
The timely diagnosis of 49, XXXXY with the combined detection of MLPA, karyotype, and QF-PCR in a newborn with multiple congenital malformations: a case report
Highlight box
Key findings
• The newborn with 49, XXXXY syndrome manifested multiple congenital malformations and was diagnosed with a combined detection of MLPA, QF-PCR, and karyotype.
What is known and what is new?
• 49, XXXXY is a rare sex chromosomal aneuploidy syndrome and is the most severe variant of 47, XXY syndrome. It is recommended that patients suffering from 49, XXXXY should be treated with EHT to improve their language and gestural development and penis size.
• The neonate in this case presented with respiratory distress and multiple malformations, so the easy and fast method of MLPA P036 mix was performed to determine the chromosome number change, then karyotyping and QF-PCR confirmed the karyotype of 49, XXXXY.
What are the implications, and what should change now?
• The infant exhibited the characteristics of both autosomal and sex chromosome aneuploidies undetected prenatally and could be rapidly screened by the economical MLPA P036 mix method, and then confirmed by the appropriate method.
Introduction
The 49, XXXXY syndrome was first reported by Fraccaro et al. in 1960 (1), based on whom the syndrome is now named. It is a rare sex chromosome polysomy, with an incidence of between 1:85,000 and 1:100,000 male births (2). Typical clinical features of 49, XXXXY usually include facial dysmorphism, developmental delay, mental retardation, skeletal and cardiac malformations, micropenis, hypogonadism, and gynecomastia (3,4). Some researchers consider 49, XXXXY to be the most severe variant of Klinefelter syndrome (KS) whose karyotype is 47, XXY (2,5). It is recommended that patients suffering from 49, XXXXY syndrome should be treated with early hormonal treatment (EHT) at preschool age (6). Testosterone replacement therapy improves the vocabulary, language, motor capability and gestural development of patients as well as their penis size but does not treat infertility, gynecomastia, or small testes (5-7).
The prenatal diagnosis of 49, XXXXY by ultrasound and non-invasive prenatal screening (NIPS) is usually non-specific and uncertain due to limited research (8,9). Thus, early accurate diagnosis after birth is essential for timely testosterone replacement therapy. The neonate may only present with a characteristic facial appearance such as upslanting palpebral fissures, hypertelorism, arched eyebrows, synophrys, cheilopalatognathus, and ear deformities (3). Due to the gradual onset of developmental delay and slowly discovered skeletal, cardiac malformation, the child usually will be typically diagnosed several months or years later (10,11). Even some people were found to be 49, XXXXY at the age of 19 years old (12,13). Diagnosis at birth is rarely reported, in these cases karyotype was performed because of the unusual sonographic signs prenatal (10), atypical genitals (14,15), or respiratory distress (16). And in our case, genetic testing was done due to neonatal asphyxia and facial dysmorphism.
Nowadays, the methods used to discover 49, XXXXY include karyotype, quantitative fluorescent polymerase chain reaction (QF-PCR) (13), fluorescence in situ hybridization (FISH) (13), chromosome microarray (17), and the karyotype were still regarded as the final definite test (15,16). Here, we applied a cost-effective, rapid, and reliable complementary method of multiplex ligation-dependent probe amplification (MLPA) (18) to screen whether the number of chromosomes was abnormal, then karyotyping was used to diagnose 49, XXXXY syndrome and QF-PCR was used to analyze the potential origin of the three extra chromosomes. We present the following case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-23/rc).
Case presentation
Our male patient was born via spontaneous vaginal delivery at 41+3 weeks’ gestation and hospitalized in the Neonatal Department due to respiratory distress. He was diagnosed with neonatal asphyxia. He was the firstborn child to a non-consanguineous, 24-year-old gravida1, para1 (G1P1) mother and a 31-year-old father. The newborn was characterized by intrauterine growth restriction (IUGR), low birth weight (2.4 Kg, below the 3rd percentile), and an Apgar score of 6 at 1 minute, 8 at 5 minutes, and 9 at 10 minutes. At clinical examination, he presented with a variety of malformations, including such as hypertelorism, epicanthal folds, a low nasal bridge, a high-arched palate, micrognathia, low-set ears, and microcephaly. He had a 1-centimeter cleft palate, micropenis, and a single transverse palmar crease on the right hand. An excrescence was also observed in front of his right ear, as shown in Figure 1.
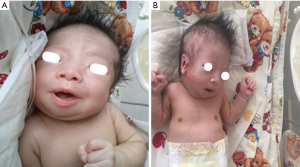
No obvious malformations were discovered on brain magnetic resonance imaging (MRI) and cranial ultrasound. Echocardiography revealed 3.4 and 7 mm atrial septal defects (ASD). The brainstem auditory evoked potential (BAEP) reflected auditory function impairment, with right and left auditory thresholds of 60 dBnHL and 30 dBnHL, respectively, which indicated lesions on the patient’s auditory neurons.
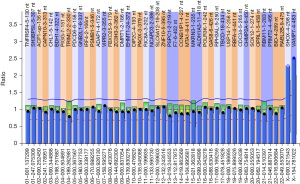
Due to his characteristic phenotype, MLPA and karyotype analysis were performed to make a definitive diagnosis. MLPA P036 subtelomeres mix 1 analysis revealed that the peaks of the X/Y chromosomal pseudo-autosomal regions (PAR) loci SHOX-PAR1 and VAMP7-PAR2 were 2.53 and 2.3, respectively, which was markedly higher than the normal average height ratio of 0.8–1.2 (Figure 2). These results indicated that the patient may possess up to three extra copies of these two X/Y chromosomal PAR genes (SHOX-PAR1 and VAMP7-PAR2). As these genes are located on different ends of the X/Y chromosome, the MLPA analysis suggested the presence of three additional X/Y chromosomes. Also, G-banding showed a karyotype of 49, XXXXY in 20 out of 20 cells counted (Figure 3), revealing that the three additional sex chromosomes are X chromosomes.
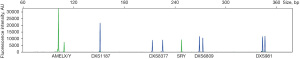
QF-PCR was performed to identify the constitution of the sex chromosome. This analysis included 4 short tandem repeat (STR) markers on the X chromosome and 2 non-polymorphic markers (AMELXY on X/Y and SRY on Y). The peak area ratio of AMELX and AMELY was 4:1, which is consistent with the numbers of X and Y chromosomes obtained from the karyotyping results. The peak ratio of 3 in 4 STR markers on the X chromosome was 1:1 and the other marker displayed only one peak (Figure 4).
The patient was followed up at 6 months of age. He had received normal care but presented developmental delays and depressed immunity. He could show certain facial expressions and make monosyllabic sounds. At 4 months of age, he could raise his head but was not stable till 6 months. He often suffered from respiratory tract infections. At 6 months, the patient was still too young to evaluate his genitalia development. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Affiliated Hospital of Guilin Medical University (No. 2020GZRLL-46). Written informed consent was obtained from the parents of the child for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
It is well known that aneuploidies that can survive to term include trisomy 13, 18, 21, and sex chromosome aneuploidies, among which trisomy 21 and sex chromosome aneuploidies are more common (19). The malformations exhibited by our patient, such as hypertelorism, epicanthal folds, a low nasal bridge, a high-arched palate, micrognathia, low-set ears, microcephaly, etc., are similar to the clinical features of autosomal aneuploidies. In addition, the condition of the patient’s micropenis was suspected to be associated with sex chromosomal abnormalities. Thus, MLPA P036 analysis was performed to screen whether a change in the number of chromosomes existed in the body of the neonate. Since the P036 kit is used to confirm the copies of the subtelomeric regions, the number of chromosomes can be speculated. Therefore, the MLPA P036 assay is fit to examine aneuploidy at the genome-wide level (20) but it could not distinguish between the X and Y sex chromosomes due to the two probes on the sex chromosome being in the PAR, which are identical in the X and Y chromosomes. Subsequently, the karyotyping results confirmed that the three additional sex chromosomes were X chromosomes. Although the result of the P036 mix could be obtained within 24 hours, the gold standard status of karyotype analysis, which is slow and laborious, could not be replaced.
The 49, XXXXY syndrome is caused by the double non-disjunction events of the X chromosome during maternal meiosis I and meiosis II, which induce four aberrant X chromosomes. Then, this egg containing the four abnormal X chromosomes combines with a normal male sperm (5). 49, XXXXY appears to be maternally derived but is unrelated to maternal age (13,21,22). Our STR-marker analysis results showed that one locus was homozygous, while the other three loci were heterozygous. The consequence had no difference from a normal female, which has two X populations. Unfortunately, we could not obtain a sample from the child’s parents to evaluate the origin of the extra chromosomes directly. However, we could suppose that the four X chromosomes were derived from one parent, as the egg and the sperm could not be abnormal at the same time. Therefore, they likely came from the non-disjunction X chromosome of the mother, which is consistent with the existing literature. For genetic counseling, there is no evidence to suggest that recurrent risk is increased higher than that of the general population. The chromosomal non-disjunction process is not likely to repeat itself in a particular family (5).
The reported prenatal ultrasound features of 49, XXXXY include cystic hygroma, microgenitalia, clubfoot, epignathus, non-immune hydrops, and hypoplastic right heart syndrome (8,9). However, there are still some fetuses, such as in our case, that only present with IUGR and low birth weight, which are easily overlooked (16). Although the NIPS tool has been widely used, it can result in some false low-risk results (9). Therefore, children with 49, XXXXY were found clinically, and some were diagnosed even in adulthood (12,13). In recent years, increasing research has shown that early intervention and EHT in preschool boys with 49, XXXXY will produce significant therapeutic effects (6). It has also been reported that androgens have a significant impact on neurodevelopment, brain function, and behavioral outcomes from as early as 16 weeks gestation to adulthood (23). Thus, once a baby is found with a variety of malformations and total developmental delays, it is necessary to immediately make a clear genetic diagnosis.
Although many more advanced technologies have emerged for 49, XXXXY diagnosis, each approach has its own advantages and disadvantages. QF-PCR is a convenient method to identify the origin of the chromosome in aneuploidy diseases by analyzing the STR polymorphism (21,22). But it is mainly used to check the number of 13, 18, 21 and sex chromosome (13,22). In addition compared to microarray analysis and FISH, MLPA provides a faster, high-throughput, and more economical means of quantifying copy numbers of up to 60 different genomic DNA sequences simultaneously (18). However, karyotype analysis is still the gold standard for the diagnosis of chromosomal diseases.
With the advent of improved detection capabilities and the resolution of genetic testing methods, a growing number of genetic variations have been discovered. Common sex chromosomal aneuploidies may contain some other smaller variations that cannot be identified with the lower resolution karyotype analysis (24). The limitation of the study is that we did not explore whether there are some other genetic variants associated with the patients’ clinical presentation.
Conclusions
The clinical manifestations of 49, XXXXY neonates may not be typical, and may only present with facial anomalies and/or ambiguous externalia. To avoid delayed diagnosis a rapid and cost-effective detection of MLPA was used to screen the subtelomere regions of the entire genome, and finally the newborn was proved to be 49, XXXXY syndrome by karyotype analysis. We hope that the present study will draw physicians’ attention to perform karyotype analysis, especially for undiagnosed infants who suffer from key features of aneuploid syndrome, including facial dysmorphism, micropenis, mental retardation, genital abnormalities, and skeletal malformations.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (No. 82060037), the Natural Science Foundation of Guangxi Province (No. 2018JJA140062), and the Guangxi Ba Gui Scholars Special Project.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-23/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-23/coif). All authors report that the present manuscript was supported by the National Natural Science Foundation of China (No. 82060037), the Natural Science Foundation of Guangxi Province (No. 2018JJA140062), and the Guangxi Ba Gui Scholars Special Project. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Affiliated Hospital of Guilin Medical University (No. 2020GZRLL-46). Written informed consent was obtained from the parents of the child for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fraccaro M, Kaijser K, Lindsten J. A child with 49 chromosomes. Lancet 1960;2:899-902. [Crossref] [PubMed]
- Peet J, Weaver DD, Vance GH. 49,XXXXY: a distinct phenotype. Three new cases and review. J Med Genet 1998;35:420-4. [Crossref] [PubMed]
- Sprouse C, Tosi L, Stapleton E, et al. Musculoskeletal anomalies in a large cohort of boys with 49, XXXXY. Am J Med Genet C Semin Med Genet 2013;163C:44-9. [Crossref] [PubMed]
- Ricciardi G, Cammisa L, Bove R, et al. Clinical, Cognitive and Neurodevelopmental Profile in Tetrasomies and Pentasomies: A Systematic Review. Children (Basel) 2022;9:1719. [Crossref] [PubMed]
- Visootsak J, Graham JM Jr. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis 2006;1:42. [Crossref] [PubMed]
- Samango-Sprouse CA, Gropman AL. Introduction: Comprehensive investigation into an international cohort of boys with 49,XXXXY. Am J Med Genet A 2021;185:3554-6. [Crossref] [PubMed]
- Samango-Sprouse CA, Gropman AL, Sadeghin T, et al. Effects of short-course androgen therapy on the neurodevelopmental profile of infants and children with 49,XXXXY syndrome. Acta Paediatr 2011;100:861-5. [Crossref] [PubMed]
- Lu YC, Huang LY, Yang YD, et al. Early prenatal diagnosis of 49,XXXXY: two case reports. J Obstet Gynaecol 2019;39:275-7. [Crossref] [PubMed]
- Putra M, Hicks MA, Abramowicz JS. False Low-Risk Single Nucleotide Polymorphism-Based Noninvasive Prenatal Screening in Pentasomy 49,XXXXY. AJP Rep 2018;8:e4-6. [Crossref] [PubMed]
- Burgemeister AL, Daumiller E, du Bois G, et al. Clinical report of 8 patients with 49,XXXXY syndrome: Delineation of the facial gestalt and depiction of the clinical spectrum. Eur J Med Genet 2019;62:210-6. [Crossref] [PubMed]
- Counts DR, Yu C, Lasutschinkow PC, et al. Evidence of intrauterine growth restriction and growth hormone deficiency in 49,XXXXY syndrome. Am J Med Genet A 2021;185:3547-53. [Crossref] [PubMed]
- Wei L, Liu Y, Sun S, et al. Case report of 49,XXXXY syndrome with cleft palate, diabetes, hypothyroidism, and cataracts. Medicine (Baltimore) 2019;98:e17342. [Crossref] [PubMed]
- Rajabzadeh M, Taheri N, Jazayeri O. 49,XXXXY syndrome: A case study and a systematic review of clinical features among the Iranian population. Clin Case Rep 2022;10:e6342. [Crossref] [PubMed]
- Fadil Iturralde JL, Marani J, Lahoz García M, et al. Genital malformation: Trigger of the diagnosis of severe variants of Klinefelter syndrome. Malformación genital: Disparador del diagnóstico de variantes severas de síndrome de Klinefelter. Rev Chil Pediatr 2020;91:111-5. [Crossref] [PubMed]
- Ng SF, Boo NY, Wu LL, et al. A rare case of ambiguous genitalia. Singapore Med J 2007;48:858-61.
- Etemadi K, Basir B, Ghahremani S. Neonatal diagnosis of 49, XXXXY syndrome. Iran J Reprod Med 2015;13:181-4.
- Samango-Sprouse C, Lasutschinkow PC, Mitchell F, et al. 49,XXXXY syndrome: A study of neurological function in this uncommon X and Y chromosomal disorder. Am J Med Genet A 2021;185:3557-66. [Crossref] [PubMed]
- Schouten J, van Vught P, Galjaard RJ. Multiplex Ligation-Dependent Probe Amplification (MLPA) for Prenatal Diagnosis of Common Aneuploidies. Methods Mol Biol 2019;1885:161-70. [Crossref] [PubMed]
- Hutaff-Lee C, Cordeiro L, Tartaglia N. Cognitive and medical features of chromosomal aneuploidy. Handb Clin Neurol 2013;111:273-9. [Crossref] [PubMed]
- Diego-Alvarez D, Rodriguez de Alba M, Cardero-Merlo R, et al. MLPA as a screening method of aneuploidy and unbalanced chromosomal rearrangements in spontaneous miscarriages. Prenat Diagn 2007;27:765-71. [Crossref] [PubMed]
- Celik A, Eraslan S, Gökgöz N, et al. Identification of the parental origin of polysomy in two 49,XXXXY cases. Clin Genet 1997;51:426-9. [Crossref] [PubMed]
- Chen CP, Chern SR, Chang CL, et al. Prenatal diagnosis and genetic analysis of X chromosome polysomy 49, XXXXY. Prenat Diagn 2000;20:754-7. [Crossref] [PubMed]
- Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol 2006;21:825-45. [Crossref] [PubMed]
- Dhangar S, Ghatanatti J, Vundinti BR. array-CGH revealed gain of Yp11.2 in 49,XXXXY and gain of Xp22.33 in 48,XXYY karyotypes of two rare klinefelter variants. Intractable Rare Dis Res 2020;9:145-50. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



