
Effects of different types of visual music on the prefrontal hemodynamics of children with autism spectrum disorder based on functional near-infrared spectroscopy
Highlight box
Key findings
• There were differences in HbO in different parts of the prefrontal lobe between the two groups of children when they were given the same visual music task. The effects of different types of visual music tasks on the frontal lobe of the brain in children with ASD are inconsistent.
What is known and what is new?
• Music therapy improves behavior, social communication, brain connectivity, and parent-child relationships in children with ASD.
• The effects of different types of visual music on the frontal lobe of the brain in children with ASD are inconsistent.
What are the implications, and what should change now?
• When treating children with ASD with visual music, the appropriate type of visual music should be selected. In the future, the sample size should be further expanded to explore more systematically which type of visual music is most beneficial to children with ASD.
Introduction
Autism spectrum disorder (ASD), characterized by deficits in social communication and the presence of restricted, repetitive behaviors or interests, is a neurodevelopmental disorder affecting approximately 2.3% children aged 8 years in the US and approximately 2.2% of adults (1). ASD children have obvious defects in visual and auditory information integration compared with normal children (2,3). The ability to integrate the visual and auditory components of social stimuli is theorized to be particularly critical to developing accurate, unified representations of the sensory world and foundational to the development of higher-order social, communication, and cognitive skills (4). Various interventions, such as applied behavioral analysis (ABA), occupational therapy, speech therapy, physical therapy, and pharmacotherapy, have been adopted to relieve ASD symptoms (5); however, most treatments have only achieved limited success. Many (but not all) patients with ASD require some form of lifelong support (6); thus, there is an urgent need to develop new ASD interventions.
Music therapy can help children with ASD improve their skills in core areas, including social interaction, verbal communication, positive behavior, and socio-emotional reciprocity. In addition, music therapy can help improve children’s social adjustment and improve the quality of parent-child relationships (7). According to a 25-section randomized controlled trial comparing music therapy with music listening, music therapy is more effective in children with ASD than listening to music (8). However, an international, multicenter, single-blind randomized controlled trial of improvisational music therapy noted that adding music therapy to the treatment of children with ASD did not improve their social impact or parental assessed social responses (9). Music therapy has shown promising efficacy in several areas, but the largest trials have produced conflicting or inconclusive results (10). Given the highly heterogeneous nature of ASDs, music therapy may be a potential therapeutic adjunct to autism. However, there is currently no neuroscientific evidence to support its benefits, and the exploration of its underlying neurobiological mechanisms may contribute to the development of more targeted and promising music therapies.
The ability to integrate information from multiple senses (e.g., auditory and visual information) is the basis for developing higher-level skills (e.g., language, communication, and social skills) (11,12). Visual music therapy is a novel multi-sensory comprehensive intervention method based on music therapy, which combines vision, hearing, and motor sensation organically through therapeutic elements such as music, pictures, and lights. Specifically, therapists interact with patients, regulate their moods, and serve a spiritual role in terms of awakening, encouraging, soothing, and catharsis through which they can alter the bad behaviors of patients and promote social adaptability (13).
Functional near-infrared spectroscopy (fNIRS) is a non-invasive optical method for brain imaging that can significantly reflect the brain hemodynamic parameters of neuronal activity by measuring neurovascular coupling (14). With high temporal resolution and reasonable spatial resolution, fNIRS can easily be applied to studies involving adolescents, adults, infants, and young children with neurological disorders by providing a non-invasive and convenient imaging environment.
At present, results on whether music therapy benefits the development of children with ASD are inconsistent, and findings on the mechanism of visual music affecting prefrontal hemodynamic changes have also not been reported (10). Broder-Fingert et al. reported that further studies need to be conducted in more controllable settings and the selection of subjects must be more carefully performed to acquire better patient-and family-centered results (10). Compared with standard care, music therapy was shown to be beneficial to people with a low level of cognitive function [Intelligence Quotient (IQ) <70], non-verbal individuals, and children less than 5 years of age (15).
Herein, fNIRS was employed to evaluate the prefrontal cortices (PFCs) of children with ASD and typical development (TD) who were subjected to different types of visual musical tasks. Moreover, the effects of different types of visual music therapy on prefrontal hemodynamics were explored, and the differences in audiovisual multi-sensory integration between the two groups of children were assessed. This study aimed to provide support for further investigation of the neurobiological motivational model of visual music intervention therapy for ASD. We present the following article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-693/rc).
Methods
Study population
From October 2020 to October 2021, 7 children with ASD diagnosed in special education schools (ASD group) were recruited, with an average age of (4.86±1.35) years, including 6 boys and 1 girl. Nine Typical developing (TD) child volunteers (TD group) were recruited from kindergartens around Beijing Rehabilitation Hospital, Capital Medical University. They were 3–7 years old with an average age of (4.78±1.20) years old, including 8 boys and 1 girl. All children were right-handed. The general information of the subjects was recorded, and the family members of all study subjects were informed about the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Beijing Rehabilitation Hospital, Capital Medical University (No. 2019bkky032). Informed consent was taken from all the patients’ guardians.
The inclusion criteria for children with ASD were as follows: (I) patients who met the diagnostic criteria of ASD in the diagnostic and statistical manual of mental disorders-5th (DSM-5) or the Autism Diagnostic Observation Schedule (ADOS). Proof of diagnosis for ASD was provided by a professional (i.e., neurologist, psychologist, psychiatrist); (II) age: 3–7 years; (III) childhood autism rating scale (CARS) score: 30–60 points; and (IV) patients who could complete the test. The study exclusion criteria were as follows: (I) presence of other neurological diseases; and (II) patients who could not complete the test; for example, had difficulty wearing a hat or could not remain quiet for 1 minute.
Moreover, the exclusion criteria for normal children were: (I) presence of any neurological or developmental diagnosis/delay, premature delivery, or other significant birth histories; (II) taking drugs for neurological or psychiatric effects; (III) patients with a history of seizures; and (IV) those a family history of ASD.
fNIRS test and visual music treatments
Test task
The test tasks were divided into resting states and 12 different types of visual music tasks. The test was conducted in a quiet, dark room. In order to ensure the relative silence of the children, we asked the children to sit on the lap of their caregivers during the test, do not talk or move, and remain relatively still. Children in both groups were given the same 12 different types of visual music tasks. To avoid interference between the two types of visual music therapy, a gap of about 60 seconds was set between each type of visual music therapy, and the tests could be completed within a week for each person. The contents of the 12 visual music tasks are shown in Table 1. The children were held by their caregivers 70 cm away from two television screens, one showing a realistic picture and the other showing one of four pictures (Figure 1).
Table 1
| Tasks | Contents |
|---|---|
| 1 | Red light + positive music + mirror |
| 2 | Red light + positive music + fantasy |
| 3 | Red light + positive music + cartoon |
| 4 | Red light + positive music + sketch |
| 5 | Green light + neutral music + mirror |
| 6 | Green light + neutral music + fantasy |
| 7 | Green light + neutral music + cartoon |
| 8 | Green light + neutral music + sketch |
| 9 | Blue light + negative music + mirror |
| 10 | Blue light + negative music + fantasy |
| 11 | Blue light + negative music + cartoon |
| 12 | Blue light + negative music + sketch |
Note: The playing repertoire of positive music, neutral music, and negative music were Dog, Bird, and Duck, respectively.

fNIRS test
The fNIRS tests were performed under two conditions: the resting state and the visual music state. The test time of the resting state was 8 min. Block design was adopted for the visual music task. The rest time was 10 s and the rest time was 10 s for the visual music task. Children in both groups were tested with the fNIRS resting state test and 12 different types of visual music therapy tasks. Each child was given a total of 13 fNIRS tests over the course of a week. To avoid mutual interference between the two music therapies, an interval of about 60 s was set between every two visual music therapy. The contents of the 12 visual music tasks are presented in Table 1. The children were held by their caregivers at a distance of 70 cm from two television screens, with one screen displaying a realistic picture and the other displaying one of four pictures (Figure 1).
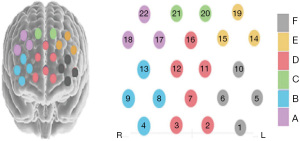
The ETG-4000 NIRS (Hitachi Medical Systems Corporation, Tokyo, Japan) was adopted to perform the fNIRS measurement, with a sampling rate of 10 Hz, 22 measurement channels, wavelengths of 695 and 830 nm, and a fixed distance of 3 cm between the emitter and the detector. The fNIRS brain activity measurement was performed by converting the absorbance differences into relative concentration changes in oxyhemoglobin (HbO), deoxyhemoglobin (HbR), and total hemoglobin according to the modified Beer-Lambert law. In this study, we primarily focused on the concentration change of HbO as it is the most sensitive indicator of regional cerebral blood flow in fNIRS measurements and is considered to have the strongest positive correlation with the blood-oxygen-level-dependent (BOLD) signal used in functional magnetic resonance imaging (fMRI) (10). Based on the International 10-20 system of electrode placement, 15 probes (eight emitters and seven detectors) were attached to a 3×5 grid overlying the PFC of the brain on a soft cap designed for children.
According to the 10-20 system, regions of interest (ROIs) were shown as follows: Channels 17, 18, and 22 were defined as A; Channels 4, 8, 9, and 13 as B; Channels 20 and 21 as C; Channels 2, 3, 7, 11, 12, and 16 as D; Channels 14, 15, and 19 as E; and Channels 1, 5, 6, and 10 as F (13). The sites and partition of the fNIRS test are shown in Figure 2.
Data analysis
FNIRS data preprocessing uses SPM toolbox for fNIRS (https://www.nitrc.org/projects/spm_fnirs/) Specific processing steps are as follows: (I) convert csv format to mat format; (II) wavelet-mdl was applied to eliminate noise, such as heartbeat and respiration; (III) the HRF low-pass filter was adopted to eliminate signal drift and burr, etc.; and (IV) the parameters required for the generalized linear model (GLM) were selected, and the resulting parameter β-values were utilized to estimate the model.
Statistical analysis
The effects of visual music for different tasks on the prefrontal lobe of children’s brains are represented by ∆HbO (∆HbO = average HbO under visual music tasks - average HbO under resting state). A t-test was performed between the β value and the baseline value (β=0) in the visual music task to determine the activation channel, which was defined as the channel with significant difference compared with the baseline. The mean value of β value of HbO in 6 areas of interest in the prefrontal lobe of the brain was taken for statistical analysis. Normal distribution test was carried out on β data. The data conforming to normal distribution was represented by mean ± standard deviation, and the data disconforming to normal distribution was represented by median (interquartile distance) [M (P25, P75)]. Multifactor analysis of variance was used to analyze the data of ASD and TD. A general linear model (GLM) was used to compare the effects of different types of visual music on HbO in the prefrontal cortex of children with ASD and TD. “A, B, C, D, E, F” were selected as dependent variables (i.e., observation variables), and light and picture were selected as fixed factors (i.e., control variables). Multiple post-hoc comparisons were made, and LSD test was selected in the assumed analysis of variance. All statistical analysis using the IBM SPSS 22.0 (https://www.onlinedown.net/soft/10064139.htm), P<0.05 is considered a significant difference.
Results
Intra-group comparison of the effects of visual music therapy on HbO in the ROIs in the prefrontal cortex (PFC) of children
According to the comparison results of children in the ASD group, the effect of the 12 different types of visual music on HbO in the brain PFCs of children with ASD was not statistically different (P>0.05). However, there was a marked difference in the ∆HbO in area F of the ROI in children with ASD under different light and music conditions (P<0.05). Specifically, the ∆HbO in area F of ASD children under red light and positive music was smaller than that under green light and neutral music (P=0.04<0.05) and blue light and negative music (P=0.033<0.05) (Figure 3A).
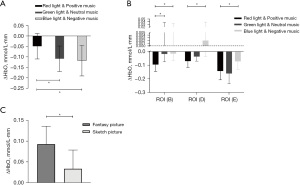
According to the comparison results of children in the TD group, the effects of the 12 different types of visual music on HbO in the brain PFCs of TD children were not significantly different (P>0.05). However, the effects of light and music on HbO in regions B, D, and E of the ROI in TD children were significantly different (P<0.05). Specifically, the activation of HbO in region B of the ROI in TD children under red light and positive music was better than that under green light and neutral music (P=0.02<0.05); and the activation degree of HbO in regions B, D, and E under red light and positive music was higher than that under blue light and negative music (P<0.05) (Figure 3B). Based on further comparisons of the effects of different pictures on HbO in the F region of the ROI in TD children, HbO activation in the F region of the ROI under fantasy pictures was preferred to that under sketch pictures (P<0.05) (Figure 3C).
Inter-group comparisons of the effect of visual music therapy on HbO in the ROIs in the PFC of children
The activation of different regions of the PFC was compared between the two groups of children based on visual music therapy. The outcomes revealed no differences in the ∆HbO in the A (F=0.621, P=0.444), B (F=0.626, P=0.442), C (F=1.145, P=0.303), and D (F=1.368, P=0.262) regions in the PFC; meanwhile, obvious differences could be observed in the ∆HbO in the E (F=5.737, P=0.031) and F (F=4.362, P=0.055) regions.
The activation of different regions of the PFC between the two groups of children was then compared based on light and music. The results showed that the ∆HbO in the prefrontal E region of the brain was lower in ASD children than that in TD children under both red light and positive music (F=7.224, P=0.018) and green light and neutral music (F=5.627, P=0.033). Under blue light and negative music, the ∆HbO in the prefrontal F region of ASD children was lower than that in TD children (F=8.120, P=0.013), while no differences were observed in the E region (F=3.425, P=0.085).
Under different pictures, the activation of different regions of the PFC was compared between the two groups of children. The outcomes showed that the ∆HbO in the prefrontal E region of the brain was lower in ASD children than that in TD children under fantasy pictures (F=7.253, P=0.017) and sketch pictures (F=6.364, P=0.024). However, a significant difference was not observed in the ∆HbO in the prefrontal E region of the brain between the two groups with mirror (F=4.037, P=0.064) and cartoon (F=3.249, P=0.093) pictures.
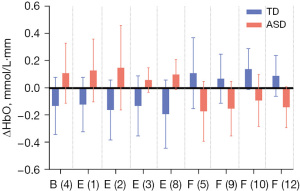
The activation of different regions of the PFC was compared between the two groups of children based on different tasks. The results demonstrated that visual musical tasks 1, 2, 3, 4, and 8 positively activated HbO in the B and E prefrontal regions of the brain in children with ASD and negatively activated HbO in TD children. Visual musical tasks 5, 9, 10, and 12 negatively activated HbO in the prefrontal F region of the brain in children with ASD and positively activated HbO in TD children.
In addition, ASD children had a higher ∆HbO in the prefrontal E region than TD children under task 1 (F=5.709, P=0.031), task 2 (F=5.302, P=0.037), task 3 (F=4.696, P=0.048), and task 8 (F=8.216, P=0.012). Children with ASD had a lower ∆HbO than TD children in the prefrontal F region of the brain under task 5 (F=5.248, P=0.038), task 9 (F=4.995, P=0.042), task 10 (F=7.290, P=0.017), and task 12 (F=9.744, P=0.008) (Figure 4).
Effects of different types of visual music on HbO2 in the PFCs of children
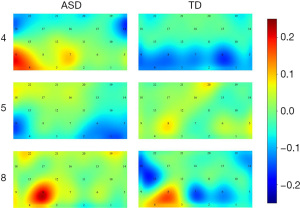
Under visual music task 4, ASD children exhibited higher ∆HbO in both the B (t=2.938, P=0.011) and D (t=2.226, P=0.044) regions in the prefrontal ROI of the brain compared with TD children. Under visual music task 5, the ∆HbO2 in the B region in the prefrontal ROI of the brain was weaker in ASD children than that in TD children (t=−2.268, P=0.040). Under visual music task 8, the ∆HbO in the B region in the prefrontal ROI of the brain was stronger in ASD children than that in TD children (t=2.162, P=0.048).
Activation of the prefrontal ROI in the two groups of children under different visual music tasks is shown in Figure 5. Briefly, the effects of different types of visual music tasks on HbO in different regions of the cerebral cortex, mainly manifested in the B, D, and E regions in the ROI cortex, were different between the two groups of children. Additionally, red light and positive music + sketch and green light and neutral music + sketch positively activated the PFC in ASD children, while green light and neutral music + mirror negatively activated the PFC in the right brain of ASD children.
Discussion
Music therapy improves behavior, social communication, brain connectivity, and parent-child relationships in children with ASD (16).
To the best of our knowledge, this is the first report using fNIRS to study the effects of different types of visual music tasks on PFC activation in children with ASD. This study shows that different types of visual music tasks have different activation levels in the prefrontal regions of the brains of children with ASD and children with TD, and different visual music tasks have different activation levels in different regions of the brain PFC of the two groups of children, which is mainly manifested as insufficient activation in children with ASD. Previous studies have reported that the dorsolateral PFC (DLPFC) plays a fundamental role in cognitive control ability (17) and is a key region involved in attention networks (18). According to Kruppa et al.’s division of the PFC and the positioning of the international 10-20 system, DLPFC is mainly region E in this study (19).
In this study, the degree of activation of HbO in ASD children was lower than that in TD children in the E region under red light and positive music and green light and neutral music, in the F region under the condition of blue light and negative music, as well as under/in the conditions/targets of fantasy and sketch pictures. Furthermore, functions such as working memory, planning, attention, and motivation were related to the E region of the PFC in the brains of children. The above findings suggested that visual music could activate ROIs in children with ASD.
Studies have shown that emotionally consistent music enhances facial emotion recognition in children with ASD, which can improve their social skills (20). This study shows that in the F region of ASD children, the degree of HbO activation was lowest under the condition of red light and positive music and highest under the condition of blue light and negative music. The F region of TD children exhibited higher activation of HbO under fantasy pictures than cartoon pictures. In regions B, D, and E of TD children, the activation degree of HbO was higher in the red light and positive music condition than that in the blue light and negative music condition. In the rFPC region of TD children, the degree of HbO activation was higher in the red light and positive music conditions than that in the green light and neutral music conditions. Moreover, the effects of different types of visual music tasks on HbO in the various regions of the cerebral cortex were different between the two groups of children, and the dissimilarities were mainly manifested in the left PFC of the brain. Specifically, red light and positive music had positive activation effects on the left PFC of the brain in ASD children, while blue light and negative music played a negative activation role. According to the international 10-20 system, ROI (E) is the left DLPFC and ROI (F) is the left FPC; meaning that red light and positive music can increase the degree of activation of the E region, while blue light and negative music can decrease the degree of activation of the F region in ASD children. Therefore, personalized visual music therapies are indicated for ASD patients with different symptoms.
Audiovisual multisensory integration (e.g., auditory and visual information) is considered to underpin the development of higher-level skills (e.g., language, communication, and social skills) (21). In this study, there were differences in the activation of HbO in the PFC of the brain in visual music tasks between ASD and TD children. These differences may explain why ASD children have poor audiovisual integration and may also be the pathogenesis of speech, communication, and social disorders. In addition, different color lights were used as a form of visual attention. Eye-tracking studies measuring gaze toward social target areas in still pictures (22), videos (23), or in vivo social interactions using computer applications (24) have revealed notable differences in individuals with autism. Thompson et al. reported that ASD children have difficulty looking at people, particularly their faces, but when they watched videos of someone singing or reading a story, they would look more at the person if they were singing and if the story was familiar to them; thus, using songs and familiar stories may be a way to help children with autism to naturally engage with others (25). Similar studies have shown that music can be used to improve emotion recognition in facial expressions and emotion induction through facial stimulation in children with high functioning ASDs (26).
In this present study, different light colors, music, and figures were used to stimulate multi-sensory audiovisual processing. Taken together with the published literature, we deduce that children with ASD have slower adaptation to color, figures, and music than TD children. Thus, it is important that the caregivers find ways to promote more natural and spontaneous engagement to allow ASD children to become accustomed to the settings before expecting beneficial results/responses. Further, adaptation should be individualized because ASD children might take a much longer time to respond due to their lower integration ability compared with TD children as well as the existing differences in integration between ASD children themselves.
In this study, the effects of visual music on hemodynamic changes in the PFCs of ASD children were initially investigated, and some directions for intervention methods were also provided. However, some limitations of this study cannot be ignored. Firstly, the PFC of the brain was divided into six ROIs in children aged 3–7 years based on previous studies and the international 10-20 system as references; These 6 partitions cannot be divided according to the adult MINI space, and can only be roughly estimated. However, as there is no uniform criterion to standardize fNIRS tests in children with ASD at present, including localization of the cerebral cortex and a method of data analysis; hence, this rough partition should be improved in the future. Secondly, this was a cross-sectional study, the sample size was small, and the conclusions lacked generalizability. Therefore, in the future, larger sample size and longitudinal data need to be used to understand the brain mechanism in ASD and explore effective intervention methods.
Conclusions
In this study, evidence of activation in the prefrontal lobe of the brain in children with ASD based on different types of visual music therapy was examined using fNIRS parameters commonly used in clinical trials. There were differences in HbO in different parts of the prefrontal lobe between the two groups of children when they were given the same visual music task. The effects of different types of visual music tasks on the frontal lobe of the brain in children with ASD are inconsistent.
Acknowledgments
Funding: This work was supported by the Research Talents Foundation of Beijing Rehabilitation Hospital Affiliated to Capital Medical University (No. 2019R-010).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-693/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-693/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-693/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Beijing Rehabilitation Hospital of Capital Medical University (No. 2019bkky032). Informed consent was taken from all the patients’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirota T, King BH. Autism Spectrum Disorder: A Review. JAMA 2023;329:157-68. [Crossref] [PubMed]
- Feldman JI, Dunham K, DiCarlo GE, et al. A Randomized Controlled Trial for Audiovisual Multisensory Perception in Autistic Youth. J Autism Dev Disord 2022; Epub ahead of print. [Crossref]
- Taylor N, Isaac C, Milne E. A comparison of the development of audiovisual integration in children with autism spectrum disorders and typically developing children. J Autism Dev Disord 2010;40:1403-11. [Crossref] [PubMed]
- Wallace MT, Woynaroski TG, Stevenson RA. Multisensory Integration as a Window into Orderly and Disrupted Cognition and Communication. Annu Rev Psychol 2020;71:193-219. [Crossref] [PubMed]
- Sharma SR, Gonda X, Tarazi FI. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol Ther 2018;190:91-104. [Crossref] [PubMed]
- Lord C, Elsabbagh M, Baird G, et al. Autism spectrum disorder. Lancet 2018;392:508-20. [Crossref] [PubMed]
- Geretsegger M, Elefant C, Mössler KA, et al. Music therapy for people with autism spectrum disorder. Cochrane Database Syst Rev 2014;2014:CD004381. [Crossref] [PubMed]
- Rabeyron T, Robledo Del Canto JP, Carasco E, et al. A randomized controlled trial of 25 sessions comparing music therapy and music listening for children with autism spectrum disorder. Psychiatry Res 2020;293:113377. [Crossref] [PubMed]
- Mayer-Benarous H, Benarous X, Vonthron F, et al. Music Therapy for Children With Autistic Spectrum Disorder and/or Other Neurodevelopmental Disorders: A Systematic Review. Front Psychiatry 2021;12:643234. [Crossref] [PubMed]
- Broder-Fingert S, Feinberg E, Silverstein M. Music Therapy for Children With Autism Spectrum Disorder. JAMA 2017;318:523-4. [Crossref] [PubMed]
- Rubio-Fernandez P, Mollica F, Jara-Ettinger J. Speakers and listeners exploit word order for communicative efficiency: A cross-linguistic investigation. J Exp Psychol Gen 2021;150:583-94. [Crossref] [PubMed]
- MacDonald K, Marchman VA, Fernald A, et al. Children flexibly seek visual information to support signed and spoken language comprehension. J Exp Psychol Gen 2020;149:1078-96. [Crossref] [PubMed]
- Zheng YE. Application of Visual Music in Music Intervention Therapy for Children with Autism. Northern Music 2019;16:245-6.
- Westgarth MMP, Hogan CA, Neumann DL, et al. A systematic review of studies that used NIRS to measure neural activation during emotion processing in healthy individuals. Soc Cogn Affect Neurosci 2021;16:345-69. [Crossref] [PubMed]
- Sharda M, Silani G, Specht K, et al. Music therapy for children with autism: investigating social behaviour through music. Lancet Child Adolesc Health 2019;3:759-61. [Crossref] [PubMed]
- Gassner L, Geretsegger M, Mayer-Ferbas J. Effectiveness of music therapy for autism spectrum disorder, dementia, depression, insomnia and schizophrenia: update of systematic reviews. Eur J Public Health 2022;32:27-34. [Crossref] [PubMed]
- Crone EA, Steinbeis N. Neural Perspectives on Cognitive Control Development during Childhood and Adolescence. Trends Cogn Sci 2017;21:205-15. [Crossref] [PubMed]
- Zwanzger P, Steinberg C, Rehbein MA, et al. Inhibitory repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex modulates early affective processing. Neuroimage 2014;101:193-203. [Crossref] [PubMed]
- Kruppa JA, Reindl V, Gerloff C, et al. Brain and motor synchrony in children and adolescents with ASD-a fNIRS hyperscanning study. Soc Cogn Affect Neurosci 2021;16:103-16. [Crossref] [PubMed]
- Wagener GL, Berning M, Costa AP, et al. Effects of Emotional Music on Facial Emotion Recognition in Children with Autism Spectrum Disorder (ASD). J Autism Dev Disord 2021;51:3256-65. [Crossref] [PubMed]
- Feldman JI, Dunham K, Cassidy M, et al. Audiovisual multisensory integration in individuals with autism spectrum disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev 2018;95:220-34. [Crossref] [PubMed]
- Nuske HJ, Vivanti G, Dissanayake C. Reactivity to fearful expressions of familiar and unfamiliar people in children with autism: an eye-tracking pupillometry study. J Neurodev Disord 2014;6:14. [Crossref] [PubMed]
- Frazier TW, Klingemier EW, Beukemann M, et al. Development of an Objective Autism Risk Index Using Remote Eye Tracking. J Am Acad Child Adolesc Psychiatry 2016;55:301-9. [Crossref] [PubMed]
- Hutchins TL, Brien A. Conversational topic moderates social attention in autism spectrum disorder: Talking about emotions is like driving in a snowstorm. Res Autism Spectr Disord 2019;26:99-110.
- Thompson GA, Abel LA. Fostering Spontaneous Visual Attention in Children on the Autism Spectrum: A Proof-of-Concept Study Comparing Singing and Speech. Autism Res 2018;11:732-7. [Crossref] [PubMed]
- Ramirez-Melendez R, Matamoros E, Hernandez D, et al. Music-Enhanced Emotion Identification of Facial Emotions in Autistic Spectrum Disorder Children: A Pilot EEG Study. Brain Sci 2022;12:704. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


