
Abdominal catheter-induced intussusception following renal transplantation in two pediatric recipients: 2 cases report and literature review
Highlight box
Key findings
• Abdominal catheter can be a lead point to induce intussusception.
What is known and what is new?
• Postoperative intussusception is rare and the risk factors need to be investigated.
• Abdominal catheter can induce intussusception as a lead point.
What is the implication, and what should change now?
• Postoperative intussusception is rare and the risk factors need to be investigated.
• Health practitioners should consider catheter as a pathologic lead point to induce intussusception if an acute abdominal disorder is observed in children.
Introduction
Intussusception is the most common cause of acute intestinal obstruction in children (1). The most prevalent kind of intussusception is idiopathic and ileocolonic intussusception (1,2), which has an incidence of 18–56 per 100,000 (3). Delayed diagnosis of intussusception may induce severe bowel obstruction, resulting in abdominal distension, dehydration, intestinal necrosis, perforation, shock, and even death (4,5). Postoperative intussusception (POI) is a rare and severe condition reported after treatment for Merkel’s diverticulum, retroperitoneal tumor, and megacolon in children (6). Pediatric intussusception following renal transplantation is scantily reported; however, the risk factors need to be investigated. Herein, we report 2 cases of post-transplant intussusceptions which were triggered by peritoneal dialysis catheter and intraperitoneal drainage catheter, respectively. We proposed that catheter can be a leading point to induce intussusception, which may have been overlooked. This experience would contribute to discerning the risk factors for POI. We present the following article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-257/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients’ parents for publication of this case report and accompanying images. Copies of the written consent are available for review by the editorial office of this journal.
Case 1
A 4.6-year-old girl with end-stage renal disease (ESRD) from nephrotic syndrome (NS) underwent peritoneal dialysis catheterization and started dialysis on 17 April 2018. She received allograft renal transplantation via an extraperitoneal approach on 16 March 2019. During the post-transplant period, the maintenance immunosuppressants included tacrolimus, mycophenolate mofetil (MMF), and prednisone. She was admitted on 17 June 2019 with intermittent pain of a 1-week duration. Her self-reported symptoms did not include nausea, vomiting, diarrhea, or abdominal distension. On admission, physical examination revealed a flat abdomen with a peritoneal dialysis catheter well fixed in the right lower quadrant.
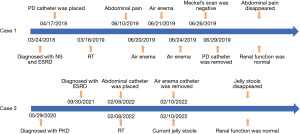
Blood routine test revealed the following: leukocytes 4.83×109/L, hemoglobin 118 g/L, and creatinine concentration 106 µmol/L, and she returned a weak positive for urine protein. In addition, here leukocytes were measured as 165×106/L in the drainage fluid. Abdominal plain film showed a peritoneal dialysis tube running in the ileocecal part and no clear signs of intestinal obstruction. Ultrasound indicated multiple concentric rings on her right abdomen. Ileocolonic intussusception was diagnosed. The intussusception was successfully managed using air enema (Figure 1). However, the child experienced 2 recurrent intussusceptions within the following 3 days. Meckel’s scan was conducted after the third successful reduction, and the result was negative. Due to recurrent episodes of intussusception, peritoneal dialysis catheter removal was performed, and no further recurrence was observed. The patient’s intermittent pain subsequently disappeared. The child’s transplanted kidney function was constantly monitored. At the end of follow-up (36 months after renal transplantation), creatinine concentration was 37 µmol/L, and urine protein was negative.
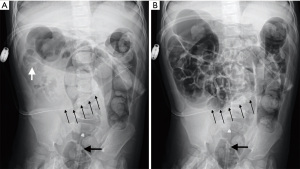
Case 2
A 1.8-year-old boy was diagnosed with polycystic kidney disease (PKD) in the first month of life. Genetic testing revealed PKD1 gene mutation and ultrasonography showed bilateral polycystic kidneys. The patient was diagnosed with ESRD at age 1.3 years and had displayed postnatal growth delays. He (weight: 11.0 kg, height: 80.0 cm) was admitted and received allogeneic renal transplantation via a transperitoneal approach on 8 February 2022. During the transplant procedure, intraperitoneal and perirenal drainage catheters were placed. The maintenance immunosuppressants included tacrolimus, MMF, and prednisone. He produced currant jelly-like stools on 10 February 2022 without vomiting or abdominal distension.
On examination, no abdominal mass or signs of peritonitis were found. The tip of the abdominal drainage catheter was palpated in the right lower quadrant. Blood leukocytes were 3.42×109/L, hemoglobin was 88 g/L, and serum creatinine was 415 µmol/L. Ultrasound revealed an epigastric mass of approximately 8.0×3.2 cm in size presenting a concentric circle sign. Ileocolonic intussusception was diagnosed. Repeated air enema reductions were unsuccessful, even with repeated massaging of the ileocecal section. The intussusception was finally managed when the abdominal drainage catheter was removed (Figure 2). The child discharged normal feces the following day, and intussusception never recurred. At 3 months after renal transplantation, his urea level was 7.4 mmol/L and serum creatinine was 30 µmol/L. Figure 3 presents the timeline of key time points, clinical manifestations, interventions, and follow-ups of the 2 cases.
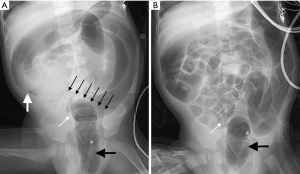
Literature review
A literature search using the keywords “renal/kidney transplantation and intussusception” in the PubMed, Web of Science, and Embase databases was conducted up to 25 February 2022. Among all the English literature, a total of 16 articles were retrieved after excluding duplicate reports. A total of 8 cases of intussusception following renal transplantation in humans had been reported in the above-mentioned databases (7-14), and 2 patients (25%) received 2 renal transplantations. Of the 8 patients, 5 (62.5%) were male and 3 (37.5%) were female, and the mean age was 37 years old. Only Beek et al. reported a case of small bowel intussusception following renal transplantation in an 11-year-old child (14). Most cases presented with bowel obstruction. Intussusception occurred more than 3 months after renal transplantation. A total of 5 cases (62.5%) occurred in the small bowel, 2 (25%) in the ileocecal region, and 1 (12.5%) in the colon. Lead points included post-transplant lymphoproliferative disorder (PTLD) in 3 patients (37.5%), and acute appendicitis, tubulovillous adenoma, tuberculosis, lymphocele, and firm adhesions each occurred in 1 patient (12.5%). All patients underwent surgeries, among whom 2 patients (25%) died, whereas 6 patients (75%) recovered uneventfully (Table 1).
Table 1
| Case | Author/Ref. | Age (year) | Sex | Symptom | Time after renal transplantation | Location of intussusception | Leading point | Type of reduction | Outcome | Geographic location |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Akl et al. (7) | 48 | M | Constipation, tenderness at right iliac region | 14 years | Ileocolic | Acute appendicitis | Surgery | Recovered | Egypt |
| 2 | Lee et al. (8) | 23 | F | Not specific | 7 years | Small bowel | PTLD | Surgery | Recovered | GBR |
| 3 | Wassmer et al. (9) | 59 | F | Septic shock | 20 years (1st transplant); 7 years (2nd transplant) | Small bowel | Tubulovillous adenoma | Surgery | Died | Switzerland |
| 4 | Atici et al. (10) | 50 | M | Intestinal obstruction | 15 years (1st transplant); 5 months (2nd transplant) | Jejunojejunal | PTLD | Surgery | Recovered | Turkey |
| 5 | Mohapatra et al. (11) | 27 | M | Fever, abdominal pain, diarrhea, mass | 3 years | Ileocolic | Tuberculosis | Surgery | Died | India |
| 6 | Alberici et al. (12) | 19 | M | Acute abdominal pain | 11 years | Ileal | PTLD | Surgery | Recovered | Italy |
| 7 | Bury et al. (13) | 59 | F | Bowel obstruction | 3 months | Colic | Lymphocele | Surgery | Recovered | Poland |
| 8 | Beek et al. (14) | 11 | M | Colicky pain, bilious vomiting, abdominal distension | 2 years | Jejunal | Firm adhesions | Surgery | Recovered | Netherlands |
M, male; F, female; PTLD, post-transplant lymphoproliferative disease.
Discussion
Intussusception is the second most common etiology of abdominal emergency after appendicitis in children (15). Idiopathic intussusception is the most common type in infants and young children. Secondary intussusception is caused by organic lesions, such as diverticula, polyps, tumors, and intestinal wall hematomas, which function as a lead point (1,2). PTLD is the most common cause of intussusception in adult kidney recipients (7,10). Here, we report 2 cases of intussusceptions in pediatric renal allograft recipients. At our center, only these 2 cases of POI occurred (460 pediatric renal transplantations were recorded and followed up), with an incidence of approximately 0.4%. To the best of our knowledge, we are the first to report catheter-induced intussusception in renal transplant recipients.
In previous literature, intussusceptions induced by feeding catheters have been reported (16-18). However, the exact mechanism of intussusception due to the involvement of catheter is not understood. Possible theories have been proposed: (I) tip of catheter acting as a pathologic leading point, (II) catheter-associated inflammatory reaction causing hypertrophy of the mucosa, which forms a leading point (19). Case 1 had proteinuria after renal transplantation due to recurrent primary kidney disease, the peritoneal dialysis catheter was retained in case of severe graft dysfunction. Recurrent intussusceptions occurred around the entrance point, and catheter-associated intraperitoneal infection was observed. In case 2, the catheter was displaced, the tip of the catheter shifted, and the intussusception was near the tip of the catheter.
As was seen in our case reports, the prevalent type of intussusception is ileocolonic intussusception. Age and duration of illness affect the clinical manifestations of ileocolic intussusception. Intussusception in younger children is characterized by bloody stools or intestinal obstruction, whereas in older children it is characterized by nonspecific abdominal pain (20). This is consistent with the presentations of our case report. Nevertheless, abdominal catheter as a pathologic lead point may be overlooked due to the lack of knowledge. Ultrasound is the preferred diagnostic method, for which the typical presentations include multiple concentric rings on transverse plane and the pseudo-kidney sign on longitudinal plane (21). However, ultrasound does not show all lead points, the need for further imaging examinations should be determined individually (22). As in our cases, catheters were not visible in the ultrasound images of intussusceptions after a thorough review. Although abdominal plain film may not reveal any abnormality, it helps to locate the catheter.
As it remains a challenging task to manage intussusception triggered by a lead point, enema reduction is recommended (22). Catheter-induced intussusception should be managed by changing the catheter. In case 1, intussusception was reducible without recurrence via pneumatic enema yet catheter removal was the premise. Renal transplantation is suggested to perform via transperitoneal approach in children weighing less than 20 kg and large kidney graft (23,24). As a drainage catheter is necessary, attention needs to be paid to its placement in this procedure. In addition, potential technologies and strategies are suggested: (I) management of catheter-associated intraperitoneal infection, (II) enteral nutrition is started after the recovery of intestinal function to avoid irregular bowel movements, and (III) selecting appropriate catheter size based on the recipient’s physique.
In this paper, we presented a novel lead point to induce intussusception. Given the severe complications resulting from delayed diagnosis and treatment, it is important to pay attention to this possibility. This finding may have significant clinical implications. It is reported that POI mostly occurs within 10 days after surgery in children, and the risk factors include surgical trauma, electrolyte imbalance, and tissue dehydration (6). Despite that we have only reported 2 cases of intussusception following renal transplantation, the experience aids our understanding of catheter as another risk factor for POI. POI is quite difficult to predict before surgery, and a delayed diagnosis may result in serious consequences; clinicians must not only be able to diagnose it swiftly, but also identify its risk factors. We hope that this article also raises attention in POI, especially when abdominal catheters are used. However, there was still a deficiency: although ultrasound confirmed intussusception in these 2 cases, the catheters were not visualized in the ultrasound images. Ultrasound does not reveal all lead points as stated previously. More cases are required to validate this point.
In conclusion, despite its rarity, if an acute abdominal disorder is observed in children following renal transplantation, intussusception should not be ignored. Catheter can be a lead point, no matter how long it has been placed. Ultrasound is capable of identifying intussusception, and abdominal plain films aid in the evaluation of catheters as a risk factor.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-257/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-257/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients’ parents for publication of this case report and accompanying images. Copies of the written consent are available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee DH, Kim SJ, Lee HJ, et al. Identifying Predictive Factors for the Recurrence of Pediatric Intussusception. Pediatr Gastroenterol Hepatol Nutr 2019;22:142-51. [Crossref] [PubMed]
- Saka R, Sasaki T, Matsuda I, et al. Chronic ileocolic intussusception due to transmural infiltration of diffuse large B cell lymphoma in a 14-year-old boy: a case report. Springerplus 2015;4:366. [Crossref] [PubMed]
- Parashar UD, Holman RC, Cummings KC, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics 2000;106:1413-21. [Crossref] [PubMed]
- Bruce J, Huh YS, Cooney DR, et al. Intussusception: evolution of current management. J Pediatr Gastroenterol Nutr 1987;6:663-74.
- Sharma P, Al-Sani F, Saini S, et al. Point-of-Care Ultrasound in Pediatric Diagnostic Dilemmas: Two Atypical Presentations of Intussusception. Pediatr Emerg Care 2019;35:72-4. [Crossref] [PubMed]
- Jiang W, Tang W, Geng Q, et al. Postoperative intussusception in infants and children: a report of seven cases. J Biomed Res 2012;26:66-8. [Crossref] [PubMed]
- Akl A, El Saftawy M, El Shaib M, et al. Colonic intussusception 14 years post kidney transplantation. Transpl Int 2017;30:528.
- Lee C, Vincentelli H, Visuri J, et al. Epstein-Barr Virus-Negative Diffuse Large B-Cell Post-transplant Lymphoma in an Epstein-Barr Virus-Positive Recipient. Cureus 2021;13:e18134. [Crossref] [PubMed]
- Wassmer CH, Abbassi Z, Ris F, et al. Intussusception in an Immunocompromised Patient: A Case Report and Review of the Literature. Am J Case Rep 2020;21:e919974. [Crossref] [PubMed]
- Atici SD, Arican C, Avci EK, et al. Intussusception Can Be the First Sign of Post-transplant Lymphoproliferative Disease. Transplant Proc 2019;51:1184-6. [Crossref] [PubMed]
- Mohapatra A, Basu G, Sen I, et al. Tuberculosis in a renal allograft recipient presenting with intussusception. Indian J Nephrol 2012;22:52-6. [Crossref] [PubMed]
- Alberici I, Mencarelli F, Melchionda F, et al. A plasmocitoma like PTLD epstein-barr virus (EBV) negative after kidney transplantation. Pediatrc Nephrol 2012;27:1829.
- Bury K, Śmietański M, Gumiela P, et al. Ventral hernia following lymphocele fenestration in a patient after renal transplantation – a case report and treatment strategy. Wideochi Inne Tech Mmaloinwazyjne 2010;5:161-5.
- Beek FJ, Rövekamp MH, Bax NM, et al. Ultrasound findings in post-operative jejunojejunal intussusception. Pediatr Radiol 1990;20:601. [Crossref] [PubMed]
- Khan S, Hartman L, Navarro YJS, et al. Pediatric Covid-19 mesenteric lymphoid hyperplasia associated intussusception: A case report and literature review. J Pediatr Surg Case Rep 2021;73:101988. [Crossref] [PubMed]
- Noake T, Yoshida S, Fujita H, et al. Intussusception during enteral nutrition: a case report. Kurume Med J 2001;48:237-40. [Crossref] [PubMed]
- Dholaria S, Lakhera KK, Patni S. Intussusception: a Rare Complication After Feeding Jejunostomy; a Case Report. Indian J Surg Oncol 2017;8:188-90. [Crossref] [PubMed]
- Komagamine J, Noritomi D. Jejunal Intussusception Caused by a Nasointestinal Ileus Tube. Eur J Case Rep Intern Med 2022;9:003161. [Crossref] [PubMed]
- Krishna S, Prabhu R, Thangavelu S, et al. Jejuno-jejunal intussusception: an unusual complication of feeding jejunostomy. BMJ Case Rep 2013;2013:bcr2013200219. [Crossref] [PubMed]
- Park IK, Cho MJ. Clinical Characteristics According to Age and Duration of Symptoms to Be Considered for Rapid Diagnosis of Pediatric Intussusception. Front Pediatr 2021;9:651297. [Crossref] [PubMed]
- Kim JW, Lee BH, Park SG, et al. Factors predicting malignancy in adult intussusception: An experience in university-affiliated hospitals. Asian J Surg 2018;41:92-7. [Crossref] [PubMed]
- Navarro OM, Daneman A, Chae A. Intussusception: the use of delayed, repeated reduction attempts and the management of intussusceptions due to pathologic lead points in pediatric patients. AJR Am J Roentgenol 2004;182:1169-76. [Crossref] [PubMed]
- Hebert SA, Swinford RD, Hall DR, et al. Special Considerations in Pediatric Kidney Transplantation. Adv Chronic Kidney Dis 2017;24:398-404. [Crossref] [PubMed]
- Badet L, Matillon X, Codas R, et al. Pediatric kidney transplantation. Prog Urol 2016;26:1045-52. [Crossref] [PubMed]


