
Impressive efficacy of the ketogenic diet in a KCNQ2 encephalopathy infant: a case report and exhaustive literature review
Highlight box
Key findings
• The introduction of the ketogenic diet in an infant harboring a “de novo” p.Ser122Leu KCNQ2 variant resulted in both seizure control and developmental milestones achievement.
What is known and what is new?
• Response to ketogenic diet in KCNQ2 mutations in general is, so far, unpredictable and variable among cases reported in literature and summarized in the text.
• To our knowledge this is the eighth case of a patient with the missense variant c.365 C>T in the KCNQ2 gene and the first case reported to be treated, successfully, with ketogenic diet.
What is the implication, and what should change now?
• Our case enlightens the need to further assess ketogenic diet’s contribution in both controlling KCNQ2-associated epilepsy and preventing KCNQ2-DEE and to further assess molecular mechanisms beyond genotype-phenotype correlation even in terms of response to treatment.
Introduction
KCNQ2 (Potassium Voltage-Gated Channel Subfamily Q Member 2) gene is located on chromosome 20q13.33 and encodes the Kv7.2 subunit of the voltage-gated potassium channels and expressed in the central and peripheral nervous system from the 22nd week of gestational age. The Kv7.2 subunits are involved in the neuronal muscarine M-current, a K+-selective, non-inactivating, slowly activating/deactivating current, which regulates negatively neuronal excitability. Thus, KCNQ2 mutations lead to a wide spectrum of neonatal-onset epilepsies, ranging from the benign familial neonatal epilepsy (BFNE) to the more severe phenotype known as KCNQ2 developmental and epileptic encephalopathy (KCNQ2-DEE) (1,2).
We describe an infant with KCNQ2 encephalopathy due to a “de novo” mutation in the S2 segment of the Kv7.2 subunit, whose seizures dramatically responded to the ketogenic diet (KD), whose administration led to both epilepsy control and neurodevelopmental milestone achievement. Furthermore, we conducted an exhaustive and systematic review of the available literature on several medical electronic databases (including Cochrane Library, Medline, PubMed Central, Scopus and Web of Science) in accordance with the “preferred reporting items for systematic review and meta-analysis” (PRISMA) (Figure 1).
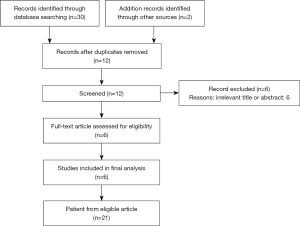
We used the following search terms: “ketogenic diet” and “KCNQ2” or “ketogenic diet” and “KCNQ2 encephalopathy” or “ketogenic diet” and “KCNQ2” and “epilepsy”. Ten articles were found from 2016 to date; 2 studies from the reference list were added from other sources. Among them, 1 was related to the KCNQ3 gene and 5 were irrelevant and were, thus, excluded. We included 6 articles (3 retrospective cohort studies, 2 case series and 1 case report) (Table 1); all the selected studies were conducted in pediatric patients. The only filters applied were English language and human studies. In literature, reports describing the KCNQ2 variant detected in our patient are limited. None of the reported subjects was treated with a KD. To date, the benefits of the KD in KCNQ2-related epilepsy have been demonstrated by some case reports and series. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-258/rc).
Table 1
| Reference/year | No. Cases | KCNQ2 variant | Age at KD start/sex | Seizure type at onset | Epilepsy syndrome | Drugs at time of KD | Seizure control | Neurological outcomes |
|---|---|---|---|---|---|---|---|---|
| Thompson et al. 2017 (3) | 1 | NA | 6 weeks/NA | DS neonatal seizures | – | Phenobarbital; levetiracetam; topiramate; clobazam | Daily seizures | Severe DD |
| Freibauer et al. 2018 (4) | 1 | De novo p.Ser247Leu plus a maternal; p.Ser374Pro | 2 weeks/male | Tonic posturing with eye deviation, apnoea, bradycardia, and oxygen desaturation. | EIMFS | Phenobarbital; phenytoin; midazolam; ketamine | No seizures | Death at 1 months and 7 days |
| Ko et al. 2018 (5) | 7 | NA | NA/male [4]; female [3] | Neonatal tonic seizures | OS [4] | Phenytoin; oxcarbazepine | IS [7] | Profound ID [7] |
| Ko et al. 2018 (6) | 6 | NA | NA/male [4]; female [2] | DS neonatal seizures | OS [6] | 2 or more not mentioned AEDs | >90% seizure reduction [5] | NA |
| Le Pichon et al. 2019 (7) | 1 | De novo p.Arg581Gln | NA/male | DS seizures | – | 1 to 3 not mentioned AEDs | No seizures | NA |
| Lee et al. 2020 (8) | 5 | NA | NA/male [2]; female [3] | GTS [2]; GTCS [2]; MS [1] | OS [5] | Phenytoin; oxcarbazepine; phenobarbital valproate; levetiracetam topiramate vigabatrin; steroids | IS [5] | Severe ID [5]; hypotonia [2]; spasticity [3]; microcephaly [1]; eye contact [2] |
| Present study 2022 | 1 | De novo p.Ser122Leu | 3 months/female | Neonatal tonic posturing asymmetric seizures with asynchronous clonic movements, apnoea and cyanosis | – | Levetiracetam; phenobarbital; valproate | No seizures | Mild DD |
KD, ketogenic diet; NA, not available; EIMFS, encephalopathy of infancy with migrating focal seizures; OS, Ohtahara syndrome; IS, infantile spasm; ID, intellectual disability; AED, antiepileptic drug; DS, drug-resistant; GTS, generalized tonic seizures; GTCS, generalized tonic-clonic seizures; MS, myoclonic seizures; DD, developmental delay.
Case presentation
A female third-child of healthy unrelated parents was admitted to our department for neonatal seizures. Her familial history was negative. At the time of gestation, her father was 38, and her mother was 35 years old. Her mother denied complications during gestation, but referred reduced fetal movements. Prenatal ultrasounds did not show fetal anomalies. The child was born at 39 weeks of gestational age by spontaneous delivery. Her birth weight was 3,600 gr, length 51 cm and head circumference 35 cm (all within normal limits). The APGAR (9) score was 8 at one and 10 at five minutes, respectively. Neither dysmorphisms nor organ involvement were noted; neurological examination revealed only mild axial hypotonia. On day two of life, she presented with seizures consisting of asymmetric tonic posturing, according to the ILAE Classification of Seizures and the Epilepsies in the Neonate: her head moving to the right side and extension of her arms to the left side or vice versa (10). Motor manifestations were associated with apnea and cyanosis and followed by crying and asynchronous clonic movements of the arms. Laboratory investigations ruled out infections and inborn error of metabolism. The brain magnetic resonance imaging (MRI) revealed only a thin corpus callosum. An early-onset epileptic encephalopathies next-generation sequencing (NGS) panel was performed. The amplitude-integrated electroencephalography (aEEG) showed a sudden rise of both lower and upper margin, followed by a marked depression of the trace. Seizures were unresponsive to levetiracetam, but remitted with phenobarbital, thus the baby was discharged home. A follow-up electroencephalogram (EEG) recorded a polymorphic rhythmic background activity in the theta-delta range with a slight interhemispheric asymmetry (amplitude of 40 µV over the right hemisphere, 20 µV over the left). Interictal multifocal spikes, predominantly in the posterior regions of her right hemisphere, were also detected during both wakefulness and sleep (Figure 2). At three months of life, she presented with seizures with a same semiology that occurred in clusters and were resistant to treatment with phenobarbital, valproate and intravenous midazolam. The physical examination showed plagiocephaly, hypotonia and poor eye contact. The ictal polygraphic-video-EEG recording revealed discharges of rhythmic theta activity in the temporal regions of the right hemisphere with a tendency to spread to the left temporal ones and, subsequently, to generalize (Figure 3). The epilepsy NGS performed in the neonatal period revealed a p.Ser122Leu (c.365 C>T) variant in the KCNQ2 gene. Her parents tested negative, suggesting that the variant was a “de novo” mutation, assumed to be pathogenetic. After diagnosis, carbamazepine was added, without consistent benefits. Therefore, we started a KD with a 4:1 ratio between the amount of lipids and carbohydrates plus protein through nasogastric tube. This led to immediate improving of her neurological condition.
Subsequently, she continued carbamazepine associated with a KD with a 3:1 ratio and achieved complete seizure freedom over time. Neurological assessments demonstrated progressive gaining of developmental milestones and oral feeding ability with only mild delay. Improvement of the Neurological Infant score was recorded at 6 months, after KD was started. Follow-up EEG recordings showed an unmodified background activity. Under carbamazepine treatment and KD regimen the baby maintained seizure remission and KD was discontinued at 20 months of age. The physical examination at 22 months of age evidenced: height and weight on 25–50th percentiles, head circumference on 40th percentile, residual plagiocephaly, mild axial hypotonia, stands up and walks with assistance, visually attentive and good social skills.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the ethical committee of the Medical Faculty of the University of Catania (IRB No. 780). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Mutations in the KCNQ2 gene are involved in more than 80% of BFNE and 10% of all early-onset epileptic encephalopathies (EOEE) (11,12). BFNE shows autosomal dominant inheritance with incomplete penetrance and a high rate of inter- and intrafamilial variability and is caused by missense, splice-region, nonsense, frameshift variants and deletion pathogenic mutations in any gene region (1). Loss-of function (LoF) mutations of KCNQ2 underlie BFNE through haploinsufficiency, resulting in a reduction of 25% of M-current activity (1). Seizures start in the first days of life and self-limit over weeks or months and are characterized by multiple episodes of asymmetric tonic posturing seizures with alternating laterality, sometimes followed by unilateral or bilateral clonic movements, associated with apnea and oxygen desaturation and rarely evolving into SE (1). Background EEG and brain MRI are unremarkable (13). Neurodevelopment is usually normal, but seizure relapse occurs in up to 25% of patients. Low dose oral carbamazepine is the first choice of treatment, leading to seizure freedom within hours from first administration (13).
KCNQ2-DEE is a much more severe neonatal-onset condition caused by “de novo” variants within critical zones of the gene. The S4 transmembrane segment, critical for channel gating, the pore domain and the A-helix and B-helix at C-terminus are the most frequently involved regions. Missense mutations cause KCNQ2-DEE and result in more than 25 % inactivation of the voltage-gated potassium channel (13). Seizure onset and semiology is the same as in BFNE but KCNQ2-DEE results in progressive hypotonia, poor general movements, no visual fixation, impaired awareness, moderate to severe developmental delay and refractory epilepsy (13,14). The interictal EEG exhibits marked abnormalities, as dysmaturity, slowing, multifocal epileptiform discharges and burst suppression. The aEEG is distinctive and consists of a sudden rise of the lower and upper margin, followed by a marked depression (14). Brain MRI can be normal or show signs of brain atrophy, hypo-myelination, thin corpus callosum or subcortical hyperintensities (14).
Sodium channel blockers (SCB) including carbamazepine, phenytoin, lidocaine and oxcarbazepine represent the best treatment option for patients with KCNQ2 mutations according to the available literature (11,12,15). In case of refractoriness to SCB, seizure control has been reported to be achieved on other antiepileptic drugs (AEDs), including phenobarbital, levetiracetam, topiramate and valproate. Refractory patients have been reported to improve on other treatments options as well, including corticosteroids, folinic acid, pyridoxine, and KD (16).
KD is a hyperlipidic, hypoglucidic, normoprotein diet regimen inducing and maintaining a chronic state of ketosis that results in neuroprotection and improvement of seizure frequency and severity by inducing an increase in cellular mitochondrial function and biogenesis, a decrease in oxidative stress, changes in potassium channels, an increase in the brain-derived neurotrophic factor (BDNF) and in the purinergic and GABAergic neurotransmission, attenuation of neuroinflammation and even changes in gut microbiota (17).
Epigenetic processes are known too to be related to changes in neuronal activity and epileptogenesis and, recently, the role of molecular mediators of epigenetic changes including non-coding RNAs, methylation of DNA and histone acetylation via de-acetylase inhibition and mitochondrial biogenesis have been hypothesized to be involved in KD’s therapeutic effects, as well (17-20); a recent transcriptional study on miRNA and mRNA’s expression of eight pediatric patients affected by intractable epilepsy has demonstrated that KD induces changes at the transcriptomic level in genes encoding ion channels, neurotransmitter receptors and synapse structural proteins, resulting in down-regulation of synaptic transmission, which is the most relevant biologic pathway related to epilepsy, and subsequent decreased excitability (17). KD’s use for early-onset intractable seizures has been described among pediatric patients with KCNQ2 mutations. In the case series by Thompson et al., a critical neonate with KCNQ2 epileptic encephalopathy refractory to multiple AEDs (phenobarbital, levetiracetam, topiramate, clobazam), was started with KD with immediate EEG organization’s improvement and seizure remission after 2 weeks (3). KD induced impressive seizure reduction, as well, in a neonate affected by encephalopathy with migrating focal seizures (EIMFS) as a consequence of a “de novo” p.Ser247Leu KCNQ2 variant plus a maternal inherited p.Ser374Pro mutation. The baby had a poor outcome regardless of the anticonvulsant poly-therapy (phenobarbital, phenytoin, midazolam and ketamine infusion, KD) (4).
Ko et al. reported on a cohort of seven patients harboring unspecified KCNQ2 mutations, six of which responded favorably to KD, vigabatrin and prednisolone, while only four of them improved with SCB (5). The same authors reported on a response rate of 83,3% at 3, 6 and 12 months after introducing KD in patients with KCNQ2 pathogenic variants (6).
In a more recent study, half of the patients with KCNQ-related epilepsy due to various unspecified mutations, gained seizure freedom initiating KD, in association with one or more AEDs (phenytoin, oxcarbazepine, phenobarbital, valproate, levetiracetam, topiramate or vigabatrin) or with steroid therapy (8). Le Pichon et al. reported an infant harboring a novel p.Arg581Gln variant in the KCNQ2 gene, who was successfully treated with KD while continuing human breast milk feeding (7).
So far, response to KD is unpredictable and variable among our case and those reported in literature; thus, it still remains unclear why some KCNQ2 mutations respond better to KD than others. Besides obvious variables, such as the adherence to KD, several plausible mechanisms have been proposed in time.
A recent preclinical study (21) demonstrated how β-hydroxybutyrate (BHB), the primary ketone body generated by ketosis, directly activates Voltage-gated potassium channels generated by KCNQ2-5 subunits, especially KCNQ2/3 heteromers via a highly conserved S5 tryptophan on KCNQ3. It might be conceivable that mutations located in “hot zones” involving the above-mentioned interaction domains affect negatively response to KD. Furthermore, some KCNQ2 variants have been demonstrated to cause an improper channel localization in addition to causing a reduction in Kv7-associated current (4) and this has been suggested as an explanation to the variable response to specific treatments, including KD. A recent preclinical in vitro study described molecular mechanisms besides the response to KD in the first missense loss-of-function pathogenic variant described within the S4 segment of Kv7.3, suggesting that the type and location of mutation predicts the response to specific treatments (22). Others suggest that the unpredictable response to KD might be, instead, mutation-independent and related to changes induced by KD at the transcriptomic level (17). Unfortunately, data available to date only allow speculative hypothesis and further preclinical and clinical studies of combined analysis of gene and miRNAs’ expression are needed to clearly assess this issue and to allow, in the future, to use KD as a targeted-therapy.
To our knowledge, the case presented is the eighth case reported in literature of the missense variant c.365 C>T in the KCNQ2 gene and the first case reported to be treated successfully with KD; functional damage results from the substitution of a polar serine residue with a non-polar leucine residue at codon 122 of the KCNQ2 protein in the extracellular loop between the S1 and S2 transmembrane segments of Kv7.2. A study conducted in oocytes demonstrated that the p.Ser122Leu sequence change causes a slower activation kinesis than wild-type voltage-gated potassium channel (23). Therefore, the p.Ser122Leu missense variant has been classified as pathogenic for KCNQ2-related disorders. Using the ClinVar (www.ncbi.nlm.nih.gov/clinvar) and RIKEE (rikee.org) databases we identified four studies describing seven patients harboring the same KCNQ2 variant of our subject (Table 2). Hunter et al. described three individuals in a pedigree, presenting with early-onset seizures and who exhibited a normal development (23).
Table 2
| Reference/year | No. cases | Inheritance | Seizure control | Neurodevelopment |
|---|---|---|---|---|
| Hunter et al. 2006 (23) | 3 | Familial | No seizures at 11 weeks after phenobarbital [1]. No seizures at 6 weeks after carbamazepine; subsequent febrile seizures [1]. No seizures after phenobarbital and phenytoin; recurrence up to 7 years [1] | Normal |
| Millichap et al. 2016 (24) | 2 | Paternal | No seizures after neonatal period | Mild DD |
| Zhang et al. 2015 (25) | 1 | De novo | NA | Profound DD and ID |
| Malerba et al. 2020 (26) | 1 | De novo | Three tonic seizures relapsed until 10 years. Seizure freedom from 10 to 12 years | Normal |
| Present study 2022 | 1 | De novo | No seizures at three months after KD and carbamazepine | Mild DD |
DD, developmental delay; NA, not available; ID, intellectual disability.
In a proband, seizures remitted at 11 weeks after initiation of phenobarbital. In the second subject seizure control was obtained with carbamazepine at 6 weeks. In the third patient, phenobarbital and phenytoin lead to remission of seizures, but they recurred until 7 years of life (23). A study of Millichap et al. reported a sibling pair harboring a p.Ser122Leu KCNQ2 variant. Epilepsy was limited to the neonatal period, whereas a mild developmental delay was noted over time. Their father suffered from neonatal seizures but he had normal intellect (24). Zhang et al. described a male with seizure onset on day 3 as a consequence of a “de novo” p.Ser122Leu mutation in KCNQ2. At the time of the study, he was three-year-old and had severe intellectual and developmental disabilities (25). A recent study of Malerba et al. reported a 12-year-old male who started with multiple tonic neonatal seizures due to a spontaneous p.Ser122Leu KCNQ2 variant. He was under oxcarbazepine and gained seizure control and normal neurodevelopment (26).
Conclusions
The present case highlights the role of the S2 transmembrane segments of the Kv7.2 subunit in the regulation of neuronal firing. We would like to propose this region of the channel as one of the KCNQ2-DEE hot zones though various phenotypes have been reported to result from the p.Ser122Leu KCNQ2 variants; our patient harboring a “de novo” KCNQ2 pathogenic variant didn’t respond to oral carbamazepine but reached complete seizure cessation and progressively achieved neurodevelopmental milestones after initiating KD. Studies describing the role of KD in pediatric KCNQ2 epilepsies are limited to small, retrospective cohorts and encourage KD’s usefulness along with one or more ongoing AEDs. None of the previously reported patients with the p.Ser122Leu variant had received KD before. Cause of genotype-phenotype variability and the subsequent possibility to predict response to specific medications still remain unclear and need further studies with particular regard to epigenetic and transcriptomic analysis. Our experience, though based on a single case report, enlightens the strong and urgent need to perform further studies to assess KD’s potential contribution in resolving KCNQ2-associated epilepsy and to determine whether it can influence positively not only epilepsy but also neurodevelopment in infants with KCNQ2-DEE. Furthermore, larger prospective studies and randomized-controlled trials (RCTs) are needed to determine whether KD’s potential benefits on neurocognitive evolution are higher when KD is started as early as possible, as proposed for seizure control and to assess whether, in the future, KD might be considered as a potential first-line treatment in patients with KNCQ2-associated epilepsy.
Acknowledgments
We wish to thank the authors in the reference list who contributed to our review by providing information about their patients.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-258/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-258/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the ethical committee of the Medical Faculty of the University of Catania (IRB No. 780). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nappi P, Miceli F, Soldovieri MV, et al. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflugers Arch 2020;472:881-98. [Crossref] [PubMed]
- Portale A, Comella M, Salomone G, et al. The Spectrum of KCNQ2- and KCNQ3-Related Epilepsy. J Pediatr Neurol 2021; [Crossref]
- Thompson L, Fecske E, Salim M, et al. Use of the ketogenic diet in the neonatal intensive care unit-Safety and tolerability. Epilepsia 2017;58:e36-9. [Crossref] [PubMed]
- Freibauer A, Jones K. KCNQ2 mutation in an infant with encephalopathy of infancy with migrating focal seizures. Epileptic Disord 2018;20:541-4. [Crossref] [PubMed]
- Ko A, Youn SE, Kim SH, et al. Targeted gene panel and genotype-phenotype correlation in children with developmental and epileptic encephalopathy. Epilepsy Res 2018;141:48-55. [Crossref] [PubMed]
- Ko A, Jung DE, Kim SH, et al. The Efficacy of Ketogenic Diet for Specific Genetic Mutation in Developmental and Epileptic Encephalopathy. Front Neurol 2018;9:530. [Crossref] [PubMed]
- Le Pichon JB, Thompson L, Gustafson M, et al. Initiating the ketogenic diet in infants with treatment refractory epilepsy while maintaining a breast milk diet. Seizure 2019;69:41-3. [Crossref] [PubMed]
- Lee S, Kim SH, Kim B, et al. Genetic diagnosis and clinical characteristics by etiological classification in early-onset epileptic encephalopathy with burst suppression pattern. Epilepsy Res 2020;163:106323. [Crossref] [PubMed]
- American Academy of Pediatrics Committee on Fetus and Newborn. The Apgar Score. Pediatrics 2015;136:819-22. [Crossref] [PubMed]
- Pressler RM, Cilio MR, Mizrahi EM, et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE Task Force on Neonatal Seizures. Epilepsia 2021;62:615-28. [Crossref] [PubMed]
- Cornet MC, Sands TT, Cilio MR. Neonatal epilepsies: Clinical management. Semin Fetal Neonatal Med 2018;23:204-12. [Crossref] [PubMed]
- Falsaperla R, Scalia B, Giugno A, et al. Treating the symptom or treating the disease in neonatal seizures: a systematic review of the literature. Ital J Pediatr 2021;47:85. [Crossref] [PubMed]
- Berg AT, Mahida S, Poduri A. KCNQ2-DEE: developmental or epileptic encephalopathy? Ann Clin Transl Neurol 2021;8:666-76. [Crossref] [PubMed]
- Vilan A, Mendes Ribeiro J, Striano P, et al. A Distinctive Ictal Amplitude-Integrated Electroencephalography Pattern in Newborns with Neonatal Epilepsy Associated with KCNQ2 Mutations. Neonatology 2017;112:387-93. [Crossref] [PubMed]
- Pisano T, Numis AL, Heavin SB, et al. Early and effective treatment of KCNQ2 encephalopathy. Epilepsia 2015;56:685-91. [Crossref] [PubMed]
- Kuersten M, Tacke M, Gerstl L, et al. Antiepileptic therapy approaches in KCNQ2 related epilepsy: A systematic review. Eur J Med Genet 2020;63:103628. [Crossref] [PubMed]
- Ruiz-Herrero J, Olaso-Gonzalez G, Serna E, et al. Transcriptomic profile of epileptic children treated with ketogenic therapies. J Integr Neurosci 2022;21:31. [Crossref] [PubMed]
- Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 2006;60:223-35. [Crossref] [PubMed]
- Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol 2017;30:187-92. [Crossref] [PubMed]
- Chriett S, Dąbek A, Wojtala M, et al. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci Rep 2019;9:742. [Crossref] [PubMed]
- Manville RW, Papanikolaou M, Abbott GW. M-Channel Activation Contributes to the Anticonvulsant Action of the Ketone Body β-Hydroxybutyrate. J Pharmacol Exp Ther 2020;372:148-56. [Crossref] [PubMed]
- Dirkx N, Miceli F, Taglialatela M, et al. The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding. Front Physiol 2020;11:570588. [Crossref] [PubMed]
- Hunter J, Maljevic S, Shankar A, et al. Subthreshold changes of voltage-dependent activation of the K(V)7.2 channel in neonatal epilepsy. Neurobiol Dis 2006;24:194-201. [Crossref] [PubMed]
- Millichap JJ, Park KL, Tsuchida T, et al. KCNQ2 encephalopathy: Features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol Genet 2016;2:e96. [Crossref] [PubMed]
- Zhang Y, Kong W, Gao Y, et al. Gene Mutation Analysis in 253 Chinese Children with Unexplained Epilepsy and Intellectual/Developmental Disabilities. PLoS One 2015;10:e0141782. [Crossref] [PubMed]
- Malerba F, Alberini G, Balagura G, et al. Genotype-phenotype correlations in patients with de novo KCNQ2 pathogenic variants. Neurol Genet 2020;6:e528. [Crossref] [PubMed]




