Goal-directed-perfusion in neonatal aortic arch surgery
Introduction
Mortality and morbidity of congenital cardiac procedures have always been an issue for cardiac surgeons since the very first operation on cardiopulmonary bypass. Technical improvement in extracorporeal circulation, increased knowledge in physiology and pathophysiology of cardiopulmonary bypass and special organ protection strategies have helped to reduce the incidence of complications and death to an acceptable rate. However, they are still present and need to be tackled every day. Since Bellinger, Newburger and Jonas published their landmark studies about neurological outcomes after arterial switch operations (1-4), perfusion strategies, especially for aortic arch corrections, have been more and more modified to avoid the potential deleterious effects of deep hypothermic circulatory arrest (DHCA) (5-9). Several alternative perfusion regimens of body and brain have been suggested and were implemented into clinical practice more or less successfully, so that we have learned a lot about possible benefits and potential new complications when mal- or hypo-perfusion of organs occur. To our opinion, monitoring and visualization of end organ oxygen supply and blood-flow is of utmost importance and not only of scientific interest.
Cerebral protection during aortic arch repair is currently performed by either deep hypothermic circulatory arrest or regional cerebral perfusion (RCP) via the innominate artery. Both completely distinct cardiopulmonary bypass techniques were unable to demonstrate a significant difference in randomized controlled trials regarding the incidence of perioperative cerebral injury or neurodevelopmental outcome (10,11). Several studies suggested that longer duration of deep hypothermic circulatory arrest is associated with neurocognitive impairment (12-14), but despite the missing evidence of a clear time limit (12,15), perioperative seizures with impaired motor development (16) and brain damage evident on MRI were consistent with RCP as well (10,17,18). Nevertheless, improved outcome reports (early and late) about shorter postoperative ventilation, improved renal function and adequate time-related neurodevelopment have been subsequently published after using RCP (15,19,20).
The question of an effective distribution and ideal quantity of cerebral blood flow, particularly in the contralateral left hemisphere, is one of the main issues about RCP, and effective neuro-monitoring in addition to visualization of flow could lower this burden (21,22). The same hypothesis pertains to the amount of infra-diaphragmatic perfusion which is potentially provided via pre-existing collaterals (via subclavian and intercostal arteries to the descending aorta), which could be different in size and may not be adequate if the patient’s core temperature is kept “too warm” (Figure 1).
The level of concern in our group, especially in complex aortic arch repair with longer arch clamping times, has brought us to the concept of total body perfusion (TBP) using an additional separate arterial pump for infradiaphragmatic perfusion of the descending aorta.
Both regional cerebral oxygen saturation from the frontal cortex (rSO2) (23,24) and time average velocity (TAV) of blood flow in the medial cerebral artery (MCA) (21,22,24-29) have been interpreted as potential surrogate indicators for cerebral perfusion during infant cardiac surgery. Low intraoperative rSO2-level may impact psychomotor development (15,30,31) and correlate with postoperative cerebral lesions diagnosed by magnetic resonance imaging (15,17,18,23,31-33). On the other hand, particularly in the context of aortic arch surgery using RCP, measuring TAV may avoid the potential dangers of excessive cerebral blood flow resulting in cerebral edema or intracranial hemorrhage (22,34).
TAV in one MCA is usually displayed continuously by transcranial Doppler ultrasonography from the temporal window. Nowadays, transfontanellar ultrasound has become routine analysis in pediatric patients whose fontanelles are not closed. It can be applied as a point-of-care method during cardiac surgery (35) and provides additional information regarding morphology of the whole brain, including detection of brain lesions, measurement of TAV and 3-dimensional (3D)-imaging of various blood vessels. It is currently our routine to investigate cerebral blood flow to both hemispheres during RCP, using combined transfontanellar/transtemporal ultrasound and bilateral frontal rSO2.
In addition, regional oxygenation below the diaphragm (rSO2) with an additional left renal somatic reflectance oximetry pad is monitored as well, but we have not yet tried to visualize renal blood flow with selective ultrasound tools (Figure 2).
This review focuses mainly on practical and theoretical issues on how to protect organs from ischemic or hypoxemic damage during complex aortic arch surgery by adequate monitoring of tissue oxygen supply, thus providing relatively adequate blood flow to target perfusion regions [goal-directed-perfusion (GDP)].
Methods
Monitoring
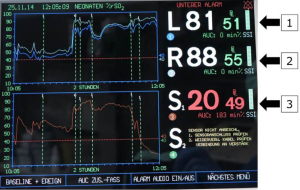
Perioperative perfusion monitoring for aortic arch repair in neonates and young infants with open fontanelles includes intraoperative combined transfontanellar/transtemporal 2D- and 3D-ultrasound imaging of both blood flow intensity in both hemispheres, and assessment of mean TAV displayed in the basilar artery (BA), bilateral internal carotid arteries (ICA), bilateral anterior cerebral arteries (ACA) and bilateral MCAs, respectively. Additionally, bilateral cerebral frontal rSO2 and subdiaphragmatic rSO2 (left kidney) are measured.
Surgical technique
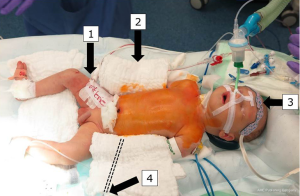
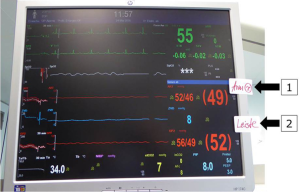
After initiation of anesthesia, arterial blood pressure monitoring lines are placed in the right radial artery and in one femoral artery. Measurement of rSO2 (%) is performed by continuous plotting of the somatic reflectance oximetry in both frontal hemispheres and subdiaphragmatic (rSO2, INVOS®; Somanetics Corporation, Troy, MI, USA). After midline sternotomy and heparinization, a 3.5-mm PTFE-tube (Gore-Tex®, Flagstaff, AZ, USA) is anastomosed to the innominate artery and cannulated with a 10F arterial cannula.
CPB is started with an estimated flow of 3.0 L/m2 BSA (175–200 mL/kg) after bicaval cannulation and patients are cooled to 28 °C rectal temperature. The pericardium is opened posterior and to the left of the inferior vena cava (IVC). The descending aorta is identified in the left pleural space after mobilization of the lingula to the left of the esophagus. In case of ductal dependent descending aortic circulation, both pulmonary arteries are snared, distal aortic perfusion is accomplished by selective cannulation of the descending aorta above the diaphragm and connected to a second roller pump: in this way, perfusion is secured to both the head and neck vessels, as well as to the lower body vasculature below the diaphragm during isolation of the arch for reconstruction. Mean radial and femoral arterial pressures are kept in the range of 35–50 mmHg. In order to induce cerebral vasodilatation for homogenous cerebral tissue cooling, a modified alpha-stat strategy with pCO2 elevation around 50–60 mmHg is used (Figure 3).

Arch vessels and the descending aorta are clamped. Cerebral protection via RCP is commenced with 30% estimated flow (52–60 mL/kg/min). The same amount of flow is provided to the infradiaphragmatic aorta and monitored via femoral arterial pressure and somatic reflectance oximetry (Figures 4,5).
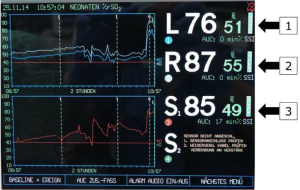
Myocardial protection is ensured by either continuous myocardial perfusion with 10% estimated flow after connecting another arterial line to the aortic root cannula (beating heart), or by cardioplegic arrest using a single shot (40 mL/kg) of cardioplegia. Arch repair includes coarctation-resection and augmentation of the aortic concavity with a patch of bovine pericardium. Patients undergoing the Norwood procedure additionally undergo atrial septectomy, division of the main pulmonary artery and Damus-Kaye-Stansel anastomosis; pulmonary perfusion is ensured by either right ventricle to pulmonary artery conduit in hypoplastic left heart syndrome or by modified Blalock-Taussig-shunt in patients with a systemic left ventricle. After the aortic and supra-aortic cross-clamps are removed, reperfusion is started until the patients are warmed up to 36 °C. Weaning from CPB is performed in the usual fashion.
Ultrasound imaging
Transfontanellar ultrasonography is investigated with a multifrequent sector probe S 4–10 (7MHz), 3D/4D curved array probe RNA 5-9-D (8MHz) and transtemporal Doppler ultrasound uses a M5S sector probe (3 MHz).
Transfontanellar examination includes B-mode scan, 2D and 3D Power- or Color-Doppler ultrasound of both hemispheres. Two-D Power- and Color-Doppler ultrasound visualizes the intensity of vessel perfusion of the main blood vessels and illustrates a functioning communication in the Circle of Willis. 3D Power- and Color-Doppler via the anterior fontanelle allows 0.5 mm cerebral tomography, and glass-body rendering of the main cerebral vessels. Pulsed-wave (Pw)-Doppler is used to measure mean TAV for intervals of 3–5 seconds. The probes are placed over the anterior fontanelle or over the right and left temporal area. The positioning and measurement with the best Color Doppler signal is selected. Ultrasound imaging with the above mentioned techniques is currently not applicable for monitoring infradiaphragmatic perfusion.
Collected data at given standard time points are compared between hemispheres (left vs. right), and between two perfusion time points (FF versus RCP) in each ipsilateral hemisphere.
Observations
Patient characteristic and outcome
Fourteen patients were monitored with a complete data-set as specified above. One patient out of this group died after the procedure; all other patients were discharged home without clinical signs of impaired neurologic function.
Cerebral sonography
Two-D and 3D Color/Power-Doppler ultrasound showed regular anatomy with a communicating Circle of Willis in all infants, with near symmetric distribution of blood flow intensity in vessels of both hemispheres during both RCP and TBP.
Comparing TAVs in contralateral vessels during both TBP and RCP, no significant differences between hemispheres were calculated, except for higher TAV in right ICA during TBP. Comparing TAVs in each vessel depending on perfusion methods, no significant differences between FF and RCP were observed. Comparison of contralateral mean levels of rSO2 did not reveal significant differences between both hemispheres, regardless of the perfusion method. Comparing rSO2 in each hemisphere between perfusion methods, there was a significant difference regarding rSO2 measured in the right frontal cortex, with higher levels during TBP when compared to RCP.
Discussion
The main concern regarding the efficiency of RCP is about adequate perfusion of the left hemisphere and the quality of subdiaphragmatic perfusion. We are one of the first groups to publish a prospective study evaluating combined transtemporal/transfontanellar ultrasound in an intraoperative setting during aortic arch repair, showing a functioning Circle of Willis for all studied patients, pointing to symmetric distribution of blood flow intensity to both cerebral hemispheres during both TBP and RCP. This impression was confirmed by bilateral comparison of cerebral TAVs and rSO2.
An exception was found for ICA-flow during TBP, which was substantiated by significantly higher TAVs in the right vessel when compared to the left. Divergent blood flow directions between patients indicate that the left ICA is being supplied from both sites, either antegrade via the hypoplastic aortic arch, or retrograde via the Circle of Willis, which is probably related to the size of the transverse arch by reflecting the amount of antegrade aortic flow. Therefore, calculated difference in TAV between both contralateral ICAs during TBP may be a result of counteracting blood flow directions in the left ICA, leading to reduction or flow signal extinction for TAV.
Difference between contralateral TAVs in both carotid arteries was evident even during RCP, however, without reaching statistical significance and with an exclusively retrograde flow-direction in the left ICA at this time point. As an explanation for that difference in flow intensity, it should be kept in mind, that even with an effective perfusion of the left hemisphere during RCP, the left ICA, when perfused from the right via Circle of Willis, presents the end of the cerebral vasculature with a “blind end” due to proximal clamping at its origin from the aortic arch, and limited run-off into the left external carotid, BA and ophthalmic artery.
Changes in direction of blood flow in the ICA following occlusion of the ipsilateral common carotid artery have been reported previously (36,37). Divergent flow-directions in the left ICA during both TBP and RCP may mirror the non-physiologic perfusion of blood vessels originating from the distal aortic arch in neonates with aortic arch hypoplasia and ductal-dependent lower body perfusion even as an inherent phenomenon. We have performed transfontanellar ultrasound sporadically in our cohort in the perioperative context and could verify a normalization of flow velocity in the left ICA and changing flow-direction in left the vertebral artery after surgery in one infant.
Assessment of bilateral SO2 did not reveal a significant difference when compared between hemispheres. Both, increased rSO2 in the right frontal cortex by comparing both methods of perfusion, and higher TAV in the right ICA by contralateral comparison during TBP, raises suspicion of increased blood flow to the right hemisphere especially during TBP. The potential dangers of excessive cerebral blood flow include cerebral edema and intracranial hemorrhage (22,25). We believe that initiation of “full-flow” bypass over the innominate artery might be responsible for early hyper-perfusion especially of the right hemisphere, which may explain why an alpha stat strategy with limitation of cerebral vasodilatation is beneficial in these patients to avoid excessive overflow. Initial “overperfusion” of the right hemisphere seems to persist during cooling despite introduction of distal aortic perfusion and adjustment of TBP between both arterial lines.
With regard to infradiaphragmatic perfusion and oxygen supply it is difficult to support statements that conclude that RCP provides adequate somatic perfusion via native collaterals, as suggested by some authors (9). After more than 15 years’ experience (including animal lab experiment and subsequent later patient observation), it is even more difficult to believe that this holds true and has been questioned by us in the past (5). A special subset of patients with large intercostal arteries may be well perfused on both sites of the diaphragm by RCP, but we would not rely on them, especially if surgery is performed under warmer conditions of moderate hypothermia around 28 °C. If you do not follow an effective strategy to perfuse the lower body with bypass, some patients will suffer from postoperative renal failure or mesenteric ischemia. We therefore rely on regional saturation plotting and femoral artery pressure monitoring during RCP, and feel very safe since we have introduced our infradiaphragmatic cannulation technique.
It is our personal bias that continuous rSO2-monitoring (NIRS) as a point of care measurement of tissue oxygenation has given us a substantial surplus in procedure safety. In analogy to our anesthesiology colleagues, we think that NIRS has become the pulse oximetry of perfusionists and cardiac surgeons.
One limitation to our observations is that TAV is only a surrogate indicator for perfusion, considering a fixed diameter of cerebral vessels during measurements. Further, the technique of transfontanellar ultrasound is investigator-dependent, and can be affected by suboptimal positioning or covering of the patient. This variation in color distribution can depend on the direction of the vessel and corresponding blood flow direction regarding the position to the probe. If the blood flow is vertical to the ultrasonic waves, a movement of blood cannot be detected. It remains unclear whether our results can be extrapolated to other RCP strategies including “real” pH-stat regime and deep hypothermia (10,12,14,15,19,22,34). A superiority to other modes of RCP or neuroprotective strategies as deep hypothermic circulatory arrest cannot be derived from our experience to date, but may become likely when gathering data from an increasing number of patients. To date, postoperative transfontanellar morphologic cerebral evaluation in five of our patients did not reveal side-dependent structural abnormalities, and did not show evidence of hyper- and/or hypo-perfusion-related injury.
In conclusion, the hypothesis of a homogenous distribution of cerebral blood flow to both hemispheres during RCP is being strengthened, using a combined transfontanellar/transtemporal approach, with 2D and 3D Color- and Power-Doppler ultrasound to visualize the Circle of Willis and the intensity of cerebral vessel perfusion during aortic arch repair. By indirect and non-invasive estimations of effective cerebral blood flow using the transcranial ultrasound methods described in our study, and regional cerebral tissue oxygenation with NIRS, it is hoped to make arch reconstruction using cardiopulmonary bypass even safer.
Acknowledgements
Most of the data and figures are related to the work by the interdisciplinary research group of Andre Rueffer and colleagues.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Bellinger DC, Wypij D, du Plessis AJ, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg 2001;121:374-83. [Crossref] [PubMed]
- Bellinger DC, Wypij D, Kuban KC, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999;100:526-32. [Crossref] [PubMed]
- Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation 1998;97:773-9. [Crossref] [PubMed]
- Bellinger DC, Rappaport LA, Wypij D, et al. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr 1997;18:75-83. [Crossref] [PubMed]
- Roerick O, Seitz T, Mauser-Weber P, et al. Low-flow perfusion via the innominate artery during aortic arch operations provides only limited somatic circulatory support. Eur J Cardiothorac Surg 2006;29:517-24. [Crossref] [PubMed]
- Rüffer A, Danch A, Gottschalk U, et al. The Norwood procedure - does the type of shunt determine outcome? Thorac Cardiovasc Surg 2009;57:270-5. [Crossref] [PubMed]
- Rüffer A, Klopsch C, Münch F, et al. Aortic arch repair: let it beat! Thorac Cardiovasc Surg 2012;60:189-94. [Crossref] [PubMed]
- Korkola SJ, Tchervenkov CI, Shum-Tim D. Aortic arch reconstruction without circulatory arrest: review of techniques, applications, and indications. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:116-25. [Crossref] [PubMed]
- Pigula FA, Gandhi SK, Siewers RD, et al. Regional low-flow perfusion provides somatic circulatory support during neonatal aortic arch surgery. Ann Thorac Surg 2001;72:401-6; discussion 406-7. [Crossref] [PubMed]
- Algra SO, Jansen NJ, van der Tweel I, et al. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation 2014;129:224-33. [Crossref] [PubMed]
- Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007;133:880-7. [Crossref] [PubMed]
- Algra SO, Kornmann VN, van der Tweel I, et al. Increasing duration of circulatory arrest, but not antegrade cerebral perfusion, prolongs postoperative recovery after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2012;143:375-82. [Crossref] [PubMed]
- Gaynor JW, Nicolson SC, Jarvik GP, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg 2005;130:1278-86. [Crossref] [PubMed]
- Myung RJ, Petko M, Judkins AR, et al. Regional low-flow perfusion improves neurologic outcome compared with deep hypothermic circulatory arrest in neonatal piglets. J Thorac Cardiovasc Surg 2004;127:1051-6; discussion 1056-7. [Crossref] [PubMed]
- Andropoulos DB, Easley RB, Brady K, et al. Neurodevelopmental outcomes after regional cerebral perfusion with neuromonitoring for neonatal aortic arch reconstruction. Ann Thorac Surg 2013;95:648-54; discussion 654-5. [Crossref] [PubMed]
- Gunn JK, Beca J, Penny DJ, et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg 2012;93:170-6. [Crossref] [PubMed]
- Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2006;131:190-7. [Crossref] [PubMed]
- McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 2007;38:736-41. [Crossref] [PubMed]
- Algra SO, Schouten AN, van Oeveren W, et al. Low-flow antegrade cerebral perfusion attenuates early renal and intestinal injury during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 2012;144:1323-8, 1328.e1-2.
- Di Eusanio M, Wesselink RM, Morshuis WJ, et al. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003;125:849-54. [Crossref] [PubMed]
- Austin EH 3rd, Edmonds HL Jr, Auden SM, et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg 1997;114:707-15, 717; discussion 715-6.
- Andropoulos DB, Stayer SA, McKenzie ED, et al. Regional low-flow perfusion provides comparable blood flow and oxygenation to both cerebral hemispheres during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 2003;126:1712-7. [Crossref] [PubMed]
- Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab 2002;22:335-41. [Crossref] [PubMed]
- Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41:1951-6. [Crossref] [PubMed]
- Polito A, Ricci Z, Di Chiara L, et al. Cerebral blood flow during cardiopulmonary bypass in pediatric cardiac surgery: the role of transcranial Doppler--a systematic review of the literature. Cardiovasc Ultrasound 2006;4:47. [Crossref] [PubMed]
- Trivedi UH, Patel RL, Turtle MR, et al. Relative changes in cerebral blood flow during cardiac operations using xenon-133 clearance versus transcranial Doppler sonography. Ann Thorac Surg 1997;63:167-74. [Crossref] [PubMed]
- Zimmerman AA, Burrows FA, Jonas RA, et al. The limits of detectable cerebral perfusion by transcranial Doppler sonography in neonates undergoing deep hypothermic low-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg 1997;114:594-600. [Crossref] [PubMed]
- Burrows FA, Bissonnette B. Monitoring the adequacy of cerebral perfusion during cardiopulmonary bypass in children using transcranial Doppler technology. J Neurosurg Anesthesiol 1993;5:209-12. [Crossref] [PubMed]
- van der Linden J, Priddy R, Ekroth R, et al. Cerebral perfusion and metabolism during profound hypothermia in children. A study of middle cerebral artery ultrasonic variables and cerebral extraction of oxygen. J Thorac Cardiovasc Surg 1991;102:103-14. [PubMed]
- Hoffman GM, Brosig CL, Mussatto KA, et al. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 2013;146:1153-64. [Crossref] [PubMed]
- Kussman BD, Wypij D, Laussen PC, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation 2010;122:245-54. [Crossref] [PubMed]
- Chen Y, Tailor DR, Intes X, et al. Correlation between near-infrared spectroscopy and magnetic resonance imaging of rat brain oxygenation modulation. Phys Med Biol 2003;48:417-27. [Crossref] [PubMed]
- Scott JP, Hoffman GM. Near-infrared spectroscopy: exposing the dark (venous) side of the circulation. Paediatr Anaesth 2014;24:74-88. [Crossref] [PubMed]
- Andropoulos DB, Stayer SA, McKenzie ED, et al. Novel cerebral physiologic monitoring to guide low-flow cerebral perfusion during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg 2003;125:491-9. [Crossref] [PubMed]
- Park YH, Song IK, Lee JH, et al. Intraoperative trans-fontanellar cerebral ultrasonography in infants during cardiac surgery under cardiopulmonary bypass: an observational study. J Clin Monit Comput 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Bates MC, Dorros G, Parodi J, et al. Reversal of the direction of internal carotid artery blood flow by occlusion of the common and external carotid arteries in a swine model. Catheter Cardiovasc Interv 2003;60:270-5. [Crossref] [PubMed]
- Tindall GT, Odom GL, Dillon ML, et al. Direction of blood flow in the internal and external carotid arteries following occlusion of the ipsilateral common carotid artery. Observations in 19 patients. J Neurosurg 1963;20:985-94. [Crossref] [PubMed]


