
An endogenous amniotic fluid-derived 10-amino acid peptide improves lung development and hyperoxia injury
Highlight box
Key findings
• The COL5A2 peptide with a GPPGEPGPPG sequence can reverse the inhibition of the proliferation and promotion of apoptosis induced by hyperoxia on MLE-12 cells.
What is known, and what is new?
• A proteomic analysis of amniotic fluid at 16–18 weeks of gestational age showed that proteins are involved in organ development. Protein-derived peptides have been confirmed to possess a range of biological activities, such as promoting cell proliferation and differentiation.
• Analysis of amniotic fluid samples after the 25th week of gestation reveals that amniotic fluid peptides play a role in lung development. Peptides are mainly derived from the precursor proteins SPP1, COL5A2, SPRR3, COL1A1, APOA1, and ANXA1.
What is the implication, and what should change now?
• Amniotic fluid peptides may be used in the future to assist in the postnatal development of preterm infants and provide new therapeutic prospects for bronchopulmonary dysplasia.
Introduction
Bronchopulmonary dysplasia (BPD) is one of the most common and serious complications of preterm birth, with long-term effects involving multiple organ systems, including adverse effects on lung function and neurodevelopment (1,2). The primary factor leading to BPD is that the developmentally immature lung is susceptible to multiple injuries which interfere with pulmonary alveolar and vascular development. The pathogenesis of BPD involves multiple aspects, such as mechanical ventilation, oxygen toxicity, chorioamnionitis, and postpartum inflammation (3,4), but essentially, it is a disease of developmental origin. Due to prematurity, the normal in utero lung growth process is interrupted, and the underdeveloped lungs cannot perform their normal functions. Although contemporary medical treatments have significantly improved the survival rate of preterm infants, some life-saving therapies, such as mechanical ventilation, often serve as enablers for BPD development while maintaining normal vital signs (5). Therefore, there is an urgent need to find substances that can assist with the lung development process from a developmental point of view to prevent and treat BPD, thus improving the quality of children’s survival.
It is widely accepted that amniotic fluid (AF), as a liquid environment in direct contact with the fetus in utero, not only protects the fetus from extrusion but is also closely related to fetal development (6). The fetal lung contributes to the formation of the AF, while the composition of AF may well reflect the stage of fetal lung development. It is well established that mechanical forces play a crucial role in the effect of AF on lung development, and oligohydramnios can lead to reduced lung expansion, thereby causing lung development retardation (7-9). However, with the development of regenerative medicine in recent years, AF-derived stem cells have also been shown to promote lung growth and maturation in models of pulmonary hypoplasia by promoting branching morphogenesis (10), stimulating lung epithelial cell differentiation, and improving alveolarization (11). Furthermore, AF-derived mesenchymal stem cells can improve lung function in rats with hyperoxia-induced pulmonary fibrosis by reducing reactive oxygen species (ROS) production, inflammatory cell infiltration, and expression of the inflammatory factor IL-6 (12).
Endogenous peptides are mainly produced by proteolysis and gene encoding and have gained much attention in recent years due to their low molecular weight and wide distribution in the body (13). By detecting the composition and changes of peptides in different biological samples, peptidomics is highly valued for the screening of biomarkers, the diagnosis of diseases, and the search for potential active peptides (14-16). Peptidomic analysis of cord blood has identified biomarkers associated with neonatal respiratory distress syndrome (17), and endogenous peptides in breast milk are essential for neonatal growth and development (18,19). As a body fluid critical for fetal development, AF has peptides associated with heart development and ventricular septal defects (20), but as yet, no studies have identified the peptides associated with lung development. Therefore, in this study, we performed a peptide omics analysis of AF to find differentially expressed peptides related to different stages of lung development to provide a new therapeutic modality for BPD.
In this paper, the characteristic changes in the polypeptide group in AF samples were studied using liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology, and the effect of the differential polypeptide GPPGEPGPPG on the function of mouse lung epithelial (MLE-12) cells was identified by an in vitro study, which provides support for the study of BPD pathogenesis and treatment. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-681/rc).
Methods
Collection of AF
We collected AF samples from pregnant women undergoing prenatal examination using ultrasound-guided amniocentesis at Dongguan Eighth People’s Hospital (Dongguan Children’s Hospital). Samples were taken for routine prenatal diagnosis, and the remaining samples were used for our experimental analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The sample collection process for this study was approved by the Medical Ethics Committee of Dongguan Eighth People’s Hospital (approval No. LL2021113001). The patients provided their written informed consent to participate in this study. Samples were divided into <25 weeks’ gestation (n=3) and ≥25 weeks’ gestation (n=6). All samples were stored at –80℃ until required for the peptide omics analysis.
Sample processing and peptide extraction
The samples stored at −80 ℃ were thawed and centrifuged at 12,000 ×g for 15 minutes. The supernatant was transferred to new tubes, and a 7:1 volume of lysate (8 M Urea/100 mM Tris-Cl, pH 8.0) was added. Then, a 10 kD aperture ultrafiltration tube was used for ultrafiltration, and the filtrate was collected. After the pH value of the solution was adjusted to about 6.0, centrifugation was performed at 12,000 ×g for 15 minutes, and the supernatant was taken for desalting. The desalted peptide solution was drained by a centrifuge concentrator and frozen at −20 ℃ for further use.
LC-MS/MS analysis
The peptides were analyzed using a Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) and an UltiMate 3000 RSLCnano System (Thermo Fisher Scientific, Waltham, MA, USA). The peptide samples were dissolved in the sample buffer, then inhaled by an autosampler, bound to the C18 trap column (3 µm, 100 µm × 20 mm), and eluted at 300 nL/min to the analytical column (2 µm, 750 µm × 150 mm). We used two mobile phases (mobile phase A: 3% DMSO, 0.1% formic acid, 97% H2O; mobile phase B: 3% DMSO, 0.1% formic acid, 97% ACN) to establish the analysis gradient. For data-dependent acquisition (DDA), each scan loop contained an MS full scan (R =70 k, AGC =3e6, max IT =20 ms, scan range =350–1,800 m/z) and 15 MS/MS scans (R =17.5 k, AGC =2e5, max IT =100 ms). The high energy collision dissociation (HCD) was 28, and the quadrupole screening window was 1.6 Da. The dynamic elimination time of the repeated ion collection was set to 35 s.
Data analysis
The identification and quantification of proteins and peptides were performed using MaxQuant (V1.6.6) software (Max Planck Institute of Biochemistry, Martinsried, Germany). Our search was conducted using the Uniprot Human Swiss-prot proteome reference database (https://www.uniprot.org). The main retrieval parameters were as follows: the item type was LFQ; Oxidation (M) and Acetyl (Protein N-term) were selected as the variable modifications; Carbamidomethyl (C) was chosen as the fixed modification, and the digestion method was “Nonspecific.” The search results were screened using a 1% false discovery rate (FDR) at the protein and peptide levels. The criteria for differential expression were fold change (FC) ≥1.2 or ≤1/1.2 and P<0.05.
Bioinformatics
The basic characteristics and physicochemical properties of the differential peptides were analyzed using the Uniprot protein database and “ProtParam” tool. In addition, to explore the potential functions of differentially expressed peptides and their precursors, Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the GO website (http://geneontology.org/) and the KEGG Orthology Based Annotation System (KOBAS) v.3.0.
Cell culture
MLE-12 cells were purchased from the Cell Bank of the Typical Culture Collection Committee of the Chinese Academy of Sciences and placed in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 100 mL streptomycin/penicillin at 37 ℃ with 5% CO2 for culture. For the subsequent cell function assay, the MLE-12 cells were divided into three groups: control, hyperoxia, and hyperoxia + GPPGEPGPPG groups. The control group cells were placed at 37 ℃ with 5% CO2 for culture, the hyperoxia group cells were placed in an oxygen container with 85% O2 at 37 ℃ with 5% CO2 for culture, and the hyperoxia + GPPGEPGPPG group cells were treated with GPPGEPGPPG (GPPGEPGPPG: culture media =1:500), then placed in an oxygen container with 85% O2 at 37 ℃ with 5% CO2 for culture.
Cell proliferation assay
MLE-12 cells were inoculated in 96-well plates at a density of 1×105 per well and cultured until they had grown to 70–80% confluence. The proliferation of MLE-12 cells was examined using 5-Ethynyl-2'-deoxyuridine (EdU) staining. The cells were then fixed with paraformaldehyde and stained with 4',6-diamidino-2-phenylindole (DAPI). Finally, the cells were imaged by fluorescence microscopy.
Mitochondrial membrane potential (MMP/ΔΨm) determination
MMP/ΔΨm was assessed by the JC-1 assay kit according to the instructions and incubated at 37 ℃ for 30 minutes. The average fluorescence intensity of red and green fluorescence in each group was subsequently examined using confocal microscopy, and the ratio of red/green fluorescence intensity was calculated to assess the loss of MMP—an early indicator of apoptosis.
Flow cytometry assay
The MLE-12 cells were rinsed twice with PBS, followed by the Annexin V-APC/7-AAD apoptosis kit, and incubated overnight in the dark at 4 ℃ with a propidium iodide (PI) staining mixture. The final test was conducted in accordance with the manufacturer’s instructions using flow cytometry.
The intracellular ROS
We assessed ROS levels in the MLE-12 cells using a ROS assay kit, incubating them with 2’7’-dichlorofluorescein diacetate (DCFH-DA) for 30 minutes at 37 ℃ in the dark. After washing with phosphate-buffered saline (PBS), the diclofenac (DCF) fluorescence intensity was measured by fluorescence microscopy.
Statistical analysis
For this study, all data were expressed as the mean ± standard error of the mean, and the statistical significance of differences between different groups was determined by one-way analysis of variance (ANOVA). A P<0.05 was considered statistically significant.
Results
Identification and screening of differential peptides in AF samples
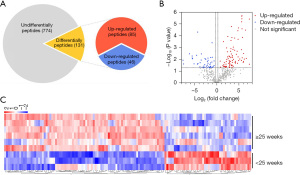
The 25th week of gestation represents the transition from the late canalicular to early saccular lung stage (21), which is an important transitional stage in lung development. We used mass spectrometry to analyze the polypeptide composition of AF at different stages of lung development. A total of 905 peptides were quantified by LC-MS/MS analysis, including 131 differentially expressed peptides (85 up-regulated and 46 down-regulated) (FC ≥1.2 or ≤1/1.2, P<0.05) in the ≥25 weeks’ gestation group compared to the <25 weeks’ gestation group (Figure 1A). Volcano mapping (Figure 1B) and hierarchical clustering (Figure 1C) show these differentially expressed peptides between the two groups. The sequence and detailed information about the differentially expressed peptides are shown in Table S1.
Characteristics of the differentially expressed peptides
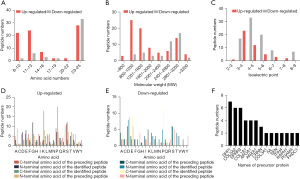
Understanding the basic characteristics and physicochemical properties of peptides will help us to carry out subsequent functional verification. Peptides are small molecular compounds made of amino acids linked to each other. The differentially expressed peptides ranged in length from 8 to 25 amino acids (Figure 2A), and the molecular weights ranged from approximately 800 to 3,200 (Figure 2B). The isoelectric points (Figure 2C) of most peptides are acidic, indicating that the number of acidic amino acids is higher than that of basic amino acids. In addition, since peptides are cleaved from precursor proteins by different enzymes at different sites, we analyzed the cleaved sites of differentially expressed peptides (Figure 2D,2E). The results showed that the dominant amino acid sites of the up-regulated peptides were Proline (P), Lysine (K), Glycine (G), and Serine (S), and the dominant amino acid sites of the down-regulated peptides were Proline (P), Aspartic acid (D), Glutamic acid (E) and Serine (S). Because different proteins may have the same conserved sequences, some peptides may come from multiple precursor proteins. Similarly, the same protein can be cleaved by different enzymes or at different sites to produce different peptides. In our study, up to 15+ peptides were derived from the precursor proteins SPP1, COL5A2, and SPRR3, and up to 9+ peptides were derived from COL1A1, APOA1, and ANXA1 (Figure 2F).
GO function and KEGG pathway analysis of the differently expressed peptides
To investigate the potential biological functions of differentially expressed peptides in AF during different stages of pregnancy, GO and KEGG pathways analyses were performed using the GO website (http://geneontology.org/) and the KOBAS v.3.0.
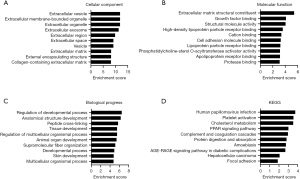
GO analysis revealed that the precursor proteins of these differential peptides are mainly located outside the cell, with the top three most significantly enriched being extracellular vesicles, extracellular membrane-bound organelles, and extracellular organelles (Figure 3A). The molecular function of these proteins is mainly involved in the extracellular matrix structural constituent, growth factor binding, and structural molecule activity (Figure 3B). The main biological processes they are involved in include regulation of the developmental process, anatomical structure development, and peptide cross-linking (Figure 3C). The KEGG pathway enrichment analysis indicated that the potential function of these differentially expressed peptides was related to the human papillomavirus infection, platelet activation, cholesterol metabolism, PPAR signaling pathway, complement and coagulation cascades, protein digestion and absorption, amoebiasis, and the AGE-RAGE signaling pathway in diabetic complications (Figure 3D).
GPPGEPGPPG improved hyperoxia-induced apoptosis and attenuated hyperoxia-induced impairment of cell viability in MLE-12 cells
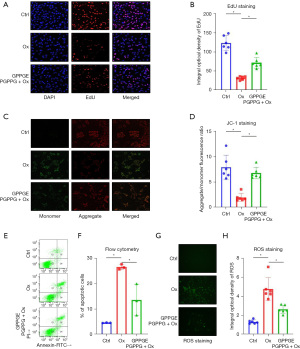
In the cell function assay, we divided MLE-12 cells into three groups: control, hyperoxia, and hyperoxia + GPPGEPGPPG, and explored the mechanism of GPPGEPGPPG in MLE-12 cells based on EdU staining, JC-1 staining, ROS assay, and flow cytometry. The EdU staining results (Figure 4A,4B) showed that the number of MLE-12 cells was significantly reduced after hyperoxia treatment compared to the control group, while the peptide GPPGEPGPPG reversed this phenomenon and promoted the proliferation of MLE-12 cells. The JC-1 kit also examined the changes in MMP /ΔΨm between the different groups, showing that the red fluorescence was weaker and the green fluorescence was stronger in the hyperoxia group, indicating that MMP/ΔΨm was reduced at an early stage of apoptosis (Figure 4C,4D). However, the green fluorescence was weaker in the hyperoxia + GPPGEPGPPG group than in the hyperoxia group, indicating that GPPGEPGPPG interfered with the effect of hyperoxia on apoptosis in MLE-12 cells. Flow cytometry revealed that GPPGEPGPPG reversed the impairment of MLE-12 cell viability by hyperoxia (Figure 4E,4F). In addition, based on the results of the ROS assays, we observed a higher accumulation of ROS in MLE-12 cells in the hyperoxia group (Figure 4G,4H).
Discussion
Human embryonic lung development is divided into five stages: embryonic, pseudoglandular, canalicular, saccular, and alveolar. Complete and normal lung development generates abundant alveoli and a rich network of capillaries, which constitute the blood and air exchange barrier and maintain the normal physiological activities of the body (22). Preterm birth interrupts normal lung development and often requires mechanical ventilation or oxygen therapy due to a lack of alveolar surfactant. Although preterm children have an increased survival rate due to the advances in these treatments, the treatments themselves can lead to BPD and even increased susceptibility to asthma and chronic obstructive pulmonary disease in adulthood (23).
The importance of the uterus as a place for fetal growth and development is undeniable. In addition to the placenta as a source of nutrients for the fetus, the role of AF as a fluid environment surrounding the fetus is also irreplaceable. AF is not just a filtrate of maternal plasma but a separate fluid environment (24) that includes fetal urine and alveolar fluid, as well as a variety of metabolites (25). Bolton et al. carried out a proteomic analysis of AF at 16–18 weeks of gestational age and identified that proteins were involved in organ development (23). Extensive experimental research has confirmed that protein-derived peptides possess a range of biological activities, such as promoting cell proliferation and differentiation (25). Peptidomics allows us to study the composition of AF peptides, determine their sequences, and further understand their potential biological functions through bioinformatics analysis (26). Unlike other studies, the innovative aspect of our research is the identification of peptides in AF to investigate their relationship with lung development so as to assist in the postnatal lung development of preterm infants and provide a new therapeutic tool for the prevention and treatment of BPD.
In this study, the analysis of the endogenous peptidome of AF samples from different gestational periods, using 25 weeks of gestation as the cut-off, resulted in the screening of 131 differential peptides (85 up-regulated and 46 down-regulated) in a ≥25 weeks’ gestation group compared to a <25 weeks’ gestation group. These differential peptides reflect changes in the synthesis, processing, and degradation of proteins in the AF during different stages of pregnancy, indirectly reflecting the physiological or pathological condition of the mother and fetus. Bioinformatics analysis of the precursor proteins of the differential peptides in this study showed that these proteins are involved in the regulation of the developmental process, anatomical structure development, and other biological processes. We specifically investigated the peptide COL5A2 that contained the GPPGEPGPPG sequence and explored the functional mechanism of GPPGEPGPPG in MLE-12 cells by in vitro assays.
In the cell function experiments, we first assessed the differences in the proliferation of MLE-12 cells treated by different methods based on EdU staining. It was clear that the number of MLE-12 cells decreased after hyperoxia treatment compared to the control group, indicating that hyperoxia inhibited the proliferation of MLE-12 cells. Conversely, the peptide GPPGEPGPPG reversed this phenomenon and promoted the proliferation of MLE-12 cells. The JC-1 kit also examined changes in MMP/ΔΨm and assessed the effect of the GPPGEPGPPG peptide and hyperoxia on apoptosis in MLE-12 cells. It was evident that the red fluorescence was weaker in the hyperoxia group, while the green fluorescence was stronger, indicating that MMP/ΔΨm was decreased in early apoptosis. However, the green fluorescence was weaker in the hyperoxia + GPPGEPGPPG group compared to the hyperoxia group. These findings indicated that GPPGEPGPPG interfered with the effect of hyperoxia on apoptosis in MLE-12 cells. Furthermore, we observed that GPPGEPGPPG attenuated the hyperoxia-induced impairment of MLE-12 cell viability. In addition, GPPGEPGPPG and hyperoxia were associated with the accumulation of ROS in MLE-12 cells. There is no report that the peptide COL5A2 can be applied in clinical. The above results suggest that the peptide COL5A2 contained the GPPGEPGPPG sequence is involved in BPD-related cell genesis and development, and it could be a potential target for the clinical treatment of BPD.
Conclusions
In conclusion, we have revealed that the composition of AF varies between gestational periods from a peptidomic perspective, and these differential peptides are expected to provide new therapeutic tools for the prevention and treatment of BPD. In vitro investigations exploring the lung development of preterm infants may improve the survival of preterm infants and reduce long-term complications.
Acknowledgments
The authors are grateful to all lab members for their suggestions and encouragement.
Funding: This work was supported by the Social Science and Technology Development Project of Dongguan, China (No. 20221800905362), and by grants from the National Natural Science Foundation of China (No. 81901512 to Xingyun Wang) and Shanghai Rising-Star Program (No. 22QB1401000 to Xingyun Wang).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-681/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-681/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-681/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The sample collection process for this study was approved by the Medical Ethics Committee of Dongguan Eighth People’s Hospital (approval No. LL2021113001). The patients provided their written informed consent to participate in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 2021;375:n1974. [Crossref] [PubMed]
- Gao SQ, Zhang XL, Du WN, et al. Systematic review and meta-analysis: the effect of bronchopulmonary dysplasia on neurodevelopment in very low birth weight premature infants. Transl Pediatr 2021;10:3023-33. [Crossref] [PubMed]
- Yang K, Dong W. Perspectives on Probiotics and Bronchopulmonary Dysplasia. Front Pediatr 2020;8:570247. [Crossref] [PubMed]
- Lal CV, Olave N, Travers C, et al. Exosomal microRNA predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight 2018;3:93994. [Crossref] [PubMed]
- Davidson LM, Berkelhamer SK. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J Clin Med 2017;6:4. [Crossref] [PubMed]
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol 2005;25:341-8. [Crossref] [PubMed]
- Kitterman JA, Chapin CJ, Vanderbilt JN, et al. Effects of oligohydramnios on lung growth and maturation in the fetal rat. Am J Physiol Lung Cell Mol Physiol 2002;282:L431-9. [Crossref] [PubMed]
- Blachford KG, Thurlbeck WM. Lung growth and maturation in experimental oligohydramnios in the rat. Pediatr Pulmonol 1987;3:328-33. [Crossref] [PubMed]
- Moessinger AC, Collins MH, Blanc WA, et al. Oligohydramnios-induced lung hypoplasia: the influence of timing and duration in gestation. Pediatr Res 1986;20:951-4. [Crossref] [PubMed]
- Khalaj K, Antounians L, Figueira RL, et al. Autophagy Is Impaired in Fetal Hypoplastic Lungs and Rescued by Administration of Amniotic Fluid Stem Cell Extracellular Vesicles. Am J Respir Crit Care Med 2022;206:476-87. [Crossref] [PubMed]
- Antounians L, Catania VD, Montalva L, et al. Fetal lung underdevelopment is rescued by administration of amniotic fluid stem cell extracellular vesicles in rodents. Sci Transl Med 2021;13:eaax5941. [Crossref] [PubMed]
- Solaiman A, Mehanna RA, Meheissen GA, et al. Potential effect of amniotic fluid-derived stem cells on hyperoxia-induced pulmonary alveolar injury. Stem Cell Res Ther 2022;13:145. [Crossref] [PubMed]
- Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today 2015;20:122-8. [Crossref] [PubMed]
- Van JAD, Clotet-Freixas S, Zhou J, et al. Peptidomic Analysis of Urine from Youths with Early Type 1 Diabetes Reveals Novel Bioactivity of Uromodulin Peptides In Vitro. Mol Cell Proteomics 2020;19:501-17. [Crossref] [PubMed]
- Lin L, Zheng J, Zheng F, et al. Advancing serum peptidomic profiling by data-independent acquisition for clear-cell renal cell carcinoma detection and biomarker discovery. J Proteomics 2020;215:103671. [Crossref] [PubMed]
- Yang J, Xiong X, Liu S, et al. Identification of novel serum peptides biomarkers for female breast cancer patients in Western China. Proteomics 2016;16:925-34. [Crossref] [PubMed]
- Hu Y, Wang J, Zhou Y, et al. Peptidomics analysis of umbilical cord blood reveals potential preclinical biomarkers for neonatal respiratory distress syndrome. Life Sci 2019;236:116737. [Crossref] [PubMed]
- Zhou Y, Zhang L, Yu Z, et al. Peptidomic analysis reveals multiple protection of human breast milk on infants during different stages. J Cell Physiol 2019; Epub ahead of print. [Crossref]
- Wang X, Yan X, Zhang L, et al. Identification and Peptidomic Profiling of Exosomes in Preterm Human Milk: Insights Into Necrotizing Enterocolitis Prevention. Mol Nutr Food Res 2019;e1801247. [Crossref] [PubMed]
- Li X, Wu LJ, Gu M, et al. Peptidomic Analysis of Amniotic Fluid for Identification of Putative Bioactive Peptides in Ventricular Septal Defect. Cell Physiol Biochem 2016;38:1999-2014. [Crossref] [PubMed]
- Toth A, Steinmeyer S, Kannan P, et al. Inflammatory blockade prevents injury to the developing pulmonary gas exchange surface in preterm primates. Sci Transl Med 2022;14:eabl8574. [Crossref] [PubMed]
- Surate Solaligue DE, Rodríguez-Castillo JA, Ahlbrecht K, et al. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2017;313:L1101-53. [Crossref] [PubMed]
- Bolton CE, Bush A, Hurst JR, et al. Lung consequences in adults born prematurely. Postgrad Med J 2015;91:712-8. [Crossref] [PubMed]
- Tong XL, Wang L, Gao TB, et al. Potential function of amniotic fluid in fetal development---novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J Chin Med Assoc 2009;72:368-73. [Crossref] [PubMed]
- Tong X. Amniotic fluid may act as a transporting pathway for signaling molecules and stem cells during the embryonic development of amniotes. J Chin Med Assoc 2013;76:606-10. [Crossref] [PubMed]
- Foreman RE, George AL, Reimann F, et al. Peptidomics: A Review of Clinical Applications and Methodologies. J Proteome Res 2021;20:3782-97. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


