
Adverse events after repair of tetralogy of Fallot: prediction models by machine learning of a retrospective cohort study in western China
Highlight box
Key findings
• Differential pressure of the right ventricular outflow tract, cardiopulmonary bypass time, and transannular patch repair are risk factors, and SPO2 is a protective factor for adverse events after complete tetralogy of Fallot repair.
What is known and what is new?
• The reference point was 116.5 minutes of CPB time, 88% of SPO2 and the reference point of differential pressure of the right ventricular outflow tract cutoff was 70 mmHg.
What is the implication, and what should change now?
• Patients should receive individualized treatment according to the patients different condition.
Introduction
Tetralogy of Fallot (TOF) is the most common cyanogenic congenital heart disease, accounting for approximately 10% of all congenital heart diseases. Early surgical repair in the first year of life is now commonly performed at most major centres. Intraoperative mortality is less than 2% to 3% when the repair is performed at an early age (1). Peritoneal dialysis and pneumonia, with a reported incidence of 62.4% (2), are still common and have become important factors that often prolong the length of hospital stay and increase expenses (3), imposing a heavy burden on society and families. In developing areas, such as western China, many patients undergo surgery after the optimal period. The mortality rate and incidence of postoperative adverse events are higher in these areas than in developed areas, just as the intraoperative mortality rate is higher in developing countries than in developed countries (1). Surgeons should make more of an effort to help parents understand the disease severity and inform them of the varying probability of complications among patients. Moreover, due to the slow spread of medical information, parents are often anxious, doubt the treatment and become argumentative when their children experience adverse events. Hence, an accurate and reliable preoperative predictive model of adverse events would be very meaningful not only for individualized treatment but also for communication between the surgeons and parents.
With the widespread application of artificial intelligence (AI) in the medical field, machine learning (ML) has rendered medical research involving prediction models more scientific and practical (4,5); however, we note that in the area of TOF, this kind of research is scarce. Therefore, we combined AI with basic clinical metrics and echocardiography to provide an accurate and reliable prediction model for clinical work. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-246/rc).
Methods
Data and patients
Clinical data for patients with TOF who underwent anatomical correction from January 2002 to January 2022 were collected. All patients underwent computed tomography angiography (CTA) and echocardiography. Patient characteristics, such as weight and age, pulmonary venous anatomy, demographics, preoperative data, intraoperative data, postoperative data and adverse events, were recorded.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of our hospital (Approval No. 2021-352), and informed consent was obtained from all patients’ parents or legal guardians.
All patients included were diagnosed with TOF (ICD-10: Q21.3) and underwent standard primary complete repair (ICD-9-CM-3: 35.81). Patients were excluded if (I) they also had pulmonary atresia, major aortopulmonary collateral arteries, or an atrioventricular (AV) septal defect; (II) they had undergone palliative procedures, such as right ventricular (RV) outflow ballooning/stenting or arterial duct stenting; or (III) they had undergone staged repair with unifocalization of the pulmonary arteries and conduit placement subsequently followed by ventricular septal defect (VSD) closure.
Definition and predictors
Adverse events included arrhythmia, peritoneal dialysis, pneumonia, and death occurring before discharge. Arrhythmia involved complete AV blockage requiring pacemaker implantation and ventricular tachycardia or supraventricular tachycardia and other arrhythmias requiring treatment. Pneumonia was defined as pneumonia developing at 48 hours or more after the initiation of mechanical ventilation, including both of the following criteria: fever (body temperature greater than 38.5 ℃) and positive cultures of sputum obtained by suction. The indications for peritoneal dialysis were as defined by Baskin (6).
Age, sex, weight, pulmonary valve annulus Z score (Z-index), transannular patch repair (TP repair), preoperative SPO2, aortic cross-clamp (ACC) time, cardiopulmonary bypass (CPB) time, opening of the pulmonary valve (PvO), differential pressure of the right ventricular outflow tract (RVOTDP or DP), pulmonary valve leaflet (PV-leaflet; two or three), annulus of the pulmonary artery (annulus), VSD size, left ventricular end-diastolic volume index (LVEDI), haematocrit (HCT), ejection fraction (EF), McGoon index (M-index), and lateral branch occlusion (branch occlusion) were used as predictors. In addition, the surgical team and the year the surgery was performed were analysed to avoid potential bias. However, these factors were not included in the models because they could not be used as available predictors.
Surgical technique
All patients underwent surgery with a standard median sternotomy and CPB. A Gore-Tex patch was used to close the VSD, and the right ventricular outflow tract (RVOT) was dredged according to the pulmonary artery anatomy (3). The pulmonary valve annulus was preserved if a Hegar bougie of the appropriate size could be passed smoothly after supravalvular and subvalvular stenoses were completely removed. If a Hegar bougie one size lower than the appropriate size could be passed and the RV/left ventricular (LV) pressure ratio (RV pressures were measured by direct needle puncture) was above 0.7, TP repair was performed.
Statistical analysis
Means and standard deviations (SDs) are used to display continuous data. The Shapiro-Wilk test was used to evaluate the normality of distributions, and an unpaired t-test was used to compare continuous variables between two groups. The Mann-Whitney U test was used to compare data for variables without a normal distribution. Data for categorical variables were compared by the chi-square test. Standardized differences less than 0.10 indicated an absolute balance (7). A multiple collinearity test was used to remove confounding variables, and least absolute shrinkage and selection operator (LASSO) regression was utilized to rank the importance of predictors. Univariate logistic regression (LR) and subgroup analyses were used in this study. The variables confirmed by the univariate LR, LASSO regression and subgroup analyses were selected as the main variables affecting the occurrence of adverse events and further analysed by propensity score matching (PSM), trend analysis and restricted cubic spline (RCS) analysis.
In the ML model, LR, Gaussian Naive Bayes (GNB), multilayer perceptron (MLP), support vector machine (SVM) and K-nearest neighbour (KNN) were chosen, and R version 3.6.3 and Python version 3.7 were used to analyse statistics. More detailed parameter settings are provided in Table S1. The whole dataset was randomly divided into training and test sets at a ratio of 4:1. The guidelines of the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) Initiative were used to report the risk prediction model (8). A receiver operating characteristic (ROC) curve was used to analyse the discrimination of each model. A comprehensive assessment by the area under the curve (AUC) of the training and test sets was performed to select the most reliable model and avoid overfitting. The Hosmer-Lemeshow test was used to identify the most reliable model (9). A Brier score less than 0.25 was considered to indicate that a model had good comprehensive properties. A formula and a dynamic nomogram that could exhibit the clinical application of the model were provided on a web page, and the clinical utility of the model was evaluated by decision curve analysis (DCA) (10).
Results
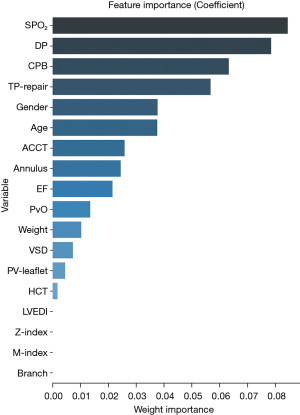
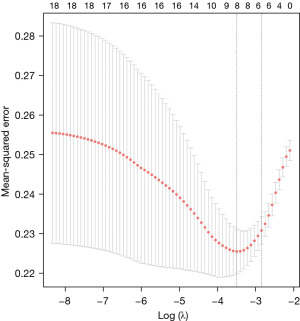
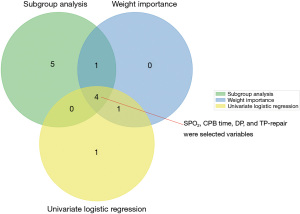
The flow chart of the study is shown in Figure 1. A total of 281 participants were included, with adverse events occurring in 131 of them. The variance of all variables was less than 10. Hence, all the variables passed the multiple collinearity test. Nearly ninety percent of the patients with adverse events underwent TP repair; the integrity of the pulmonary valve was preserved in one-third of patients with no adverse events (P=0.01). Differences in SPO2, CPB time, age, PvO, DP, annulus, M-index, Z-index, TP repair and LVEDI were observed between the two groups. Only the distributions of branch occlusion and HCT were well balanced (Table 1). Significant differences in DP (P=0.003), CPB time (P=0.016), sex (P=0.047), TP repair (P=0.037) and SPO2 (P=0.001) were detected by univariate LR (Table 2). Significant differences in the year of surgery [odds ratio (OR) =0.938–1.021, P=0.325] and surgical team (OR =0.379–2.156, P=0.827) were not observed. The order of feature importance was SPO2, DP, CPB, TP repair, sex, age, etc. (Figure 2). The detailed variables of feature importance are shown in Table S2. The minimum criterion lambda had a value of 0.058, showing that excessive convergence would not occur when analysing no more than 6 variables in detail (Figure 3). A Venn diagram was used to select the main variables according to the three analytical methods mentioned above, resulting in the selection of SPO2, CPB time, DP, and TP repair (Figure 4).
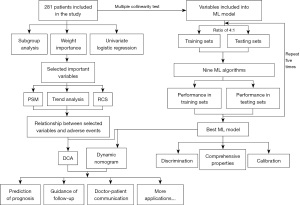
Table 1
| Variable | Adverse event (n=131) | Non-adverse event (n=150) | P value | Standardized differences |
|---|---|---|---|---|
| Gender, n | Male/female (88/43) | Male/female (85/65) | 0.08 | 0.22 |
| Age (months), median (IQR) | 12 (8.0–17.0) | 12 (8.0–25.0) | <0.001* | 0.73 |
| Weight (kg), median (IQR) | 8.9 (7.5–10) | 9 (7.0–10.6) | 0.09 | 0.20 |
| HCT (%), median (IQR) | 42.8 (37.9–48.7) | 40.75 (36.78–45.78) | 0.71 | 0.04∆ |
| SPO2 (%), median (IQR) | 84 (75–91) | 89 (81.75–95.00) | <0.001* | 0.52 |
| CPB-time (min), median (IQR) | 119 (106–140) | 113 (98.75–128.0) | 0.01* | 0.69 |
| ACC-time (min), median (IQR) | 78 (68–87) | 75 (65–86.25) | 0.06 | 0.19 |
| EF (%), median (IQR) | 64 (61–69) | 66 (61–71) | 0.25 | 0.14 |
| VSD (mm), median (IQR) | 13.5 (12–15) | 13.30 (12.0–15) | 0.46 | 0.10 |
| Z-index, median (IQR) | −1.82 (−2.75, −1.26) | −1.46 (−2.4, −0.22) | 0.01* | 0.67 |
| M-index, median (IQR) | 1.38 (1.23–1.64) | 1.50 (1.28–1.71) | 0.04* | 0.52 |
| LVEDI (mL/m2), median (IQR) | 41.76 (33.49–53.05) | 49.80 (37.36–59.48) | 0.01* | 0.35 |
| PvO (cm), median (IQR) | 4.7 (3.7–5.4) | 5.0 (4.1–6.2) | <0.001* | 0.47 |
| DP (mmHg), median (IQR) | 71 (60–86) | 65 (55–81) | <0.001* | 0.83 |
| Annulus (cm), median (IQR) | 9.2 (7.8–10.0) | 10.0 (8.5–12.0) | <0.001* | 0.48 |
| PV-leaflet, n | Yes/no (50/81) | Yes/no (54/96) | 0.71 | 0.23 |
| TP-repair, n | Yes/no (115/16) | Yes/no (103/47) | 0.01* | 0.46 |
| Branch occlusion, n | Yes/no (10/121) | Yes/no (12/138) | 0.99 | 0.01∆ |
*, P<0.05; ∆, standardized differences less than 0.10 indicated absolute balance. IQR, interquartile range; HCT, haematocrit; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; EF, ejection fraction; VSD, ventricular septal defect; Z-index, pulmonary valve annulus Z score; M-index, McGoon index; LVEDI, left ventricular end-diastolic volume index; PvO, opening of the pulmonary valve; RVOTDP or DP, differential pressure of the right ventricular outflow tract; PV-leaflet, pulmonary valve leaflet; TP repair, transannular patch repair.
Table 2
| Factors | OR | 95% CI (lower) | 95% CI (higher) | P value |
|---|---|---|---|---|
| Annulus | 0.915 | 0.651 | 1.255 | 0.549 |
| DP | 1.024 | 1.008 | 1.04 | 0.003* |
| PvO | 0.947 | 0.742 | 1.199 | 0.655 |
| LVEDI | 1.003 | 0.987 | 1.019 | 0.683 |
| M-index | 1.036 | 0.453 | 2.381 | 0.933 |
| Z-index | 1.027 | 0.668 | 1.625 | 0.899 |
| VSD | 1.055 | 0.942 | 1.184 | 0.356 |
| EF | 0.971 | 0.926 | 1.017 | 0.212 |
| ACC-time | 0.974 | 0.944 | 1.003 | 0.084 |
| CPB-time | 1.025 | 1.005 | 1.046 | 0.016* |
| SPO2 | 0.949 | 0.92 | 0.978 | 0.001* |
| HCT | 0.998 | 0.974 | 1.012 | 0.757 |
| Weight | 0.986 | 0.817 | 1.189 | 0.879 |
| Age | 0.986 | 0.957 | 1.015 | 0.324 |
| PV-leaflet | 1.184 | 0.677 | 2.076 | 0.554 |
| Gender | 1.757 | 1.013 | 3.085 | 0.047* |
| TP-repair | 2.193 | 1.063 | 4.68 | 0.037* |
| Branch occlusion | 0.966 | 0.338 | 2.704 | 0.948 |
*, P<0.05. OR, odds ratio; CI, confidence interval; RVOTDP or DP, differential pressure of the right ventricular outflow tract; PvO, opening of the pulmonary valve; LVEDI, left ventricular end-diastolic volume index; M-index, McGoon index; Z-index, pulmonary valve annulus Z score; VSD, ventricular septal defect; EF, ejection fraction; ACC, aortic cross-clamp; CPB, cardiopulmonary bypass; HCT, haematocrit; PV-leaflet, pulmonary valve leaflet; TP repair, transannular patch repair.
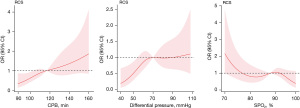
CPB time and DP were identified as the main risk factors for adverse events. The reference point [hazard ratio (HR)/OR =1] was 116.5 minutes of CPB time or 70 mmHg of DP (Figure 5). The results indicated that the risk of adverse events would significantly increase with a CPB time longer than 116.5 minutes or a DP higher than 70 mmHg. SPO2 was a protective factor, with a reference point of 88% (Figure 5).
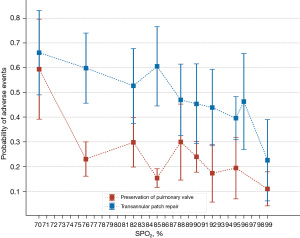
Sixty-three patients were included in the TP repair group and pulmonary valve preservation group after PSM (available online: https://cdn.amegroups.cn/static/public/tp-22-246-1.xlsx). The incidence of adverse events in the TP repair group was slightly higher than that in the pulmonary valve preservation group [(30/63) 47.6% vs. (20/63) 31.7%, P=0.07].
We also performed a trend analysis to observe whether different TP repair strategies would influence adverse events in different SPO2 groups (Figure 6). The results show that pulmonary valve preservation can reduce the incidence of adverse events at any oxygen saturation.
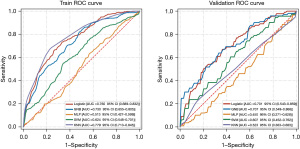
According to the principle of AI models, we included all variables in model construction. The best-performing models for the training set were the LR model and GNB model, with mean AUCs of 0.760 and 0.730, respectively. The performances of the LR model and GNB in the test set were also good, with mean AUCs of 0.701 and 0.707, respectively (Figure 7). The details of the five testing processes are shown in Tables S3,S4.
Next, we focused on the LR model. To analyse the discrimination capability of the model, we performed a detailed analysis of the individual LR model. The specificity, sensitivity and accuracy were 0.716, 0.732 and 0.721, respectively, and the AUC was 0.760. Therefore, the discrimination of the model was good (Figure S1).
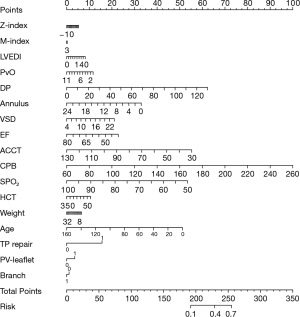
Model calibration was evaluated by the Hosmer-Lemeshow test (Figure S2). No significant difference was found between the observed and expected values (mean absolute error =0.061). Furthermore, the model showed good comprehensive performance, with a Brier score of 0.129. Coefficients of variables were used to construct a nomogram (Figure 8) to predict adverse events after radical correction of TOF. The dynamic nomogram can be used online (https://ml-cqmu.shinyapps.io/DynNomapp-TOF/), and the detailed parameters of the model can also be found on this webpage.
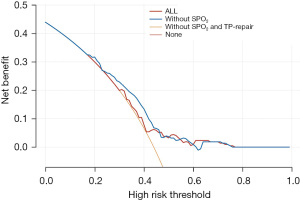
SPO2 and TP repair were important parameters according to the clinical application and analysis in our study. Hence, DCA was performed for the adverse events and showed that the model combined with SPO2 and TP repair promotes clinical decision making (Figure 9).
Discussion
Adverse events require additional drug intervention, with some of them even necessitating reoperation, which greatly increases surgical trauma to the patient and prolongs the ICU or hospital stay. However, we still have little knowledge regarding the related issues, such as the risk factors for adverse events and the cut-off points of such risk factors. With such unanswered questions, we chose the above complications as assessment criteria and constructed a prediction model.
Considering that a model to predict adverse events is urgently needed, we collected the clinical data of 281 children. After screening, we successfully constructed 5 prediction models using various AI methods. The LR model and GNB model were found to have relatively stable performances in both the training set and the test set. Since the algorithm of the LR model is simpler and easy for clinicians to use, we paid more attention to the detailed performance of the LR model. The LR model was evaluated by cross validation, ROC analysis, calibration curves, and DCA. As the predictors used in our study are available at the completion of surgery, prediction using this model can be carried out early, without a long follow-up time. To our knowledge, this is the first model to use AI for the prediction of adverse events in cases of TOF. This model can guide better preoperative evaluations of patients’ conditions.
Early repair of TOF is generally advised to alleviate myocardial fibrosis and mitochondrial damage to myocardial cells, which result from long-term anoxia and correlate positively with SPO2 and the pressure of the RVOT (11-13). Studies have reported the timing of the repair in a large cohort of infants and found the optimal age to be between 3 and 11 months (14,15). Despite this, patients are individuals, and different patients have different disease conditions. Not all patients will be suitable for the same surgical strategy or undergo surgery at the same age, especially in Chongqing, a city in western China with less-developed medical technology. Thus, some objective and definite predictors are needed to narrow the age at surgery.
In the past, parents may have found it difficult to choose an appropriate time for their child to undergo surgery. With the help of this prediction model, the SPO2 and RVOT pressure cut-off points can be used to divide patients into high-risk (e.g., SPO2 lower than the cut-off point) and low-risk (e.g., SPO2 higher than the cut-off point) groups preoperatively. For patients whose preoperative SPO2 is higher than 88% or whose RVOT pressure is lower than 70 mmHg, parents can be advised based on evidence that an older age (such as older than 6 months), at which CPB is more tolerable, would be more suitable for the surgery. For patients whose preoperative SPO2 is lower than 88% or whose RVOT pressure is higher than 70 mmHg, a younger age would be recommended to alleviate myocardial fibrosis and mitochondrial damage to myocardial cells.
With the help of this model, better communication with patients can be conducted preoperatively, allowing parents to understand the rate of adverse events and the severity of disease. Due to the complex haemodynamics of TOF, communication with parents regarding haemodynamics and cardiac abnormalities may not resolve their concerns; thus, a more effective method is needed. This model offers visualization and is concrete, making the information easier to understand. More accurate data also allow for improved trust during communication.
Analysis of risk factors for postoperative adverse events can guide the medical community to improve surgical strategies, avoid, minimize or even eliminate risk factors, and ultimately reduce the incidence of adverse events. For example, to decrease the CPB time, if a monocusp is needed for TP repair, another group of surgeons would perform this work during the operation. Based on our findings, TP repair should be carefully selected during the operation, and the pulmonary annulus should be retained as much as possible. For patients whose pulmonary annulus may be destroyed, monocusps are strongly recommended, and the RV/LV pressure ratio should also be considered.
Unsurprisingly, preoperative SPO2 and RVOT pressure were risk factors. Hypoxia and RVOT obstruction lead to pathological changes such as RV hypertrophy and myocardial interstitial fibrosis (16). Chronic hypoxia is a major stimulus for myocardial remodelling and causes detrimental histopathological alterations in cardiac myocytes and fibrosis (13,17). Previous studies have shown varying degrees of histopathological changes and mild fibrosis in muscle specimens from TOF patients (11,18). Some studies found the function of both the left and right ventricles to be damaged (19), which is also believed to be related to myocardial fibrosis (20). Notably, TP repair can lead to pulmonary regurgitation (PR), inducing RV volume overload and often progressive RV dilation, which may include the development of tricuspid regurgitation and RV dysfunction.
In addition, we analysed the influences of the year in which the surgery was conducted and differences in the surgical team on the outcome. The results showed that the year in which surgery was conducted and differences in the surgical team did not influence the outcome. The reasons for these findings might be as follows: firstly, all the patients underwent standard primary complete repair, and the major procedure has not radically changed over that time. Secondly, the distribution of patients by disease severity was not well balanced. In earlier years, many patients with severe TOF did not undergo surgery. Therefore, the basic conditions of earlier patients are actually better than those of more recent patients. Additionally, improvements in surgical skills and perioperative management in recent years may be offset by disease severity. Thirdly, our hospital is the largest children’s hospital in southwestern China. Primary complete repair is a mature procedure, and all the involved surgeons and nurses were experienced. We particularly emphasize that the use of the proposed model requires more external validation, especially for institutions with less surgical experience. The year of surgery and differences in the surgical team may affect the outcome and should not be completely ignored at these institutions. We hope that all doctors who perform TOF surgery have received strict training; otherwise, they may be the greatest risk factor for postoperative adverse events.
Many studies have analysed the length of hospital stay as an adverse event. However, the length of hospital stay is determined by various complications during hospitalization, and the measure itself is not the fundamental influencing factor; therefore, we did not choose length of stay as a main factor. Additionally, we did not select low cardiac output syndrome or vasopressor inotropic score as risk factors because the diagnosis of postoperative low cardiac output syndrome remains unclear; some studies in the literature refer to subjective factors (2,3,21), such as clinical symptoms, which will affect the diagnosis of low cardiac output syndrome.
There were certain limitations to our study. Firstly, it was a retrospective single-centre analysis because it was carried out in a less-developed area, which is limited by economic and technical aspects; the risk factors in this study were relatively basic indices, and tissue Doppler or postoperative magnetic resonance imaging assessments of cardiac function were not performed. Secondly, ML, which assumes sophisticated algorithms, provides references for clinical diagnosis, treatment and prediction of potential risks and requires a large sample size and external verification (22), and studies of paediatric surgery frequently cannot meet these requirements. Hence, classical algorithms such as LR should not be completely abandoned and still have good application in paediatric surgery research.
Conclusions
In summary, we found that CPB time, DP, and TP repair are risk factors for adverse events after complete repair of TOF. The risk of adverse events increases significantly when the CPB time is longer than 105 minutes or the RVOT pressure is higher than 70 mmHg. SPO2 is a protective factor, with a reference point of 88%; the risk of adverse events increases significantly when SPO2 is lower than 88%. ML models were established to predict the incidence of adverse events.
Acknowledgments
We are grateful to all the doctors and nurses in the Cardiothoracic Surgery Department for their help. We would also like to thank American Journal Experts (AJE) for their assistance in revising our paper for language.
Funding: This work was supported by the Extreme Smart Analysis platform (https://www.xsmartanalysis.com/) and was supported by the Basic Research in Key Laboratory of Ministry of Education (Youth Program) (No. YBRP-202119).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-246/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-246/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-246/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-246/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics committee of our hospital (approval number: 2021-352), and informed consent was obtained from all patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Ven JPG, van den Bosch E, Bogers AJCC, et al. Current outcomes and treatment of tetralogy of Fallot. F1000Res 2019;8:F1000 Faculty Rev 1530;
- Li S, Zhang Y, Li S, et al. Risk Factors Associated with Prolonged Mechanical Ventilation after Corrective Surgery for Tetralogy of Fallot. Congenit Heart Dis 2015;10:254-62. [Crossref] [PubMed]
- Mercer-Rosa L, Elci OU, DeCost G, et al. Predictors of Length of Hospital Stay After Complete Repair for Tetralogy of Fallot: A Prospective Cohort Study. J Am Heart Assoc 2018;7:e008719. [Crossref] [PubMed]
- Lassau N, Ammari S, Chouzenoux E, et al. Integrating deep learning CT-scan model, biological and clinical variables to predict severity of COVID-19 patients. Nat Commun 2021;12:634. [Crossref] [PubMed]
- Rajpurkar P, Chen E, Banerjee O, et al. AI in health and medicine. Nat Med 2022;28:31-8. [Crossref] [PubMed]
- Baskin E, Gulleroglu KS, Saygili A, et al. Peritoneal dialysis requirements following open-heart surgery in children with congenital heart disease. Ren Fail 2010;32:784-7. [Crossref] [PubMed]
- Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med 2007;26:5512-28. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [Crossref] [PubMed]
- Grant SW, Collins GS, Nashef SAM. Statistical Primer: developing and validating a risk prediction model. Eur J Cardiothorac Surg 2018;54:203-8. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Elkin EB, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak 2008;8:53. [Crossref] [PubMed]
- Chowdhury UK, Jha A, Ray R, et al. Histopathology of the right ventricular outflow tract and the relation to hemodynamics in patients with repaired tetralogy of Fallot. J Thorac Cardiovasc Surg 2019;158:1173-1183.e5. [Crossref] [PubMed]
- Bassareo PP, Deidda M, Calcaterra G, et al. Right ventricular diastolic function in post-surgical Tetralogy of Fallot patients: A pilot study to make a comparison between echocardiography and cardiac MRI. Int J Cardiol Congenit Heart Dis 2021;4:100135.
- Alpat S, Yilmaz M, Onder S, et al. Histologic alterations in tetralogy of Fallot. J Card Surg 2017;32:38-44. [Crossref] [PubMed]
- Martins IF, Doles IC, Bravo-Valenzuela NJM, et al. When is the Best Time for Corrective Surgery in Patients with Tetralogy of Fallot between 0 and 12 Months of Age? Braz J Cardiovasc Surg 2018;33:505-10. [Crossref] [PubMed]
- Powell AW, Mays WA, Chin C. Functional Capacity Is Affected by Younger Age of Repair in Tetralogy of Fallot Patients But Not by Era of Repair. World J Pediatr Congenit Heart Surg 2019;10:715-21. [Crossref] [PubMed]
- Fabian O, Gebauer R, Koblizek M, et al. Histopathological evidence of aortopathy in newborns and infants with Tetralogy of Fallot at the time of the surgical repair. Cardiovasc Pathol 2019;40:59-64. [Crossref] [PubMed]
- Munkhammar P, Carlsson M, Arheden H, et al. Restrictive right ventricular physiology after tetralogy of Fallot repair is associated with fibrosis of the right ventricular outflow tract visualized on cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2013;14:978-85. [Crossref] [PubMed]
- Negi SL, Mandal B, Singh RS, et al. Myocardial protection and clinical outcomes in Tetralogy of Fallot patients undergoing intracardiac repair: a randomized study of two cardioplegic techniques. Perfusion 2019;34:495-502. [Crossref] [PubMed]
- Terol C, Kamphuis VP, Hazekamp MG, et al. Left and Right Ventricular Impairment Shortly After Correction of Tetralogy of Fallot. Pediatr Cardiol 2020;41:1042-50. [Crossref] [PubMed]
- Haggerty CM, Suever JD, Pulenthiran A, et al. Association between left ventricular mechanics and diffuse myocardial fibrosis in patients with repaired Tetralogy of Fallot: a cross-sectional study. J Cardiovasc Magn Reson 2017;19:100. [Crossref] [PubMed]
- Ergün S, Genç SB, Yıldız O, et al. Predictors of a complicated course after surgical repair of tetralogy of Fallot. Turk Gogus Kalp Damar Cerrahisi Derg 2019;28:264-73. [Crossref] [PubMed]
- Liang H, Tsui BY, Ni H, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med 2019;25:433-8. [Crossref] [PubMed]


