1 to 5, groups 5 to 1, and groups 1 to 5. After that, the results were statistically analyzed according to CLSI EP07-A2.
Methodological comparison
No less than 200 whole-blood specimens were randomly selected from CH outpatients and inpatients, followed by testing using the BC-7500CS and the Upper Ottoman-1000 (the control instrument), respectively. SAA results were recorded, and the r values were calculated by Passing-Bablok regression analysis.
SAA-D function
The SAA-D function of the BC-7500CS was turned on and specimens with an SAA concentration >100 mg/L were selected and tested in the CD + SAA mode. Next, 800 µL of a blood specimen was pipetted and manually diluted with DS diluent at a ratio of 1:5 (6-fold dilution). The diluted specimen was then tested in the CD + SAA mode of the Mindray BC-7500CS, and the original concentration was calculated using the formula shown in 2.3.5. Passing-Bablok regression analysis was used to compare the data, and a Bland–Altman plot was drawn.
Statistical analysis
Statistical analysis was performed using SPSS 27.0 and graphs were created using GraphPad Prism 8. The SD and CV% were calculated to evaluate repeatability and precision. The correlation [correlation coefficient (r) and P value] between the BC-7500CS and Upper Ottoman-1000 was evaluated using the linear regression equation. P<0.05 was considered statistically significant.
Results
Basic performance
Background (limit of blank)
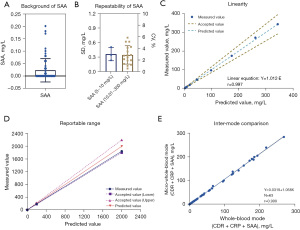
Figure 1A shows the SAA background results, which at 0.14 mg/L was in line with the manufacturer’s claim (≤2 mg/L).
Repeatability
The results of sample reproducibility are shown in Figure 1B. A total of 20 SAA samples were selected, of which 3 samples were 5–10 mg/L and 17 samples were 10.01–350 mg/L. Among them, SAA ≤10 mg/L samples had SD <1 mg/L (Figure 1B, left Y axis), and SAA ≥10.1 mg/L samples had CV <8% (Figure 1B, right Y axis). The above results all met the manufacturer’s claims.
Precision
The quality control of two concentration levels were measured twice a day for two rounds, for 20 consecutive days of testing, and a total of 160 tests were collected. The mean of SAA control materials with normal and pathological concentration were 10.17 and 49.80 mg/L, respectively. Table 1 shows the SD and CV of repeatability, between run, within day, between day, and within laboratory precision.
Table 1
| Control Level | N | Mean, mg/L | Repeatability | Between run | Within day | Between day | Within Laboratory | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD | CV | SD | CV | SD | CV | SD | CV | SD | CV | |||||||
| Normal | 80 | 10.17 | 0.2351 | 2.3% | 0.3795 | 3.7% | 0.4465 | 4.4% | 0.6808 | 6.7% | 0.8142 | 8.0% | ||||
| Pathological | 80 | 49.80 | 0.7267 | 1.5% | 0.6840 | 1.4% | 0.9980 | 2.0% | 1.6305 | 3.3% | 1.9117 | 3.8% | ||||
SAA, serum amyloid A; SD, standard deviation; CV, coefficient variation.
Linearity
The linearity results for SAA of BC-7500CS are shown in Figure 1C with a linear range of 2.265–343.475 mg/L (r>0.99). Regression equations showed that the measured values were in high agreement with the theoretical values.
Reportable range
As shown in Figure 1D, a specimen with a high theoretical SAA concentration of 2,000 mg/L was measured 3 times after dilution, with a mean SAA concentration of 184.87 mg/L. The mean SAA concentration was substituted into the formula in Section 2.3.4, and the original concentration was calculated as 1,932.38 mg/L, with a relative deviation of −7.63% from the theoretical concentration.
Intermode comparison
A total of 63 whole-blood specimens were collected to compare the results of SAA detection in whole-blood mode and micro-whole-blood mode. The results showed that the mean concentration of SAA was 48.96 mg/L in whole-blood mode and 52.09 mg/L in micro-whole-blood mode, with a correlation coefficient of 0.999, an absolute deviation of 3.13, and a relative deviation of 6.01% (Figure 1E).
Interference study
The interference of common clinical interfering substances (BIL, TG, VitC, RF, HAMA and HGB) on SAA concentration was verified (Figure 2). BIL <1,750 mmol/L, TG <22 mmol/L, VitC < 8,522.7 μmol/L, RF <65 IU/mL, HAMA <1,000 IU/mL, and HGB <203.1 g/L had no obvious interference on SAA concentration.

Methodological comparison
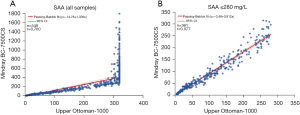
Venous blood specimens of 549 outpatients and inpatients were randomly selected and tested using the Mindray BC-7500CS and the Upper Ottoman-1000. As shown in Figure 3A, the Upper Ottoman-1000 exhibited a hook effect when SAA concentration was >280 mg/L. When SAA concentration was ≤280 mg/L, the detection results of the Mindray BC-7500CS were well correlated with those of the Upper Ottoman-1000: y= −0.48+0.912x (r=0.977) (Figure 3B).
Clinical applicability
Specimens with SAA concentration >280 mg/L were selected from the specimens mentioned above to observe the distribution of their clinical origins. Specimens with high SAA concentration mainly came from the intensive care unit (ICU) and cardiovascular surgery ICU (Figure 4A), and the diseases related to these specimens were largely coronary atherosclerotic heart disease and some malignancies.
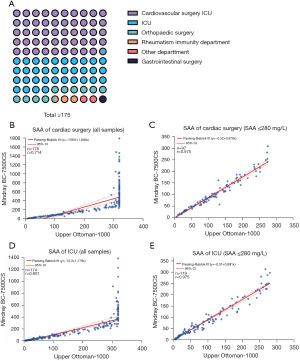
A total of 81 specimens with high SAA concentration came from the cardiovascular surgery ICU, and 55 came from the ICU. The specimens from these 2 departments were subjected to correlation analysis. As shown in Figure 4, many specimens from the ICU exceeded the upper detection limit of the Upper Ottoman-1000 (Figure 4B), but when SAA concentration was ≤280 mg/L (Figure 4C), the SAA detection results of the Mindray BC-7500CS were well correlated with those of the Upper Ottoman-1000 (r=0.975). Similar results were identified in the cardiovascular surgery ICU specimens, with many specimens containing high SAA concentration exceeding the upper limit detection of the Upper Ottoman-1000 (Figure 4D). When SAA concentration was ≤280 mg/L (Figure 4E), the SAA detection results of the Mindray BC-7500CS were well correlated with those of the Upper Ottoman-1000 (r=0.975).
Verification of the SAA-D function
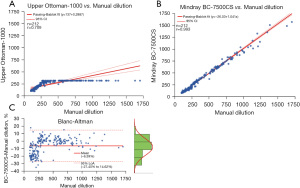
A total of 212 specimens were collected to verify the SAA-D function of the Mindray BC-7500CS, and the measured results of SAA diluted by the SAA-D module were compared with those after manual dilution. Figure 5A shows the Passing-Bablok analysis of Upper Ottoman-1000 and manual dilution, where a hook effect starts to appear after >280 mg/L. Figure 5B displays the Passing-Bablok analysis of SAA-D and manual dilution, showing a good correlation (r=0.993). The Bland–Altman plot of SAA-D and manual dilution is shown in Figure 5C.
Discussion
SAA is an acute-phase protein that increases in response to infection, injury, inflammation, or cancer. It can also serve as an evaluation indicator for the diagnosis, prognosis, and posttreatment follow-up of multiple diseases. SAA level increases notably with the progression of cancer (10-15), as well as in patients with rheumatoid arthritis or osteoarthritis (16-18). SAA also changes markedly and shows a rapid response in cardiovascular diseases, reflecting the disease activity well (19). The clinical applicability of SAA detection has broadened over time, and it is receiving more attention from clinicians. Thus, accurate SAA detection is necessary.
In this study, the basic performance of the Mindray BC-7500CS, including background, precision, linear range, intermode comparison, interfering substances, and methodological comparison, were tested. The results indicated that the Mindray BC-7500CS had good basic performance. The background of SAA was 0.14 mg/L, well below that claimed by the manufacturer (≤ 2 mg/L). When SAA was in the range of 5–10 and 10.01–350 mg/L, the corresponding SD and CV met the manufacturers’ claims. The CV of quality control (QC) precision was less than 8%. The linear range of SAA concentrations was 2.265–343.475 mg/L (r>0.997). The regression equation indicated that the measured values were essentially consistent with the theoretical values, suggesting the BC-7500CS could meet most clinical laboratory measurement requirements. The ability of the BC-7500CS to detect specimens with high SAA concentration was tested by determining its reportable range, and the results showed that the relative deviation of the original concentration from the theoretical concentration was <10%. The correlation coefficient of SAA detection results between whole-blood mode and micro-whole-blood mode was 0.999. The test for interfering substance effects showed that BIL <1,750 mmol/L, TG <22 mmol/L, VitC < 8,522.7 μmol/L, RF <65 IU/mL, HAMA <1,000 IU/mL, and HGB <203.1 g/L had no obvious interference on the measured SAA concentration, suggesting that the Mindray BC-7500CS had good anti-interference ability and reported accurate SAA results.
In the comparability evaluation of SAA, whole-blood specimens were randomly selected from outpatients and inpatients and tested for SAA using both the Mindray BC-7500CS and Upper Ottoman-1000. Comparison of the results revealed that the Upper Ottoman-1000 exhibited a hook effect when SAA concentration was >280 mg/L, its detection results were underestimated, and some specimens exceeded its upper detection limit (Figure 3). In specimens with an SAA concentration of ≤280 mg/L, the detection results of the Mindray BC-7500CS were well correlated with those of the Upper Ottoman-1000 (r=0.977). Since a hook effect was shown by the Upper Ottoman-1000 when SAA concentration was >280 mg/L, leading to an underestimated result, the specimens with an SAA concentration >280 mg/L among the 552 specimens described previously were analyzed in this study. The results showed that the specimens with an SAA concentration of >280 mg/L came mainly from the cardiovascular surgery ICU (49.71%) and the ICU (34.29%). We evaluated the clinical applicability of the Mindray BC-7500CS for specimens from these 2 departments. Figure 4 shows that the detection results of the Mindray BC-7500CS in blood specimens from the cardiovascular surgery ICU and the ICU were correlated with those of the Upper Ottoman-1000 (both r>0.9) when SAA concentration was <280 mg/L. Specimens with high SAA concentration exceeding the upper detection limit of the Upper Ottoman-1000 could be measured by the SAA-D function of the Mindray BC-7500CS. Therefore, the Mindray BC-7500CS could accurately detect specimens with a low SAA concentration and also specimens with an SAA concentration exceeding the upper detection limit of the Upper Ottoman-1000.
Although specimens with an SAA concentration exceeding the upper detection limit of the Upper Ottoman-1000 could be detected by the SAA-D function of the Mindray BC-7500CS, its accuracy needed to be verified. The SAA-D function was evaluated with manual dilution as the reference. Specifically, a specimen with an SAA concentration >100 mg/L was selected and tested in the SAA-D mode of the Mindray BC-7500CS. The specimen was then manually diluted and tested in the normal SAA mode of the Mindray BC-7500CS. When we compared the 2 results, there was an excellent correlation between SAA-D and manual dilution, and there were no false-negative or false-positive results and no significant outliers. Thus, the SAA-D function was sufficient for meeting the detection requirements of specimens with high SAA concentration in most departments.
Healthy individuals have an overall SAA concentration of approximately 1–2 mg/L in serum, which can be elevated by 1,000 times during the acute exacerbation of severe infections and chronic inflammatory diseases (3). That is, the concentration of SAA in serum can reach 1,000–2,000 mg/L, much higher than the upper limit of some instruments. SAA, as a typical positive acute-phase protein, is extensively utilized in the auxiliary diagnosis of infectious diseases and also considered an evaluation indicator during the recovery period of infectious diseases (20). SAA can also serve as an independent risk factor for cardiovascular diseases, assisting in the early diagnosis, prevention, and treatment of cardiovascular diseases (19,21). High SAA is an independent risk factor for tumorigenesis and is also closely related to the prognosis of overall survival of tumor patients (22,23), consistent with the distribution of specimens with high SAA concentration in this study (Figure 3). Taken together, research findings show that high SAA is closely associated with the occurrence, prognosis, and severity of various diseases.
One protocol for detecting specimens with high SAA concentration starts with predilution of the specimens. The hook effect can be reduced through 10-to-21-fold predilution of the specimen. Nonetheless, this method may introduce pre-analysis variation and thus affect the accuracy of the results of specimens with low SAA concentration. To address the hook effect of high-concentration SAA, the Mindray BC-7500CS identifies specimens with high SAA concentration according to the kinetic characteristics of antigen-antibody reaction and then dilutes these specimens. Hence, the impact of the hook effect on the results as well as the pre-analysis variation that may affect the detection accuracy for specimens with low SAA concentration can be avoided, ensuring the accurate detection of SAA at various concentrations. It should be noted that the SAA-D module does increase the use of reagent since it automatically dilute the blood samples and run another test when detecting the high value SAA, which is the main drawback of the system.
In summary, the Mindray BC-7500CS not only performed well for SAA detection, but also have good performance in other basic performance (24). It could accurately detect SAA at low concentrations and also detect SAA at concentrations that exceeded the upper detection limit of other instruments through its SAA-D function. These capabilities are of great value for accurate clinical auxiliary diagnosis and will lay a solid foundation for further research on the interactions between SAA and various clinical diseases and the mechanisms underlying them.
Conclusions
The SAA module of the Mindray BC-7500CS had excellent performance, good precision and accuracy of detection, a wide measurement range, and could simultaneously obtain complete blood counts. Thus, the Mindray BC-7500CS could be used by the majority of clinical departments with SAA detection requirements. In addition, the SAA-D function could accurately detect SAA at high concentrations, and so it is applicable for auxiliary diagnosis of diseases and also valuable for scientific research.
Acknowledgments
Funding: This study was funded by Nanjing Medical University (No. NMUB20210078).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-661/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-661/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-661/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of the Children’s Hospital of Nanjing Medical University (No. 202008096-1). Due to the study’s retrospective nature, the requirement to obtain signed informed consent from the patients was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sack GH Jr. Serum amyloid A - a review. Mol Med 2018;24:46. [Crossref] [PubMed]
- Sack GH Jr. Serum Amyloid A (SAA) Proteins. Subcell Biochem 2020;94:421-36. [Crossref] [PubMed]
- De Buck M, Gouwy M, Wang JM, et al. Structure and Expression of Different Serum Amyloid A (SAA) Variants and their Concentration-Dependent Functions During Host Insults. Curr Med Chem 2016;23:1725-55. [Crossref] [PubMed]
- Lin HY, Tan GQ, Liu Y, et al. The prognostic value of serum amyloid A in solid tumors: a meta-analysis. Cancer Cell Int 2019;19:62. [Crossref] [PubMed]
- Djurec M, Graña O, Lee A, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci U S A 2018;115:E1147-56. [Crossref] [PubMed]
- Cook MB, Barnett MJ, Bock CH, et al. Prediagnostic circulating markers of inflammation and risk of oesophageal adenocarcinoma: a study within the National Cancer Institute Cohort Consortium. Gut 2019;68:960-8. [Crossref] [PubMed]
- Griffiths K, Pazderska A, Ahmed M, et al. Type 2 Diabetes in Young Females Results in Increased Serum Amyloid A and Changes to Features of High Density Lipoproteins in Both HDL(2) and HDL(3). J Diabetes Res 2017;2017:1314864. [Crossref] [PubMed]
- Thompson JC, Wilson PG, Shridas P, et al. Serum amyloid A3 is pro-atherogenic. Atherosclerosis 2018;268:32-5. [Crossref] [PubMed]
- Azurmendi L, Lapierre-Fetaud V, Schneider J, et al. Proteomic discovery and verification of serum amyloid A as a predictor marker of patients at risk of post-stroke infection: a pilot study. Clin Proteomics 2017;14:27. [Crossref] [PubMed]
- Fourie C, Shridas P, Davis T, et al. Serum amyloid A and inflammasome activation: A link to breast cancer progression? Cytokine Growth Factor Rev 2021;59:62-70. [Crossref] [PubMed]
- Davis TA, Conradie D, Shridas P, et al. Serum Amyloid A Promotes Inflammation-Associated Damage and Tumorigenesis in a Mouse Model of Colitis-Associated Cancer. Cell Mol Gastroenterol Hepatol 2021;12:1329-41. [Crossref] [PubMed]
- Ghweil AA, Osman HA, Hassan MH, et al. Validity of serum amyloid A and HMGB1 as biomarkers for early diagnosis of gastric cancer. Cancer Manag Res 2020;12:117-26. [Crossref] [PubMed]
- Ignacio RMC, Gibbs CR, Kim S, et al. Serum amyloid A predisposes inflammatory tumor microenvironment in triple negative breast cancer. Oncotarget 2019;10:511-26. [Crossref] [PubMed]
- Li Z, Hou Y, Zhao M, et al. Serum amyloid a, a potential biomarker both in serum and tissue, correlates with ovarian cancer progression. J Ovarian Res 2020;13:67. [Crossref] [PubMed]
- He LN, Fu S, Zhang X, et al. Baseline and early changes in circulating Serum Amyloid A (SAA) predict survival outcomes in advanced non-small cell lung cancer patients treated with Anti-PD-1/PD-L1 monotherapy. Lung Cancer 2021;158:1-8. [Crossref] [PubMed]
- Zhou J, Dai Y, Lin Y, et al. Association between serum amyloid A and rheumatoid arthritis: A systematic review and meta-analysis. Semin Arthritis Rheum 2022;52:151943. [Crossref] [PubMed]
- Nys G, Cobraiville G, Servais AC, et al. Targeted proteomics reveals serum amyloid A variants and alarmins S100A8-S100A9 as key plasma biomarkers of rheumatoid arthritis. Talanta 2019;204:507-17. [Crossref] [PubMed]
- de Seny D, Cobraiville G, Charlier E, et al. Acute-phase serum amyloid a in osteoarthritis: regulatory mechanism and proinflammatory properties. PLoS One 2013;8:e66769. [Crossref] [PubMed]
- Xie X, Ma YT, Yang YN, et al. Genetic polymorphisms of serum amyloid A1 and coronary artery disease risk. Tissue Antigens 2015;85:168-76. [Crossref] [PubMed]
- Badawi R, Asghar MN, Abd-Elsalam S, et al. Amyloid A in Serum and Ascitic Fluid as a Novel Diagnostic Marker of Spontaneous Bacterial Peritonitis. Antiinflamm Antiallergy Agents Med Chem 2020;19:140-8. [Crossref] [PubMed]
- Shi QM, Meng FJ, Yue JW, et al. Diagnostic value of serum amyloid A and C-reactive protein for predicting acute aortic dissection. Zhonghua Yi Xue Za Zhi 2021;101:1275-81. [Crossref] [PubMed]
- Zhou J, Sheng J, Fan Y, et al. Association between serum amyloid A levels and cancers: a systematic review and meta-analysis. Postgrad Med J 2018;94:499-507. [Crossref] [PubMed]
- Lai Y, Li Y, Gao L. Serum amyloid A protein in cancer prognosis: a meta-analysis and systematic review. Transl Cancer Res 2021;10:2255-64. [Crossref] [PubMed]
- Lin Z, Lin Q, Yu P, et al. Performance evaluation of routine blood and C-reactive protein analysis using Mindray BC-7500 CRP auto hematology analyzer. Ann Transl Med 2022;10:588. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)


