
Impact of COVID-19 on the incidence of respiratory viral infections and clinical characteristics of associated febrile seizures
Highlight box
Key findings
• Despite epidemiological changes in respiratory viral infections, the clinical features and prognosis of febrile seizure (FS) before and during the coronavirus disease 2019 (COVID-19) pandemic were comparable.
What is known and what is new?
• During the COVID-19 pandemic, mitigation measures have contributed to changes in the incidence of respiratory viral infections.
• In children with FS, substantial reduction in the incidence of influenza virus infections and significant increase in the incidence of parainfluenza virus infections were observed during the COVID-19 pandemic.
• During the COVID-19 pandemic, clinical features and outcomes of FS were similar to those before the COVID-19 pandemic.
What is the implication, and what should change now?
• Despite changes in the incidence, seasonality, and viral causes of FS during the COVID-19 pandemic, strategies to manage children with FS need not differ before and during the COVID-19 pandemic.
Introduction
Febrile seizures (FSs) are seizures that occur during febrile episodes without any evidence of seizure-provoking central nervous system (CNS) infections, trauma, or metabolic abnormality, affecting 2–5% of children between 6 months and 5 years of age (1). FSs are considered benign and generally have no long-term neurodevelopmental complications in the majority of cases (1). FSs are most frequently associated with upper respiratory viral infections. Any respiratory viruses, including rhinovirus, influenza virus, enterovirus, parainfluenza virus, and coronavirus, can cause FS (2). FSs have been linked to seasonal viral epidemics, that follow a pattern similar to that seen with common respiratory pathogens (3,4).
Since the first case of severe pneumonia caused by a novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] was reported in December 2019 (5), the pandemic of coronavirus disease-2019 (COVID-19) has continued unabated. The SARS-CoV-2 is primarily transmitted from person to person, either through direct contact or droplet, spread by coughing or sneezing from an infected individual (6). To mitigate the spread of the virus, non-pharmacological interventions (NPIs), including personal behavioral changes, such as wearing a mask and frequent handwashing, and maintaining social distance, were implemented worldwide: social distancing has removed children from schools, day care centers, and other contact with peers (7). This led to a significant decrease in the transmission of common infectious pathogens among children (8). Moreover, the overall incidence of respiratory infections in South Korea significantly decreased after the implementation of the COVID-19 mitigation measures in February 2020 (9). Accordingly, these measures may have influenced the incidence of respiratory viral infections causing a parallel change in the incidence and clinical characteristics of FS. A nationwide study in South Korea using national health insurance data demonstrated that the incidence of FS decreased by 38% in 2020, compared to that in 2010–2019 (10).
In this study, we aimed to evaluate the altered incidence and clinical characteristics of FS in a real-life clinical setting during the COVID-19 pandemic, when the seasonal distribution of respiratory viral infections causing a majority of cases of FS had changed, and to compare them to those observed before the pandemic began. Furthermore, we aimed to identify the patterns of FS incidence and clinical characteristics with specific respiratory viral pathogens. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-406/rc).
Methods
We conducted a retrospective medical chart review of pediatric patients between the age of 6 and 60 months who presented with FS at Incheon St. Mary’s Hospital (Incheon, South Korea) and Daejeon St. Mary’s Hospital (Daejeon, South Korea) between March 2016 and February 2022. Seizures caused by acute CNS infections, other intracranial lesions, and metabolic causes, including electrolyte imbalance and hypoglycemia, were excluded from the study. Patients were identified using the diagnostic codes for FS, convulsion, and epilepsy. The diagnostic codes used for patient identification included R56.0 (FS), R56.8 (convulsions), and G40 (epilepsy), according to the 10th version of the International Classification of Diseases and Related Health Problems (ICD-10). We reviewed the medical records thoroughly and confirmed that the patients fit the definition of FS. During the study period, each seizure episode accompanied by fever in the same patient was regarded as a separate episode if fever and seizure were absent for ≥7 and ≥24 hours, respectively, between seizures. Clustered seizures occurring within 24 hours were considered as a single episode, and the number of seizures within 24 hours was investigated. Additionally, the illness was considered the same when the same viral pathogen was confirmed within 4 weeks.
The retrieved data included sex, age of FS onset, seizure type, seizure frequency and duration, the presence of prolonged (>15 min) or clustered FS, the time interval between fever onset and seizure occurrence, the season when FS occurred, a clinical diagnosis for fever and accompanying symptoms suggesting infection, a family history of FS or epilepsy, electroencephalography (EEG) and brain magnetic resonance imaging (MRI) findings, and the development of subsequent epilepsy up to May 2022. The seasons of the year were defined as follows: spring, March to May; summer, June to August; autumn, September to November; and winter, December to February. The results of blood, urine, and stool cultures, rapid influenza detection tests (RIDTs), and multiplex polymerase chain reaction (mPCR) tests were also analyzed. Commercial kits that were used to perform RIDTs and mPCR tests on nasopharyngeal swab samples are as follows: Sofia Influenza A+B Fluorescent Immunoassay (Quidel Corp., San Diego, CA, USA) and AdvanSureTM RV real-time RT-PCR kit (LG Life Sciences, Seoul, South Korea), respectively, at Incheon St. Mary’s Hospital, and Alere BinaxNOW® Influenza A & B Card (Abbott, IL, USA) and AllplexTM Respiratory Panel (RP) 1, 2, 3 (Seegene Inc., Seoul, South Korea), respectively, at Daejeon St. Mary’s Hospital. The RIDTs were performed routinely during influenza outbreaks (usually from December to April in Korea). Since the mPCR tests were expensive and time-consuming, the tests were performed only for hospitalized patients presenting with fever or respiratory symptoms unless the guardian refused testing. During the COVID-19 pandemic, the commercial kits that were used to perform a polymerase chain reaction (PCR) test for SARS-CoV-2 on nasopharyngeal and oropharyngeal swab samples are as follows: STANDARDTM M nCoV Real-Time Detection kit (SD BIOSENSOR, Osong, South Korea) and Real-Q 2019-nCoV Detection kit (BioSewoom, Seoul, South Korea) at Incheon St. Mary’s Hospital, and Allplex™ SARS-CoV-2 Assay (Seegene Inc., Seoul, Korea) at Daejeon St. Mary’s Hospital.
According to the timing of FS occurrence, the enrolled children were classified into two groups: before the COVID-19 pandemic group (comprising of those with FS episodes between March 2016 to February 2020) and during the COVID-19 pandemic group (comprising of those with FS episodes between March 2020 to February 2022). In South Korea, the first patient diagnosed with COVID-19 was reported on January 20, 2020, followed by a surge in the number of cases with COVID-19 at the end of February 2020. The demographic and clinical characteristics of FS, and the distribution of detected respiratory viruses were compared between the two groups.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 for Windows (IBM Corp., Armonk, NY, USA). Numerical data, including age, were compared using Mann-Whitney test. Categorical data, such as the demographic and clinical characteristics as well as the distribution of respiratory viral pathogens, were analyzed using Fisher’s exact test or the chi-square test. Fisher’s exact test was used when more than 20% of cells had expected frequencies <5. Statistical significance was set at P<0.05.
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Catholic Medical Center (No. OC22RASI0007) and individual informed consent for this retrospective analysis was waived.
Results
Demographic and clinical characteristics of children with FS
Altogether, 988 episodes with a diagnosis of FSs were identified between March 2016 and February 2022, consisting of 865 (87.6%) and 123 episodes (12.4%) before and during the COVID-19 pandemic, respectively (Table 1). As shown in Figure 1, the occurrence of FSs decreased during the COVID-19 pandemic compared to that before the pandemic. During the pandemic, the incidence of FSs increased dramatically in the autumn (35.0% vs. 16.2%), whereas the same decreased significantly in the spring (29.8% vs. 18.7%) (P<0.001, Table 1).
Table 1
| Characteristics | Before the COVID-19 pandemic (n=865) |
During the COVID-19 pandemic (n=123) |
P value |
|---|---|---|---|
| Males | 510 (59.0) | 64 (52.0) | 0.145 |
| Age of FS onset, mon, median (IQR) | 23.7 (17.4–34.1) | 22.8 (17.5–30.3) | 0.438 |
| Season | <0.001 | ||
| Spring | 258 (29.8) | 23 (18.7) | |
| Summer | 208 (24.0) | 21 (17.1) | |
| Autumn | 140 (16.2) | 43 (35.0) | |
| Winter | 259 (29.9) | 36 (29.3) | |
| Classification of FS | 0.985 | ||
| Simple FS | 590 (68.2) | 84 (68.3) | |
| Complex FS | 275 (31.8)† | 39 (31.7) | |
| >15 min | 36 (4.2) | 5 (4.1) | |
| Recur within 24 h | 187 (21.6) | 26 (21.1) | |
| Focal seizure | 57 (6.6) | 8 (6.5) | |
| Seizure types | |||
| Generalized | 789 (91.2)‡ | 110 (89.4) | 0.518 |
| Others | 78 (9.0)‡ | 13 (10.6) | 0.578 |
| Number of seizure attacks | 0.006 | ||
| 1 | 469 (54.2) | 83 (67.5) | |
| ≥2 | 396 (45.8)§ | 40 (32.5)¶ | |
| Time interval between fever onset and seizure occurrence | 0.569 | ||
| <24 h | 757 (87.5) | 104 (84.6) | |
| <72 h | 92 (10.6) | 17 (13.8) | |
| ≥72 h | 16 (1.8) | 2 (1.6) | |
| Abnormal EEG result | 22/225 (9.8) | 5/41 (12.2) | 0.964 |
| Focal epileptiform discharges | 18 (2.1) | 2 (1.6) | |
| Generalized epileptiform discharges | 0 (0.0) | 1 (0.8) | |
| Multifocal epileptiform discharges | 1 (1.1) | 0 (0.0) | |
| Focal background slowing | 2 (2.1) | 2 (1.6) | |
| Generalized background slowing | 1 (1.1) | 0 (0.0) | |
| Abnormal brain MRI result | 2/103 (1.9)†† | 3/26 (11.5)‡‡ | 0.055 |
| Family history of FS | 261 (30.2) | 44 (35.8) | 0.208 |
| Family history of epilepsy | 16 (1.8) | 1 (0.8) | 0.711 |
| Subsequent diagnosis of epilepsy | 20 (2.3) | 0 (0.0) | 0.160 |
| Clinical diagnosis of fever | 0.374 | ||
| Upper respiratory tract infection | 527 (60.9) | 70 (56.9) | |
| Lower respiratory tract infection | 72 (8.3) | 7 (5.7) | |
| Hand, foot, and mouth disease/herpangina | 63 (7.3) | 5 (4.1) | |
| Acute gastroenteritis | 35 (4.0) | 7 (5.7) | |
| Urinary tract infection | 11 (1.3) | 1 (0.8) | |
| Exanthem subitem | 47 (5.4) | 10 (8.1) | |
| Fever without a focus | 99 (11.4) | 20 (16.3) | |
| Kawasaki disease | 1 (0.1) | 0 (0.0) | |
| Others§§ | 10 (1.2) | 3 (2.4) | |
Data are presented as N (%) or median (interquartile range). †, five patients in the Before the COVID-19 pandemic group had recurrent seizures within 24 hours lasting for >15 minutes; ‡, two patients in the Before the COVID-19 pandemic group experiencing recurrent seizures had generalized and other types of seizures during the same febrile illness; §, frequency of episode was as follows: 2 (179 patients, 20.7%), 3–5 (174 patients, 20.1%), and >5 (43 patients, 5.0%); ¶, frequency of episode was as follows: 2 (23 patients, 18.7%) and 3–5 (17 patients, 13.8%); ††, one patient of periventricular leukomalacia (with subsequent diagnosis of epilepsy) and the other of old lacunar infarcts in bilateral thalami and left corona radiata; ‡‡, one patient of periventricular leukomalacia, another of right parieto-occipital encephalomalacic changes, and the other of cavernous malformation in right temporal lobe; §§, postvaccination fever or fever caused by the varicella-zoster virus infection. COVID-19, coronavirus disease 2019; FS, febrile seizure; mon, month; IQR, interquartile range; EEG, electroencephalography; MRI, magnetic resonance imaging.

No statistically significant between-group differences were observed in sex composition, the median age at onset of FS, and seizure types (Table 1). The proportion of children having their first FS during the COVID-19 pandemic was significantly higher compared to those before the pandemic (67.5% vs. 54.2%, respectively; P=0.006, Table 1). The seizure duration, time interval between fever and FS onset, presence of abnormal findings on EEG and the brain MRI, presence of a family history of FSs and epilepsy, and the development of subsequent epilepsy were not significantly different before and during the COVID-19 pandemic (Table 1). Upper respiratory tract infection was the most common cause of fever in both groups, accounting for 60.9% and 56.9% of FS episodes before and during the pandemic, respectively. The distribution of clinical diagnoses of fever between the two groups showed no statistically significant differences (Table 1). During the study period, 7 patients experienced repeated FS before the pandemic (8 episodes) and during the pandemic (8 episodes), respectively. Upon excluding these patients from analysis, no statistically significant inter-group differences were observed in terms of the clinical characteristics of FS (Table S1). Of the 552 patients (55.9%) with a first FS, no statistically significant differences in clinical characteristics of FS were observed between before the pandemic group (n=469) and during the pandemic group (n=83) (Table S2).
Identification of causative respiratory viruses in children with FS
Before the pandemic started (n=865), RIDTs and mPCR tests were performed for 366 (42.3%) and 314 (36.3%) episodes, respectively. During the COVID-19 pandemic (n=123), however, RIDTs were not conducted and mPCR tests were performed in 64 (52.0%) episodes. All children during the COVID-19 pandemic underwent PCR testing for SARS-CoV-2, out of which four (3.3%) of them were diagnosed with COVID-19. Regarding the RIDTs conducted before the pandemic, 83 (22.7%) were positive for the influenza virus. Regarding mPCR tests, there were no statistically significant differences in the rates of positivity (71.3% before the pandemic vs. 70.3% during the pandemic), and coinfection with two or more respiratory viruses (44.2% before the pandemic vs. 33.3% during the pandemic) between the two periods of assessments (Table 2). In addition, no statistically significant inter-group differences were observed among patients who underwent RIDTs and/or mPCR tests (Table S3).
Table 2
| Variables | Before the COVID-19 pandemic | During the COVID-19 pandemic | P value |
|---|---|---|---|
| RIDT | n=366 | n=0 | |
| Negative | 283 (77.3) | 0 (0.0) | NA |
| Positive | 83 (22.7) | 0 (0.0) | |
| mPCR test | n=314 | n=64 | |
| Negative | 90 (28.7) | 19 (29.7) | 0.869* |
| Positive | 224 (71.3) | 45 (70.3) | |
| Coinfection | 99 (44.2) | 15 (33.3) | 0.178** |
| Single virus infection | 125 (55.8) | 30 (66.7) | |
| Influenza virus | 8 (6.4) | 0 (0.0) | 0.355 |
| Respiratory syncytial virus | 7 (5.6) | 1 (3.3) | >0.999 |
| Adenovirus | 15 (12.0) | 2 (6.7) | 0.529 |
| Rhinovirus | 53 (42.4) | 12 (40.0) | 0.811 |
| Metapneumovirus | 9 (7.2) | 0 (0.0) | 0.207 |
| Coronavirus | 10 (8.0) | 0 (0.0) | 0.211 |
| Bocavirus | 6 (4.8) | 4 (13.3) | 0.103 |
| Parainfluenza virus | 13 (10.4) | 11 (36.7) | 0.001 |
| Enterovirus | 4 (3.2) | 0 (0.0) | >0.999 |
Data are presented as N (%). *, comparison on the proportions of negative and positive mPCR test results between Before the COVID-19 pandemic and During the COVID-19 pandemic groups; **, comparison on the proportions of single virus infection and virus coinfection between before the COVID-19 pandemic and during the COVID-19 pandemic groups. RIDT, rapid influenza detection test; mPCR, multiplex polymerase chain reaction; COVID-19, coronavirus disease-2019; NA, not available.
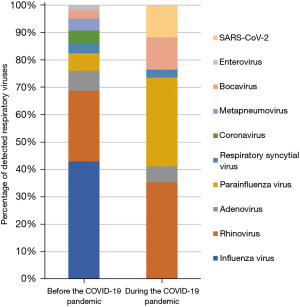
Interestingly, influenza viruses were identified in 88 episodes (80 and 5 on RIDT and mPCR testing, respectively, and 3 each in both) before the COVID-19 pandemic, while it was undetected in none of the episodes during the pandemic (P<0.001). For episodes in which a single respiratory virus was identified by an mPCR test, the detection rates of rhinovirus, the second most common respiratory virus before the COVID-19 pandemic, did not differ significantly after the pandemic commenced (42.4% vs. 40.0%, P=0.811). Meanwhile, increased detection rates of bocavirus (4.8% vs. 13.3%, P=0.103) and parainfluenza viruses (10.4% vs. 36.7%, P=0.001) were observed during the pandemic, compared to that before the pandemic, respectively (Table 2). Figure 2 illustrates the distribution of respiratory viruses observed before and during the COVID-19 pandemic.
Discussion
This was a relatively large-scale study in a real-life clinical setting that investigated the incidence and clinical characteristics of FS during the COVID-19 pandemic. The overall incidence of FS was observed to decrease during the pandemic. The distribution of detected respiratory viral infections in FS underwent significant changes after the COVID-19 pandemic began. Despite the changes in the detected respiratory viral illnesses, there were no significant differences in seizure characteristics and outcomes between children with FS before and during the pandemic.
In this study, the occurrence of FS considerably decreased during the COVID-19 pandemic compared to that before the pandemic. This was confirmed from another real-life clinical setting in South Korea that used national health insurance data to substantiate its findings (10). Likewise, Chiu et al. (11) showed a significant reduction in pediatric seizure-related emergency department attendance during the COVID-19 pandemic in Hong Kong, with a more drastic reduction observed in children aged 0–6 years who are particularly prone to FS. Furthermore, in western countries, similar decreasing trends of seizure- and FS-related emergency department visits were recorded in the early period of the COVID-19 pandemic (12,13).
Strict infection control measures, such as wearing a mask, handwashing, social distancing, and staying at home measures, may have reduced the burden of febrile illnesses and, consequently, the incidence of FSs. However, in the United States, seizure-related emergency department attendance in children returned to the pre-COVID-19 pandemic levels in 2021 (13). As a result of the relaxation of NPIs, a resurgence in the incidence of FSs is expected, and appropriate arrangements to cope with this should be made.
Respiratory viral infections are one of the most predominant causes of fever in FSs (2). Although the most common virus detected in children with FS varies across studies (2,4,14,15), the seasonal distribution of the viruses tended to correspond to those observed in community-acquired respiratory tract infections when FSs developed (4,16). In the current study, the influenza virus was the most commonly isolated virus associated with FS before the COVID-19 pandemic; in contrast, no cases of influenza were identified during the pandemic. Rhinovirus, which is another common virus implicated in FS before the COVID-19 pandemic, remained a significant cause during the pandemic. During the COVID-19 pandemic, a significant decrease in the incidence of influenza was noted in the community in many countries, including South Korea, while rhinovirus infections continued (17-19). Whereas the detection rates of bocavirus and parainfluenza viruses were significantly higher during the COVID-19 pandemic than before the pandemic in this study. Regarding the seasonal prevalence of bocavirus in South Korea, this trend was particularly noticed between the autumn of 2020 and the spring of 2021 (17). Furthermore, the community outbreak of parainfluenza viruses was identified in the autumn of 2021 in Korea, contrary to the usually increased prevalence in the spring and summer (17). During the COVID-19 pandemic, parainfluenza viruses triggered more FS in the autumn than in the spring and summer, and consequently, the corresponding incidence of FS in the autumn was significantly increased during the COVID-19 pandemic. This anomalous behavior of the parainfluenza virus in the autumn could be attributed to several external factors, such as gradual phasing-out restrictions due to increased vaccination rate beginning in October 2021 and increased social mingling during Korean Thanksgiving in September 2021 (20). As a result, the trend of respiratory viruses isolated in the pediatric population with FS represents the circulating respiratory viruses in the community during the COVID-19 pandemic, which was significantly different from that noted before the pandemic began. Given the resurgence of respiratory pathogens, it is imperative to plan for unanticipated viral epidemics of unpredictable magnitudes. SARS-CoV-2 was detected in 3.3% of FS episodes during the COVID-19 pandemic until February 2022 in this study. With the dramatic rise in the incidence of FS observed with the emergence of the omicron variant of SARS-CoV-2 (21) and its subsequent dominance in Korea after late January 2022, the resultant change in the incidence of FS in 2022 should be further investigated.
Our study demonstrated that statistically significant between-group differences were not observed in the clinical presentation and outcomes of FS before and during the COVID-19 pandemic, despite distributional changes in respiratory viral infections during the COVID-19 pandemic. In compliance with previous studies (4,14), our results revealed no significant association between the type of isolated respiratory virus and the prognosis of FS. This could be attributed to the common inflammatory response against viruses rather than the specific role of the virus in the pathogenesis of FS. However, the proportion of children with two or more attacks of FS was significantly fewer during the COVID-19 pandemic than before the pandemic began. Parents whose children had suffered FSs before are better prepared to deal with recurrent FSs; therefore, their concern for acquiring nosocomial COVID-19 might have outweighed the need to visit the hospital for recurring FS. Our study also showed that there were no statistically significant inter-group differences in the clinical characteristics of FS among patients with a first FS.
This study has certain limitations. First, due to the retrospective nature of the study, some of the data was unavailable, and RIDTs and mPCR tests were omitted in more than half of the enrolled children, which could have resulted in underreporting of the actual figures. However, no statistically significant inter-group differences were observed among patients who underwent viral tests. In addition, unknown confounding variables in the distribution of respiratory viral pathogens in FS might remain unrecognized in this retrospective study. Although the diagnoses of FS were based on the ICD-10 codes in medical records, discrepancies between diagnostic codes and actual disease might occur in patients with FS. Additionally, there is a possibility that the circumstances during the COVID-19 pandemic may impede patients from visiting the hospital, potentially resulting in underreporting of the actual occurrence of FS. Third, the true distributional pattern of detected respiratory viral pathogens could not be evaluated due to potential bias caused by differences in the commercial mPCR test kits between centers. For instance, parainfluenza virus type 4 and enteroviruses were not detected by the mPCR test kits at Incheon St. Mary’s Hospital. Since parainfluenza virus type 3 has been the most prevalent serotype among types 1–4 in Korea and was largely responsible for the epidemic in the autumn of 2021 (20), the actual impact of undetected parainfluenza virus type 4 was considered to be marginal. Furthermore, considering comparable incidences of hand, foot, and mouth disease and herpangina, both of which are clinical phenotypes of enterovirus infection, before and during the COVID-19 pandemic, the omission of enterovirus testing in one hospital may have had little impact on the results. Moreover, as the data was gathered from two referral hospitals, the results may not be representative of the general population. Lastly, the relatively shorter follow-up period in our study among patients who had FS during the pandemic compared to those before the pandemic was insufficient to evaluate the subsequent development of epilepsy. Thus, larger studies or collaborative trials with longer duration are warranted to accurately elucidate the impact of the COVID-19 outbreak on the incidence of respiratory viral infections and seizure characteristics of FS.
Conclusions
The occurrence of FS declined during the COVID-19 pandemic when strict NPIs were in place within the community. In children with FS, a substantial reduction in the incidence of influenza virus infections was observed during the pandemic, while a significantly high incidence of parainfluenza virus infections was observed during the pandemic. The distribution of detected respiratory viruses in children with FS was similar to that observed in the community during the COVID-19 pandemic. Despite changing incidence of detected viruses, the clinical characteristics and outcomes of FS were comparable before and during the pandemic. Therefore, the strategies to manage children with FS need not differ before and during the COVID-19 pandemic.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-406/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-406/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-406/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Catholic Medical Center (No. OC22RASI0007) and individual informed consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics 2008;121:1281-6. [Crossref] [PubMed]
- Millichap JG, Millichap JJ. Role of viral infections in the etiology of febrile seizures. Pediatr Neurol 2006;35:165-72. [Crossref] [PubMed]
- Manfredini R, Vergine G, Boari B, et al. Circadian and seasonal variation of first febrile seizures. J Pediatr 2004;145:838-9. [Crossref] [PubMed]
- Han JY, Han SB. Febrile Seizures and Respiratory Viruses Determined by Multiplex Polymerase Chain Reaction Test and Clinical Diagnosis. Children (Basel) 2020;7:234. [Crossref] [PubMed]
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382:727-33. [Crossref] [PubMed]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. [Crossref] [PubMed]
- Jones P, Menon A, Hicken A, et al. Global adoption of personal and social mitigation behaviors during COVID-19: The role of trust & confidence. PLoS One 2021;16:e0256159. [Crossref] [PubMed]
- Hatoun J, Correa ET, Donahue SMA, et al. Social Distancing for COVID-19 and Diagnoses of Other Infectious Diseases in Children. Pediatrics 2020;146:e2020006460. [Crossref] [PubMed]
- Ahn JG. Epidemiological changes in infectious diseases during the coronavirus disease 2019 pandemic in Korea: a systematic review. Clin Exp Pediatr 2022;65:167-71. [Crossref] [PubMed]
- Park KH, Choe YJ, Shim Y, et al. Decrease in incidence of febrile seizure following social distancing measures: A national cohort study in South Korea. Pediatr Infect Vaccine 2021;28:144-8. [Crossref]
- Chiu TGA, Leung WCY, Zhang Q, et al. Changes in pediatric seizure-related emergency department attendances during COVID-19 - A territory-wide observational study. J Formos Med Assoc 2021;120:1647-51. [Crossref] [PubMed]
- Davico C, Marcotulli D, Lux C, et al. Where have the children with epilepsy gone? An observational study of seizure-related accesses to emergency department at the time of COVID-19. Seizure 2020;83:38-40. [Crossref] [PubMed]
- Sapkota S, Caruso E, Kobau R, et al. Seizure- or Epilepsy-Related Emergency Department Visits Before and During the COVID-19 Pandemic - United States, 2019-2021. MMWR Morb Mortal Wkly Rep 2022;71:703-8. [Crossref] [PubMed]
- Francis JR, Richmond P, Robins C, et al. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr 2016;16:202. [Crossref] [PubMed]
- Rudolph H, Gress K, Weiss C, et al. General Characteristics of Children with Single- and Co-Infections and Febrile Seizures with a Main Focus on Respiratory Pathogens: Preliminary Results. Pathogens 2021;10:1061. [Crossref] [PubMed]
- Han DH, Kim SY, Lee NM, et al. Seasonal distribution of febrile seizure and the relationship with respiratory and enteric viruses in Korean children based on nationwide registry data. Seizure 2019;73:9-13. [Crossref] [PubMed]
- Shi HJ, Kim NY, Eom SA, et al. Effects of Non-Pharmacological Interventions on Respiratory Viruses Other Than SARS-CoV-2: Analysis of Laboratory Surveillance and Literature Review From 2018 to 2021. J Korean Med Sci 2022;37:e172. [Crossref] [PubMed]
- Li ZJ, Yu LJ, Zhang HY, et al. Broad Impacts of Coronavirus Disease 2019 (COVID-19) Pandemic on Acute Respiratory Infections in China: An Observational Study. Clin Infect Dis 2022;75:e1054-e1062. [Crossref] [PubMed]
- Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1305-9. [Crossref] [PubMed]
- Kim HN, Yoon SY, Lim CS, et al. Phylogenetic analysis of human parainfluenza type 3 virus strains responsible for the outbreak during the COVID-19 pandemic in Seoul, South Korea. J Clin Virol 2022;153:105213. [Crossref] [PubMed]
- Cloete J, Kruger A, Masha M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health 2022;6:294-302. [Crossref] [PubMed]


