
Genetic background and clinical characteristics of infantile hyperammonemia
Highlight box
Key findings
• There were significant differences in the genetic spectrum, clinical features, clinical course, and outcomes between infants with different hyperammonemia onset ages.
What is known and what is new?
• Urea cycle defects, organic acidemia, and fatty acid oxidation disorders are the most common inborn errors of metabolism associated with hyperammonemia.
• Genetic spectrum, clinical features, clinical course, and outcomes vary with age of infantile hyperammonemia onset.
• Genetic features underlying cholestasis may be associated with hyperammonemia.
What is the implication, and what should change now?
• Further studies regarding the genetic profile and related metabolic pathways of HA should be carried out.
• Early NGS analysis is needed if HA is suspected.
Introduction
Hyperammonemia (HA) refers to an increased blood ammonia level, which can cause irreversible damage to the central nervous system, especially in pediatric patients (1). Normal plasma ammonia levels in premature newborns, full-term newborns, infants, and children decrease with time, but their reference values have not been well defined thus far (2-4). In this study, we used plasma ammonia ≥100 µmol/L as the diagnostic threshold for infantile HA.
HA is primarily caused by severe liver diseases, infections, and certain drugs. However, in children, especially infants, inborn errors of metabolism (IEM) play an important role in the development of HA (5-8). IEM is a heterogeneous group of disorders with complex clinical manifestations. Most patients with inherited HA exhibit non-specific symptoms, such as poor feeding, lethargy, dyspnea, or hypothermia, which rapidly progress to convulsions or coma; these general symptoms make it difficult to differentiate HA from conditions such as sepsis (9,10). The time window for clinical diagnosis is very short, and timely etiology-based therapy is critical. The diagnostic rate of IEM has improved with the use of mass spectrometry. However, the spectrum of diseases that can be detected by mass spectrometry is limited, and the detection results are affected by several factors such as patient condition and the treatment given, which may cause misdiagnosis and underdiagnosis (11).
Next-generation sequencing (NGS) is applicable to a wide spectrum of diseases, and the outcomes are generally not affected by atypical clinical manifestations and laboratory tests, which can be used for the early diagnosis of disease and indicate possible underlying genetic causes (12). NGS has been applied to the early diagnosis and precision therapy of many disorders, such as infantile epilepsy, neonatal metabolic acidosis, and neonatal hypernatremia (13-15). For infantile HA, precision treatment based on orphan drugs, liver stem cell transplantation, gene therapy, and prenatal treatment have been increasingly reported (16-21). All of those previous studies were based on the application of genetic analysis to diagnosis, and NGS was the key diagnostic method used (21).
The known genetic causes of infantile HA mainly include urea cycle disorder (UCD), organic acidemia (OA), and fatty acid oxidation disorder (FAOD) (6-8,22,23). A wide range of genes have been identified and reported in the human phenotype ontology associated with infantile HA (24-26). However, in clinical practice, the status of several other genes, such as ABCC8 (27) and GALT (28,29), in HA remains unknown. In the present study, we conducted a retrospective analysis of a cohort with genetically confirmed diagnosis of infantile HA to expand its genetic background. To the best of our knowledge, this is the most comprehensive study to determine the genetic background of infantile HA. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-359/rc).
Methods
Study population
We enrolled infants diagnosed with HA at the Children’s Hospital of Fudan University from January 2016 to June 2020. The inclusion criteria were as follows: (I) HA onset before 1 year of age, (II) plasma ammonia level ≥100 µmol/L as indicated by more than two tests during the same hospital stay, and (III) genetic diagnosis using NGS. The exclusion criteria were as follows: (I) liver failure secondary to severe infection, respiratory failure or graft-versus-host disease, severe gastrointestinal bleeding, long-term total parenteral nutrition, and history of valproate use; (II) maternal autoimmune conditions; and (III) failure to obtain informed consent from the parents. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Research Ethics Committee of the Children’s Hospital of Fudan University (No. 2015-130). Written informed consent was obtained from patients’ parents or legal guardians.
Clinical subgroup classification
Clinical information was obtained from the medical record system. The clinical course of each case was classified as follows: (I) refractory, the last blood ammonia level during one hospital stay ≥100 µmol/L after treatment; (II) controllable, the last blood ammonia level during one hospital stay <100 µmol/L after treatment; and (III) self-limiting, the last blood ammonia level during one hospital stay <100 µmol/L without treatment. Follow-up information was extracted from the outpatient medical record system, and cases with missing follow-up information were followed up via telephone.
NGS
Genomic DNA was extracted from the blood samples of patients using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) and enriched using the Agilent (Santa Clara, CA, USA) ClearSeq Inherited Disease panel kit for 2,742 gene sequencing or the Agilent SureSelect XT Human All Exon V5 for clinical exome sequencing. NGS was performed using the Illumina HiSeq2000/2500 platform. The identified variants were classified based on the American College of Medical Genetics (ACMG) guidelines (30). The detected causal variants were confirmed by performing Sanger sequencing on a Biosystems 3500 DNA Analyzer and analyzed using Mutation Surveyor V4.0.9. More details are available in our previous studies (15,31).
Statistical analysis
The data were analyzed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables with non-normal variables are reported as median [interquartile range (IQR)]. Categorical variables are presented as frequencies and percentages. The chi-square test or Fisher’s exact test was used for comparison. Significance was set at P<0.05.
Results
Study population
We enrolled 85 infants with hyperammonemia who had a definite genetic diagnosis. The median age of onset was 54 days (IQR, 10–114 days). The study included 54 male (64%, 54/85) and 31 female (36%, 31/85) patients who were divided into two subgroups according to age of onset: neonate group (32 cases, 38%) and infant group (53 cases, 62%). The dominant phenotypes (a patient may have had more than one clinical phenotype) were neurological abnormality (31%), glucose metabolism disturbance (26%), metabolic acidosis (24%), respiratory failure (15%), and electrolyte disturbance (14%) (Table 1).
Table 1
| Clinical features | Total, N=85, n [%] | Neonatal, N=32, n [%] | <1 year, N=53, n [%] | P value |
|---|---|---|---|---|
| Metabolic condition spectrum | ||||
| OA | 12 [14] | 10 [31] | 2 [4] | 0.001 |
| FAOD | 5 [6] | 5 [16] | 0 [0] | 0.006 |
| UCD | 6 [7] | 4 [13] | 2 [4] | 0.416 |
| Cholestasis | 47 [55] | 6 [19] | 41 [77] | <0.001 |
| Clinical features | ||||
| Peak NH3 ≥500 μmol/L | 9 [11] | 8 [25] | 1 [2] | 0.003 |
| Neurologic abnormality | 26 [31] | 21 [66] | 5 [9] | <0.001 |
| Respiratory failure | 13 [15] | 11 [34] | 2 [4] | <0.001 |
| Circulatory failure | 6 [7] | 4 [13] | 2 [4] | 0.192 |
| Hepatic failure | 7 [8] | 0 [0] | 7 [13] | 0.042 |
| Severe infection | 8 [9] | 5 [16] | 3 [6] | 0.146 |
| Malformation | 9 [11] | 4 [13] | 5 [9] | 0.935 |
| Metabolic acidosis | 20 [24] | 14 [44] | 6 [11] | 0.001 |
| Hyperlactacidemia | 6 [7] | 3 [9] | 3 [6] | 0.668 |
| Glucose metabolic disturbance | 22 [26] | 13 [41] | 9 [17] | 0.016 |
| Electrolyte disturbance | 12 [14] | 9 [28] | 3 [6] | 0.010 |
| Treatment | ||||
| Arginine | 31 [36] | 16 [50] | 15 [28] | 0.051 |
| CRRT | 2 [2] | 2 [6] | 0 [0] | 0.133 |
| Precision medicine | 30 [35] | 16 [50] | 14 [26] | 0.027 |
| Clinical course of HA | ||||
| Self-limited | 23 [27] | 5 [16] | 18 [34] | 0.065 |
| Controllable | 44 [52] | 14 [44] | 30 [57] | 0.251 |
| Refractory | 18 [21] | 13 [41] | 5 [9] | 0.001 |
| Clinical outcomes | ||||
| Improved | 56 [66] | 12 [38] | 44 [83] | <0.001 |
| Withdrew treatment | 22 [26] | 15 [47] | 7 [13] | 0.001 |
| Died | 7 [8] | 5 [16] | 2 [4] | 0.098 |
OA, organic acidemia; FAOD, fatty acid oxidation disorder; UCD, urea cycle disorder; CRRT, continuous renal replacement therapy; Precision medicine refers to special formula or diet, drugs and liver transplantation; HA, hyperammonemia.
Genetic spectrum of infantile HA
Collectively, 136 pathogenic or likely pathogenic (P/LP) variants were identified in 33 genes, with 110 reported variants and 26 novel variants (Table S1). Of the genes identified, fourteen have been associated to HA (42%, 14/33), with SLC25A13 and MUT being the top two detected genes (22%, 18/85). Nineteen genes, which have not been previously reported with HA, were detected in this study (58%, 19/33), of which JAG1 and ABCC8 were the most frequently mutated genes (16%, 14/85).
Recurrent conditions included cholestasis (55%, 47/85), OA (14%, 12/85), UCD (7%, 6/85), and FAOD (6%, 5/85), with SLC25A13, MUT, CPS1, and SLC25A20 being the most frequently detected genes in each classification, respectively.
Genetic features of infantile HA depending on onset age
Subgroups with different ages of onset showed different conditions. The proportions of OA (P=0.001), FAOD (P=0.006), and cholestasis (P<0.001) showed significant differences between the two subgroups (Table 1). MUT (19%, 6/32) was the most frequently detected gene in the neonatal group, whereas in the post-neonatal group, the most frequently mutated gene was SLC25A13 (21%, 11/53).
In cases of refractory HA (21%, 18/85), all mutated genes in the neonatal group were reported with HA, with MUT being the most frequently identified gene (Figure 1). In the post-neonatal group, mutated ABCB11, CYP7B1, and MPV17, which have not been previously reported with the HA phenotype, were associated with refractory HA; and all these cases presented with primary liver failure.
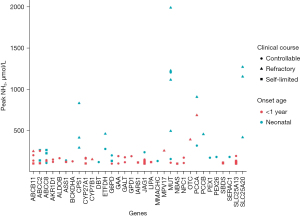
In controllable or self-limiting HA cases (79%, 67/85), mutations in SLC25A13, JAG1, and ABCC8 were detected in more than five cases, accounting for 37% (25/67) of all cases. Mutated SLC25A13 and most mutated JAG1 (7/8) were detected in the infantile group, and all cases presented a cholestatic phenotype. In the neonatal group, ABCC8 was the most frequently mutated gene, and all cases presented with hypoglycemia and mild to moderate elevation of liver enzymes. Notably, mutated JAG1 and ABCC8 with the hypoglycemic phenotype have not been previously reported with HA.
Clinical characteristics of inherited HA according to the age of onset
To investigate the characteristics of inherited HA according to the age of onset, we compared the clinical phenotypes of the neonatal and infantile groups. We found significant differences in the clinical features, clinical course, and outcomes between the two subgroups.
In the neonatal subgroup, 25% cases (8/32) presented with a peak plasma ammonia level ≥500 µmol/L, compared to 2% cases in the infantile group (1/53, P=0.003). Neonatal patients with HA showed higher rates of neurological abnormalities (P<0.001), respiratory failure (P<0.001), metabolic acidosis (P=0.001), glucose metabolism disturbance (P=0.016), and electrolyte disturbances (P=0.010) than infantile patients (Table 1). Infants with HA were more likely to present with hepatic failure than neonates (P=0.042).
In our study, 35% (30/85) patients received precision medicine, including special formula or diet, drugs and liver transplantation (details in Table S1). There is no significant statistical difference between neonatal group (16/32, 50%) and infant group (14/53, 26%, P=0.027).
Among the neonatal subgroup, 41% patients (13/32) presented with a refractory clinical course, compared to just 9% patients in the infant group (5/53, P=0.001). Clinical outcomes in the infant group were generally better than those in the neonatal group, with 83% patients showing improvement (44/53) and only 13% patients (7/53) withdrawing the treatment in consideration of the poor prognosis. In contrast, in the neonatal group, only 38% (12/32) patients had good prognoses (P<0.001), and 47% (15/32) patients withdrew from the treatment (P=0.001) given the poor prognosis.
Discussion
We observed a significantly different genetic spectrum between patients with neonatal and post-neonatal HA. In our cohort, the genetic spectrum of neonatal HA mainly included OA, FAOD, and UCD, while the proportion of these disorders was lower in the post-neonatal subgroup, which is consistent with the etiological profile reported previously (6-8,22,23). Defects in the function of any enzyme or carrier involved in the urea cycle can cause primary HA. HA caused by OA and FAOD is secondary to the functional inhibition of enzymes involved in the urea cycle by their metabolites and the reduction in substrates required for urea synthesis (5). We speculate that this difference in the genetic spectrum between subgroups based on the age of onset is because the severity of most cases of UCD, OA, and FAOD is related to the degree of enzyme-related defects in the corresponding metabolic pathways (32). Patients with reduced enzyme activity may have an earlier onset of the disease, exhibit more severe clinical presentations (higher plasma ammonia level), and may not survive the neonatal period. In other words, patients with UCD, OA, or FAOD onset after 1 month of age may exhibit lower ammonia levels, and therefore, were not included in our cohort. This also explains why patients in the neonatal subgroup showed higher rates of a refractory clinical course and poor prognosis. Furthermore, there are relatively few UCD patients in our study, the reason may be that UCD patients died too quickly to be included in this study.
In this study, hereditary liver disease was the main genetic cause of HA onset before one year of age. Neonatal-onset type II citrullinemia (MIM 605814, SLC25A13) (neonatal intrahepatic cholestasis caused by citrin deficiency, NICCD) is the most common hereditary liver disease in this study. NICCD is known to cause mild, self-limiting HA. NICCD pathogenesis involves citrin deficiency caused by the pathogenic variants of SLC25A13, which leads to the insufficient transfer of aspartic acid into the cytoplasm and affects its utilization for urea cycle substrate synthesis (10). Other cholestasis-related genes, such as JAG1, ABCC2, and ABCB11, have not been reported to cause HA phenotype. In our study, most patients with cholestasis did not show significantly elevated transaminase levels, suggesting that in patients presenting with HA along with cholestasis, the phenotype may be related to cholestasis itself.
Pathogenic variants of ABCC8 were frequently observed in our cohort (7%, 6/85). Neonatal diabetes caused by ABCC8 defects may be associated with HA phenotype (27). The authors speculated that elevated serum leucine and glutamic acid levels during ketoacidosis promote the oxidative deamination of glutamic acid to increase blood ammonia levels. However, in our study, the six patients with ABCC8 defects presented with hyperinsulinemia rather than diabetes. Therefore, ABCC8-specific pathogenesis must be clarified further. HA can present with hyperinsulinism/hyperammonemia syndrome (MIM 606762, GLUD1), wherein an excessive increase in the activity of GLUD1-encoded glutamate dehydrogenase (GDH) increases glutamate oxidative deamination and glutamate consumption, which leads to a reduction in N-acetylglutamate (NAG), the activator of the urea cycle rate-limiting enzyme, thus indirectly affecting urea synthesis (33). GDH overactivity can increase the ATP/ADP ratio in islet cells, thereby closing the K+-ATP channel, depolarizing the cell membrane, and opening the calcium channel, leading to insulin release. Variants of ABCC8 cause abnormalities in the K+-ATP channel in islet cells (34), which may be associated with GDH activity to some extent.
Two patients in our cohort had galactosemia (MIM 230400, GALT). Although one patient had liver failure, the HA was controllable. In a previous case report of galactosemia, a neonatal patient with mildly elevated transaminase levels developed transient HA. The authors speculated that this may be due to the toxic effects of galactose on hepatocytes (28). In a phenotype-genotype analysis of five patients with galactosemia, one patient with HA carried the c. 687 G >A variant as one of the patients in the cohort of our present study (29). This perhaps suggests a correlation between this GALT variant and HA; however, further studies are needed.
We also observed HA in three children with hemolytic anemia and G6PD deficiency (favism) (MIM 300908, G6PD), including one with cholestasis, one with severe infection, and another with bilirubin encephalopathy. Since patients exhibiting severe hemolysis (such as severe fracture and trauma) may present with HA (10), HA in patients with G6PD deficiency may be related to severe hemolysis.
This study expanded the spectrum of pathogenic variants associated with infantile HA and enriched its possible genetic background. However, this study has certain limitations. First, we must admit that our definitions of clinical subgroup classification may have some potential conflicts. Second, NGS cannot detect all pathogenic variants, and thus, its use in a clinical setting may lead to underdiagnosis. Third, we did not elucidate the mechanism underlying the correlation between the variants detected in this study and HA; hence, further research is needed to fully understand this relationship. Finally, this was a retrospective single-center study with few participants and limited phenotypic data, which may result in bias. Therefore, a prospective multi-center study including more participants should be carried out in the future.
Conclusions
In this study, we performed NGS to reveal the genetic background and clinical characteristics of infantile HA. In clinical practice, when IEM is considered, blood ammonia should be routinely tested. We recommend early NGS analysis if HA is suspected. Based on the toxic effects of various metabolic products on hepatocytes and the extensive linkages between various metabolic pathways, the genetic profile and related metabolic pathways of HA deserve further study.
Acknowledgments
Funding: This work was supported by the Shanghai Municipal Science and Technology Major Project (Grant No. 20Z11900600).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-359/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-359/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-359/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Braissant O, McLin VA, Cudalbu C. Ammonia toxicity to the brain. J Inherit Metab Dis 2013;36:595-612. [Crossref] [PubMed]
- Madigan T, Block DR, Carey WA, et al. Proposed Plasma Ammonia Reference Intervals in a Reference Group of Hospitalized Term and Preterm Neonates. J Appl Lab Med 2020;5:363-9. [Crossref] [PubMed]
- Häberle J. Clinical practice: the management of hyperammonemia. Eur J Pediatr 2011;170:21-34. [Crossref] [PubMed]
- Cormack BE, Jiang Y, Harding JE, et al. Plasma ammonia concentrations in extremely low birthweight infants in the first week after birth: secondary analysis from the ProVIDe randomized clinical trial. Pediatr Res 2020;88:250-6. [Crossref] [PubMed]
- Walker V. Ammonia metabolism and hyperammonemic disorders. Adv Clin Chem 2014;67:73-150. [Crossref] [PubMed]
- Vergano SA, Crossette JM, Cusick FC, et al. Improving surveillance for hyperammonemia in the newborn. Mol Genet Metab 2013;110:102-5. [Crossref] [PubMed]
- Chow SL, Gandhi V, Krywawych S, et al. The significance of a high plasma ammonia value. Arch Dis Child 2004;89:585-6. [Crossref] [PubMed]
- Abily-Donval L, Dupic L, Joffre C, et al. Management of 35 critically ill hyperammonemic neonates: Role of early administration of metabolite scavengers and continuous hemodialysis. Arch Pediatr 2020;27:250-6. [Crossref] [PubMed]
- Häberle J, Burlina A, Chakrapani A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis 2019;42:1192-230. [Crossref] [PubMed]
- Summar ML, Mew NA. Inborn Errors of Metabolism with Hyperammonemia: Urea Cycle Defects and Related Disorders. Pediatr Clin North Am 2018;65:231-46. [Crossref] [PubMed]
- Ozben T. Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem mass spectrometry. Clin Chem Lab Med 2013;51:157-76. [Crossref] [PubMed]
- Park KJ, Park S, Lee E, et al. A Population-Based Genomic Study of Inherited Metabolic Diseases Detected Through Newborn Screening. Ann Lab Med 2016;36:561-72. [Crossref] [PubMed]
- Hu L, Yang L, Yan K, et al. Importance of Early Genetic Sequencing in Neonates Admitted to NICU with Recurrent Hypernatremia: Results of a Prospective Cohort Study. Neonatology 2022;119:103-10. [Crossref] [PubMed]
- Ma H, Tang Z, Xiao F, et al. Neonatal Metabolic Acidosis in the Neonatal Intensive Care Unit: What Are the Genetic Causes? Front Pediatr 2021;9:727301. [Crossref] [PubMed]
- Yang L, Kong Y, Dong X, et al. Clinical and genetic spectrum of a large cohort of children with epilepsy in China. Genet Med 2019;21:564-71. [Crossref] [PubMed]
- Sugiyama Y, Shimura M, Ogawa-Tominaga M, et al. Therapeutic effect of N-carbamylglutamate in CPS1 deficiency. Mol Genet Metab Rep 2020;24:100622. [Crossref] [PubMed]
- Chakrapani A, Valayannopoulos V, Segarra NG, et al. Effect of carglumic acid with or without ammonia scavengers on hyperammonaemia in acute decompensation episodes of organic acidurias. Orphanet J Rare Dis 2018;13:97. [Crossref] [PubMed]
- Spada M, Porta F, Righi D, et al. Intrahepatic Administration of Human Liver Stem Cells in Infants with Inherited Neonatal-Onset Hyperammonemia: A Phase I Study. Stem Cell Rev Rep 2020;16:186-97. [Crossref] [PubMed]
- Wang L, Yang Y, Breton C, et al. A mutation-independent CRISPR-Cas9-mediated gene targeting approach to treat a murine model of ornithine transcarbamylase deficiency. Sci Adv 2020;6:eaax5701. [Crossref] [PubMed]
- Wilnai Y, Blumenfeld YJ, Cusmano K, et al. Prenatal treatment of ornithine transcarbamylase deficiency. Mol Genet Metab 2018;123:297-300. [Crossref] [PubMed]
- Wang HJ, Zhou WH. Intervention of neonatal genetic diseases in the era of precision medicine: challenges and opportunities. Zhonghua Er Ke Za Zhi 2018;56:244-6. [PubMed]
- Arbeiter AK, Kranz B, Wingen AM, et al. Continuous venovenous haemodialysis (CVVHD) and continuous peritoneal dialysis (CPD) in the acute management of 21 children with inborn errors of metabolism. Nephrol Dial Transplant 2010;25:1257-65. [Crossref] [PubMed]
- Deodato F, Boenzi S, Rizzo C, et al. Inborn errors of metabolism: an update on epidemiology and on neonatal-onset hyperammonemia. Acta Paediatr Suppl 2004;93:18-21. [Crossref] [PubMed]
- Jiang HH, Guo Y, Shen X, et al. Neonatal maple syrup urine disease in China: two novel mutations in the BCKDHB gene and literature review. J Pediatr Endocrinol Metab 2021;34:1147-56. [Crossref] [PubMed]
- Ali A, Almesmari FSA, Dhahouri NA, et al. Clinical, Biochemical, and Genetic Heterogeneity in Glutaric Aciduria Type II Patients. Genes (Basel) 2021;12:1334. [Crossref] [PubMed]
- Köhler S, Gargano M, Matentzoglu N, et al. The Human Phenotype Ontology in 2021. Nucleic Acids Res 2021;49:D1207-17. [Crossref] [PubMed]
- Thakkar AN, Muranjan MN, Karande S, et al. Neonatal diabetes mellitus due to a novel ABCC8 gene mutation mimicking an organic acidemia. Indian J Pediatr 2014;81:702-4. [Crossref] [PubMed]
- Cheung KL, Tang N, Hsiao KJ, et al. Classical galactosaemia in Chinese: A case report and review of disease incidence. J Paediatr Child Health 1999;35:399-400. [Crossref]
- Yang RL, Tong F, Hong F, et al. Analysis of newborn screening for galactosemia and genotype-phenotype of confirmed galatosemia cases. Zhonghua Er Ke Za Zhi 2017;55:104-9. [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Yang L, Dong X, Peng X, et al. Evaluation of turn around time and diagnostic accuracy of the next generation sequencing data analysis pipeline version 2 of Children's Hospital of Fudan University. Chinese Journal of Evidence Based Pediatrics 2018;13:118-23.
- Wakiya T, Sanada Y, Urahashi T, et al. Impact of enzyme activity assay on indication in liver transplantation for ornithine transcarbamylase deficiency. Mol Genet Metab 2012;105:404-7. [Crossref] [PubMed]
- Stanley CA. Hyperinsulinism/hyperammonemia syndrome: insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol Genet Metab 2004;81:S45-51. [Crossref] [PubMed]
- Arya VB, Aziz Q, Nessa A, et al. Congenital hyperinsulinism: clinical and molecular characterisation of compound heterozygous ABCC8 mutation responsive to Diazoxide therapy. Int J Pediatr Endocrinol 2014;2014:24. [Crossref] [PubMed]


