
A mid- and long-team follow-up study of the application of single-valved bovine pericardium patches in right ventricular outflow tract reconstruction
Highlight box
Key findings
• Single-valved bovine pericardium patch (svBPP) has good durability and is an ideal material for right ventricular outflow tract (RVOT) reconstruction.
What is known and what is new?
• Allogeneic valved conduits, porcine-valved Dacron conduits, bovine jugular vein conduits, and many other modified valved conduits have no growth potential and are associated with high reoperation rate.
• Autologous tissue was used as the base of RVOT, and the RVOT was reconstructed by using svBPP. This technique significantly reduced pulmonary regurgitation and protected right heart function; meanwhile, it had growth potential and significantly reduced the reoperation rate.
What is the implication, and what should change now?
• In the future, for patients without ventricle to pulmonary artery connection, the left atrial appendage can be used to connect the right ventricle with the base of RVOT, and the RVOT can be reconstructed with svBPP, so as to improve the prognosis of these patients.
Introduction
Valve problems in newborns, infants, and children cannot be treated by valve replacement due to growth and developmental requirements. Thus, repair with a growth-accommodating valve or annulus has long been pursued in cardiac surgery. Valve replacement for pulmonary stenosis or atresia is the most common procedure among patients with congenital heart disease. However, right heart dysfunction due to valvular incompetence following reconstruction of the right ventricular outflow tract (RVOT) has never been improved due to valve dysplasia or defect. In 1964, Rastelli et al. created the right ventricle-to-pulmonary artery (RV-PA) conduit (1). Since then, allogeneic valved conduits, porcine-valved Dacron conduits, bovine jugular vein conduits, and many other modified valved conduits have emerged, greatly improving the availability and durability of conduits and overcoming the problem of valve regurgitation; however, the reoperation rate remains high due to the lack of growth potential of these conduits. With the rapid advances in tissue engineering technology, bovine pericardium (BP) has become an excellent transplant material due to its good biocompatibility and bioactivity. The use of single-valved bovine pericardium patch (svBPP) in RVOT reconstruction allows the pulmonary arteries to maintain enhanced growth ability and good anti-reflux function, which either reduces the need for reoperation or maximizes the time to reoperation. In our current study, BalMonocTM (Balance Medical, Beijing, China) svBPP was used to reconstruct the RVOT across the annulus, and the long-term follow-up results were summarized. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-97/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Guangdong Women and Children Hospital (IRB approval number: 202301044) and written informed consent was obtained from all study participants or their legal guardians. A total of 88 patients undergoing RVOT reconstruction using svBPP between October 2010 and August 2020 were enrolled. Preoperative assessments including cardiac color ultrasound, cardiac computed tomography (CT) as well as calculation of M rate, Nakata index, and left ventricular end-diastolic volume index (LVEDVI) were performed before surgery, and treatment protocols were developed accordingly. A corrective surgery was indicated for all patients. Upon the completion of the corrective surgery, svBPP was applied for RVOT reconstruction. For children without right ventricle to pulmonary artery connection (RVPA connection), réparation à l'Etage ventriculaire (REV) or Barbero-Marcial procedure was performed to reconstruct the connection.
The surgery was performed under anesthesia and cardiopulmonary bypass (CPB). Effective postoperative monitoring and regular follow-up after discharge were implemented. Length of hospital stay, duration of assisted ventilation, and postoperative complications were recorded during the perioperative period. The cardiac ultrasound-related indicators during the follow-up visits included ejection fraction (EF), right ventricular end-diastolic diameter (EDD), pulmonary regurgitation, and pulmonary artery stenosis. Valvular regurgitation and stenosis were evaluated according to the European Association of Echocardiography recommendations (2). Patients over 8 years old underwent cardiac magnetic resonance imaging (MRI) to evaluate their right heart function.
Surgical methods
After endotracheal intubation, combined intravenous and inhalation anesthesia was applied. A sternal median incision was made, and the operation was completed under induced hypothermia (range: 28 to 32 ℃) during CPB. Custodiol HTK® cardioplegia solution (Cardiolink, Barcelona, Spain) was uniformly applied for controlled aortic root perfusion. After the stopping of the heart, the RVOT and the main pulmonary artery were incised longitudinally, and the hypertrophic septal band and parietal band as well as parts of the supraventricular crest were removed. Polyester patch (PP) or svBPP were used to repair intracardiac malformations. For patients without a pulmonary trunk, Barbero-Marcial procedure (left or right atrial appendage was used to create the base of the pulmonary artery) or REV was performed to establish the base of RVOT-pulmonary artery connection.
Selection and trimming of single valve
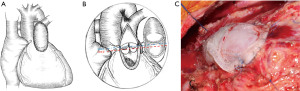
- For patients whose valve leaflets were small or absent, the single valve model (A) needed to be equal to or slightly greater than 1.5 times the preset diameter of pulmonary artery, and the base of the autologous pulmonary artery (annulus) should be equal to the svBPP A (Figure 1).
- For cases with 2 leaflets, the single valve model (A) was equal to the preset diameter of pulmonary artery, or the single-valve model (A) was slightly larger than r1 + r2 (Figure 2).
Suturing of svBPP
After the intracardiac malformation was repaired and the RVOT was unblocked, svBPP was selected using the above method. The svBBP was sutured using the 5-0 or 6-0 Prolene sutures, and the ventricle to pulmonary artery connection was reconstructed. The installed height of the free margin of the single valve was 3–5 mm higher than the normal annulus (Figure 3).
Statistical analysis
Statistical analysis was performed using SAS 9.4 software package (SAS Institute, Cary, NC, USA). Continuous variables included number, means, standard deviations, and minimum and maximum values. Categorical data were presented as frequency and percentage. Survival data were expressed using medians, and survival rates were estimated using the Kaplan-Meier method. All P values were 2-tailed, and a P value of less than 0.05 was considered statistically significant.
Results
A total of 88 patients were enrolled in this study, there were 61 males (69.3%) and 27 females (30.7%), with a median age of 6.9 months (range: 0.4 to 348 months) and a median body weight of 6.45 kg (range: 2.65 to 48 kg), and the conditions included tetralogy of Fallot (n=49), pulmonary atresia/ventricular septal defect (n=13, including 5 cases of type I, 6 cases of type II, and 2 cases of type III), permanent truncus arteriosus (n=7, including 4 cases of type I, 2 cases of type II, and 1 case of type III), double outlet right ventricle complicated by pulmonary artery stenosis (n=7), transposition of great arteries/ventricular septal defect/pulmonary artery stenosis (n=6), and pulmonary atresia/intact ventricular septum (n=6). The RVOT was reconstructed using svBPP (BalMonoc), with the median model size being 14 mm (range: 10 to 20 mm). These patients were aged 0–1 year in 65 cases (73.9%), 1–10 years in 18 cases (20.5%), and >10 years in 5 cases (5.7%). A total of 5 patients (5.7%) died during the perioperative period.
The average time to aortic occlusion was 69±29.9 minutes (range: 13 to 157 min; median: 62.5 min). The mean CPB duration was 139±54.6 minutes (range: 61 to 335 min; median: 126 min). The mean duration of postoperative assisted ventilation was 142±138.3 hours (range: 4 to 716 h; median: 116 h). The average hospital stay was 25±11.8 days (range: 10 to 71 days; median: 21 days).
The surgical procedures included simple transannular RVOT reconstruction (n=59), Barbero-Marcial procedure (n=19), and REV (n=10). The staged surgeries were performed after modified Blalock-Taussig shunt (n=10) or RVOT reconstruction (n=10). Concurrent surgeries included unifocalization operation (n=3), Slide operation (n=2), diaphragmatic hernia repair (n=2), and surgical correction of vascular ring anomaly (n=2). Early complications included pleural effusion, cardiac insufficiency, respiratory insufficiency, chylothorax, and atelectasis, all of which were cured. Early postoperative ultrasound revealed severe pulmonary stenosis in 1 case, moderate stenosis in 3 cases, mild stenosis in 4 cases, and no stenosis in 75 cases. Pulmonary regurgitation was not found in 36 patients; however, there was 1 case of moderate pulmonary regurgitation and 46 cases of mild pulmonary regurgitation. After discharge, 83 patients (94.3%) were effectively followed up, with an average follow-up time of 61±36.4 months (range: 2 to 134 months; median: 50 months). During the follow-up period, 1 patient died after a long period of time (accidental death due to vaccination) and 1 patient underwent reoperation (reoperation due to infective endocarditis 2 months after surgery). The 1-, 5-, and 10-year survival rates were 98.8%, 98.8%, and 98.8%, respectively, and the reintervention-free rates for the same time intervals were 98.8%, 98.8%, and 98.8%, respectively (Figures 4,5). The last follow-up ultrasound revealed severe pulmonary stenosis in 0 cases, moderate stenosis in 2 cases, mild stenosis in 7 cases, and no stenosis in 73 cases. Pulmonary regurgitation was not found in 12 patients; however, there were 2 cases of severe pulmonary regurgitation, 20 cases of moderate pulmonary regurgitation, and 48 cases of mild pulmonary regurgitation.
The last follow-up ultrasound showed that the right ventricular anteroposterior diameter was 11.1–24.6 mm (median: 17.5 mm; mean: 16.8±3.2 mm; Z-value range: −1.12 to 1.34 mm), pulmonary annular diameter was 12.2–36.7 mm (median: 19.25 mm; mean: 20.4±5.5 mm; Z-value range: −1.63 to 5.65 mm), systolic longitudinal displacement of the lateral tricuspid annulus was 11.3–19.8 mm (median: 16.3 mm; mean: 16±2.1 mm), and the pulmonary valve velocity was 0.8–4.4 m/s (median: 1.6 m/s; mean: 1.75±0.73 m/s) (Table 1).
Table 1
| Item | Mean ± SD | Median | Range |
|---|---|---|---|
| Age (M) | 21±48.6 | 6.9 | 0.4–348 |
| Weight (kg) | 8±7.5 | 6.45 | 2.65–48 |
| Number of single patch (mm) | 14±1.7 | 14 | 10.0–22.0 |
| Cardiopulmonary bypass time (min) | 139±54.6 | 126 | 61–335 |
| Cross-clamp time (min) | 69±29.9 | 62.5 | 13–157 |
| Mechanical ventilation time (min) | 142±138.3 | 116 | 4–716 |
| Hospital stay (d) | 25±11.8 | 21 | 10–71 |
| Right ventricular anteroposterior diameter (mm) | 16.8±3.2 | 17.5 | 11.1–24.6 |
| Pulmonary annular diameter (mm) | 20.4±5.5 | 19.25 | 12.2–36.7 |
| Systolic longitudinal displacement of the lateral tricuspid annulus (mm) | 16±2.1 | 16.3 | 11.3–19.8 |
| Pulmonary valve velocity (m/s) | 1.75±0.73 | 1.6 | 0.8–4.4 |
A total of 19 children underwent MRI (Table 2). Peripheral pulmonary stenosis included left pulmonary artery stenosis (n=1), right pulmonary artery stenosis (n=3), and left and right pulmonary artery stenosis (n=2). Balloon dilation was performed in 4 patients.
Table 2
| Item | Range | Median | Mean ± SD |
|---|---|---|---|
| Interval since surgery (years) | 2.0–10.0 | 7 | 7.3±2.2 |
| RVEDVI (mL/m2) | 38–137.2 | 70.9 | 74.5±24.1 |
| Right ventricular ejection fraction (%) | 36–69 | 54 | 53.1±9.7 |
| Pulmonary regurgitation rate (%) | 3.22–58.7 | 23.58 | 24.1±14.7 |
| Peak flow velocity (cm/s) | 57.78–184.16 | 123.4 | 122.5±36.3 |
| Left ventricular end-systolic volume index (mL/m2) | 32.4–90.4 | 60.5 | 60.9±13.8 |
| Left ventricular ejection fraction (%) | 42–72 | 65 | 61.9±8.4 |
MRI, magnetic resonance imaging; RVEDV, right ventricular end-diastolic volume index.
Discussion
For patients with complex congenital heart diseases such as tetralogy of Fallot and double outlet right ventricle complicated by pulmonary artery stenosis, transannular patches are often required to widen the RVOT in addition to the resection of hypertrophic muscle bundles. However, almost all early valve-free patches cause pulmonary valve regurgitation to varying degrees (3). Research has confirmed that pulmonary valve regurgitation will lead to a significant decrease in right heart function. Pediatric patients may experience acute right heart failure in the early postoperative period. Even mild regurgitation may lead to progressive right-sided heart dysfunction. Furthermore, irreversible myocardial damage may occur (4). Although valved conduits have a good closure function, they have no growth potential and are associated with high reoperation rate; thus, they are not suitable for children (5,6). It has been widely recognized that autologous tissues should be spared as much as possible to avoid RVOT stenosis (7). The use of single-valve patches for RVOT reconstruction can reduce the regurgitation in the pulmonary valve and protect the right heart function, which has become the mainstream surgical procedure for RVOT reconstruction (8). In our current study, svBPP was applied for RVOT reconstruction, with satisfactory effectiveness.
Single-valve patches are sewn from biological tissue or synthetic patches with single leaflets. The single leaflets currently used mainly include autologous pericardium, bovine pericardium, porcine pericardium, allogenic aortic valve, and polytetrafluoroethylene (PTFE) single valve. Autologous pericardium tends to shrink during sewing, whereas allogenic aortic valve tissue is difficult to harvest. Furthermore, the anti-calcification treatment of animal-derived tissue materials directly affects the durability of single valves. The difference in the metabolism level in the human body is a key factor that leads to the different degree of calcification. Earlier calcification of tissue valves in younger patients is one of the main features of congenital heart disease (9). At present, the lower age limit for bioprosthetic valve implantation is 50 years, which broadly excludes pediatric patients (10). The device used in our current study was the BalMonocTM svBPP, which uses an innovative process to introduce a coordination compound with free positive radicals to complete tissue crosslinking. It augments tissue materials while reducing the risk of calcification. Its efficacy has been clinically validated in adult valve surgeries. In particular, as a leaflet material, it has been applied in bioprosthetic valve replacement in younger patients with rheumatic heart disease. Therefore, this product is suitable for RVOT reconstruction in children with congenital heart disease. In our current study, svBPP effectively eliminated or reduced pulmonary valve regurgitation, thereby protecting right heart function. With the adoption of a standard manufacturing process, BalMonocTM svBPP is durable and has customized specifications, which helps to shorten the operative time, increases surgical precision, and ensures more accurate connection with surrounding tissues.
In our present study, the survival rates and reoperation-free rates were higher than those previously reported in the literature. Reoperation was required in only 1 case, which was due to infective endocarditis rather than valve dysfunction. In 2 patients who experienced severe pulmonary regurgitation, neither obvious right heart dysfunction nor right ventricular enlargement was found, and no further surgical intervention has been offered to date.
For patients with permanent truncus arteriosus, REV (which drags the pulmonary trunk directly to the right ventricular incision to reconstruct the RVOT) was performed, which satisfactorily addressed the problem of pulmonary artery and RVOT reconstruction, thereby ensuring the growth potential of the pulmonary artery after reconstruction and avoiding the high reoperation rate caused by the valved conduits (11). However, REV is not feasible in all patients. For example, the usable pulmonary arteries are too short and small, the left and right pulmonary arteries become too thin after Lecompt procedure due to the thick shared artery (neo-aorta), and there are type B/C pulmonary atresia with a heterogenous enlarged anterior branch of the right ventricular branch of the coronary artery above the right ventricular resection margin. The modified Barbero-Marcial procedure is more suitable for these pediatric patients (12-14), during which the posterior edge of the pulmonary artery incision is sutured with the left atrial appendage or the upper edge of the right atrial appendage, and the lower edge of the left atrial appendage or the right atrial appendage is sutured with the upper edge of the right ventricular incision to reconstruct the posterior wall of pulmonary artery, which can well address the above problem. In our current study, among 26 patients who received operation on the left atrial appendage or the right atrial appendage, 3 patients underwent reoperation due to the stenosis of the main pulmonary artery, which further confirmed the good growth potential of autologous tissues such as atrial appendages. Among our 88 patients, the incidence of pulmonary artery stenosis was much lower than that in patients who received valved conduits.
To better evaluate the right heart function, we performed cardiac MRI on some patients and calculated the relevant parameters including pulmonary regurgitation rate, right ventricular EF, and right ventricular end-diastolic volume index. It was found that the right heart function was satisfactorily maintained in our series. However, the single valve has no growth potential. With the growth of pulmonary arteries, incomplete closure will inevitably worsen in the long term. Fortunately, by such time, the patients would have become adults or close to adulthood, and valve replacement may be performed to avoid a second thoracotomy.
In conclusion, the standard svBPP is a good biomaterial. When used in RVOT reconstruction, it can reduce pulmonary regurgitation and protect right heart function. However, the long-term outcomes need to be further investigated in more cases.
Study limitations
In our center, 3 methods including BalMonocTM svBPP, manual sewing of autologous pericardium, and manual sewing of Gore polymers have been applied for RVOT reconstruction in 88, 126, and 22 patients, respectively. Therefore, our current study on BalMonocTM svBPP was limited by its small sample size (n=88) and relatively short follow-up period. Our future studies will be based on the analyses of multi-center data and the comparisons of different materials.
Conclusions
As shown in the mid- and long-term follow-up studies, BalMonocTM svBPP has good performance in RVOT reconstruction. It can effectively eliminate or reduce pulmonary valve regurgitation and protect right heart function. Both REV and the modified Barbero-Marcial procedure can enable growth potential and reduce reoperation rate. However, long-term results require analysis through more complete follow-up and multicenter studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-97/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-97/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-97/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-97/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Guangdong Women and Children Hospital (IRB approval number: 202301044) and written informed consent was obtained from all study participants or their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rastelli GC, Ongley PA, Davis GD, et al. Surgical repair for pulmonary valve atresia with coronary-pulmonary artery fistula: report of case. Mayo Clin Proc 1965;40:521-7. [PubMed]
- Van Berendoncks A, Van Grootel R, McGhie J, et al. Echocardiographic parameters of severe pulmonary regurgitation after surgical repair of tetralogy of Fallot. Congenit Heart Dis 2019;14:628-37. [Crossref] [PubMed]
- Kawashima Y, Naito Y, Kitamura S, et al. Use of large homograft artery with semilunar valve for correction of tetralogy of fallot. A follow-up study. J Thorac Cardiovasc Surg 1974;67:685-93. [Crossref] [PubMed]
- Ilbawi MN, Idriss FS, DeLeon SY, et al. Factors that exaggerate the deleterious effects of pulmonary insufficiency on the right ventricle after tetralogy repair. Surgical implications. J Thorac Cardiovasc Surg 1987;93:36-44. [Crossref] [PubMed]
- Wang X, Bakhuis W, Veen KM, et al. Outcomes after right ventricular outflow tract reconstruction with valve substitutes: A systematic review and meta-analysis. Front Cardiovasc Med 2022;9:897946. [Crossref] [PubMed]
- Hirai K, Baba K, Goto T, et al. Outcomes of Right Ventricular Outflow Tract Reconstruction in Children: Retrospective Comparison Between Bovine Jugular Vein and Expanded Polytetrafluoroethylene Conduits. Pediatr Cardiol 2021;42:100-8. [Crossref] [PubMed]
- Wu M, Fan C, Liu J, et al. Individualized right ventricular outflow tract reconstruction using autologous pulmonary tissue in situ for the treatment of pulmonary atresia with ventricular septum defect. Rev Cardiovasc Med 2022;23:85. [Crossref] [PubMed]
- Kumar M, Turrentine MW, Rodefeld MD, et al. Right Ventricular Outflow Tract Reconstruction With a Polytetrafluoroethylene Monocusp Valve: A 20-Year Experience. Semin Thorac Cardiovasc Surg 2016;28:463-70. [Crossref] [PubMed]
- Bourguignon T, Bouquiaux-Stablo AL, Loardi C, et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg 2014;148:2004-2011.e1. [Crossref] [PubMed]
- Bourguignon T, Espitalier F, Pantaleon C, et al. Bioprosthetic mitral valve replacement in patient aged 65years or younger: long-term outcomes with the Carpentier-Edwards PERIMOUNT pericardial valve. Eur J Cardiothorac Surg 2018;54:302-9. [Crossref] [PubMed]
- Nemoto S, Ozawa H, Sasaki T, et al. Repair of persistent truncus arteriosus without a conduit: sleeve resection of the pulmonary trunk from the aorta and direct right ventricle-pulmonary artery anastomosis. Eur J Cardiothorac Surg 2011;40:563-8. [Crossref] [PubMed]
- Barbero-Marcial M, Riso A, Atik E, et al. A technique for correction of truncus arteriosus types I and II without extracardiac conduits. J Thorac Cardiovasc Surg 1990;99:364-9. [Crossref] [PubMed]
- Barbero-Marcial M, Tanamati C. Alternative nonvalved techniques for repair of truncus arteriosus: Long-term results. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 1999;2:121-30. [Crossref] [PubMed]
- Barbero-Marcial M, Tanamati C, Atik E, et al. Intraventricular repair of double-outlet right ventricle with noncommitted ventricular septal defect: advantages of multiple patches. J Thorac Cardiovasc Surg 1999;118:1056-67. [Crossref] [PubMed]
(English Language Editor: J. Jones)






