
The comparisons of procalcitonin and age between mild and severe Hand, foot, and mouth disease
Highlight box
Key findings
• Age and blood PCT are valuable predictors of disease severity.
What is known and what is new?
• Severe complications of head, foot, and mouth disease can be fatal.
• Age and blood PCT are valuable predictors of disease severity.
What is the implication, and what should change now?
• Early identification of severe cases may reduce mortality.
Introduction
Hand, foot, and mouth disease (HFMD) is a common pediatric infectious disease with high prevalence worldwide. Its typical clinical symptoms include fever, painful oral ulcerations, and macula or papule vesicular rashes on the hands and feet (1). Although it is frequently a self-limiting disease, it can be dangerous and even fatal in rare cases. Patients can develop serious complications such as acute flaccid paralysis encephalitis, meningitis, myocarditis, brain edema, neurogenic pulmonary edema, and pulmonary hemorrhage. Recently, atypical HFMD caused by coxsackievirus A6 has gradually increased. Most cases occurred in children under 5 years of age, and some cases developed onychomycosis during recovery (2). According to previous studies, the incidence rate of severe cases in mainland China is 1.74% and the mortality rate is 0.055%. Younger age and poor living conditions are also associated with a higher risk of death (3,4). The clinical manifestations of HFMD in children vary, as the infection outcomes depend on the pathogen as well as the host immune state. At present, there is no approved antiviral treatment for HFMD. The management of mild cases includes symptomatic and supportive treatment aimed at reducing body temperature, controlling pain, and promoting adequate oral hydration. Intravenous immunoglobulin (IVIG) has been recommended for the treatment of severe infections because evidence suggests its significant benefits in reducing the associated inflammation of the central nervous system. However, the systematic use of IVIG remains controversial (5). Thus, the early identification of severe cases is crucial. Some studies have shown that brain natriuretic peptide (BNP) and procalcitonin (PCT) can be used as early biomarkers for severe or fatal HFMD (6-8). PCT is a biochemical marker of bacterial sepsis with excellent diagnostic and prognostic value for its early identification, Moreover, PCT levels is also thought to be associated with the severity of novel coronavirus-19 (COVID-19) (9,10) and other infectious diseases (11). However, most studies just focused on stage 3 and stage 4 severe HFMD, changes in the first and second stages of HFMD are rarely compared. Therefore, in this study, we mainly focused on comparing the PCT levels in the first and second stages.
Hence, we performed a retrospective study to compare the clinical characteristics of HFMD in patients admitted to our hospital in Ningbo, China, between January 2020 and August 2021. We aimed to establish which clinical parameters can be routinely assessed to identify severe cases in their early stages. We present the following article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-146/rc).
Methods
Study design
We enrolled 183 patients with HFMD at the Ningbo Women and Children’s Hospital between January 2020 and August 2021. The study inclusion criteria were as follows: (I) diagnosis of HFMD based on the latest guidelines (12); (II) onset of symptoms within 48 hours; and (III) normal heart, liver, and kidney function. The exclusion criteria were as follows: (I) severe cardiac, hepatic, or renal dysfunction; (II) poor treatment compliance; and (III) severe and critical patients. The clinical diagnostic criteria and stages of HFMD are classified as follows:
Stage 1 (rash stage): mainly characterized by fever; rash on hands, feet, mouth, and buttocks; cough; runny nose; loss of appetite. Some patients may only show rash or herpetic angina, and others may have no rash.
Stage 2 (nervous system involvement stage): characterized by central nervous system damage manifested as mental retardation, drowsiness, sucking weakness, increased startle responses, headache, vomiting, irritability, limb shaking, muscle weakness, and neck rigidity.
Stages 3 and 4 correspond to the critical cases of cardiopulmonary failure. To ensure the interests of the patients, this study only included children with mild disease in stage 1 and severe disease in stage 2. The symptoms and signs during hospitalization and the laboratory examination results were recorded. This study was approved by the Ethics Committee of Ningbo Women and Children’s Hospital (approval No. EC2022-M005). Given the retrospective nature of this case-control study, the requirement for informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Measurement of lymphocyte subsets
After hospital admission, 2 mL of morning fasting venous blood was collected from all children. The samples were treated with sodium citrate (1:9) as an anticoagulant and examined within 2 hours. Peripheral blood T lymphocytes (CD3+, CD3+CD8+, and CD3+CD4+ cells), B lymphocytes (CD19+ cells), and natural killer (NK) cells (CD56+16+ cells) were detected using an immunofluorescence multi-parameter flow cytometry analyzer (American BD Company, Franklin Lake, New Jersey, USA). Leucocyte count was recorded as the percentage of total lymphocytes. Further, the ratio of CD4+ cells to CD8+ cells was also calculated.
Measurement of PCT and BNP
At hospital admission, 2 mL of venous blood was collected, left at room temperature for 30 minutes, and centrifuged at 3,000 r/min for a minimum of 10 minutes. The serum was then collected in a 1.5 mL tube and stored at −80 ℃. The levels of serum PCT and BNP were detected by electrochemiluminescence using the Shenzhen Pumen (A, 4th Floor, Building 15, Yijing Company, 1008 Songbai Road, Nanshan District, Shenzhen) ecl8000 chemiluminescence instrument. Serum PCT ≥0.5 ng/mL was used as the positive criteria for evaluating the results, and serum BNP ≥250 ng/mL was considered abnormal.
Statistical analysis
SPSS 20.0 version (SPSS Inc., IBM, Chicago, IL, USA) was used for data processing and analysis, and the differences of each factor in the two groups were analyzed. Count data were expressed as rate or percentage, and χ2 test was used for comparison between groups. The measurement data in accordance with normal distribution were expressed by mean ± standard deviation (SD). One-way analysis of variance was used for the comparison between the two groups. Statistical significance was set at P<0.05.
Results
Patients’ clinical characteristics
A total of 186 patients with HFMD were enrolled in our study. However, three patients with serious complications met the exclusion criteria (Figure 1). Therefore, 183 patients with HFMD completed the study, among which 76 were assigned to the mild group and 107 to the severe group (Figure 1). Approximately 57.4% of the patients were males. There was no statistically significant difference in sex between the two groups. The mean age of the patients in the mild group was higher than that of the patients in the severe group (2.39±1.57 vs. 1.65±0.94 years, respectively; P=0.000).
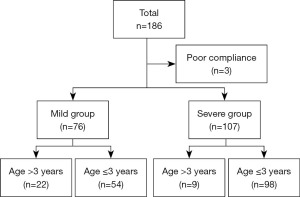
Further, 95.9% of patients presented with fever in the two groups. All patients had oral lesions and skin rashes. The PCT and BNP levels were significantly higher in the severe group (P=0.032 and P=0.021, respectively; Table 1). Regarding the lymphocyte subsets, the proportion of Suppressor T cells (CD3+CD8+) was significantly lower in the severe group (P=0.043), while peripheral blood T lymphocytes (CD3+), T helper cells (CD3+CD4+), NK cells (CD16+56+), B lymphocytes (CD19+), and the CD4/CD8 ratio were identical in the two groups (Table 2). Finally, the occurrence of fever and rash, the white blood cell (WBC) count, and the levels of hypersensitive C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum total bilirubin (TBIL), and creative kinase isoenzyme (CKMB) at hospital admission were not statistically different between the two groups (P>0.05, Table 1).
Table 1
| Characteristics | Mild group (N=76) | Severe group (N=107) | P value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 38 (50.0) | 67 (62.6) | 0.097 |
| Female | 38 (50.0) | 40 (37.4) | 0.097 |
| Age (years), mean ± SD | 2.39±0.18 | 1.65±0.09 | 0.000* |
| Symptoms, n (%) | 0.727 | ||
| Fever | 92 (95.8) | 89 (97.8) | |
| Skin rash | 76 (100.0) | 107 (100.0) | |
| Oral lesions | 96 (100.0) | 91 (100.0) | |
| WBC count (×109/L), mean ± SD | 14.48±0.80 | 14.54±0.63 | 0.942 |
| CRP (mg/L), mean ± SD | 21.14±3.29 | 19.82±2.10 | 0.722 |
| BNP (ng/mL), mean ± SD | 389.31±41.42 | 546.18±53.42 | 0.032* |
| PCT (ng/mL), mean ± SD | 0.346±0.061 | 0.568±0.067 | 0.021* |
| ALT (IU/L), mean ± SD | 18.763±1.67 | 18.579±0.754 | 0.912 |
| AST (IU/L), mean ± SD | 39.38±1.85 | 39.96±1.30 | 0.791 |
| TBIL (mmol/L), mean ± SD | 6.54±0.324 | 7.17±0.313 | 0.170 |
| CKMB (µg/mL), mean ± SD | 2.42±0.17 | 2.76±0.14 | 0.115 |
Oral lesions: oral ulcers, vesicles, or both. *, statistically significant values (P<0.05). WBC, white blood cell; SD, standard deviation; CRP, hypersensitive C-reactive protein; BNP, brain natriuretic peptide; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, serum total bilirubin; CKMB, creatine kinase isoenzyme.
Table 2
| Lymphocyte subsets (%) | Mild group (N=76) | Severe group (N=107) | P value |
|---|---|---|---|
| CD3+CD8+, mean ± SD | 22.534±0.724 | 20.685±0.566 | 0.043* |
| CD3+, mean ± SD | 62.755±1.15 | 61.166±0.74 | 0.226 |
| CD3+CD4+, mean ± SD | 43.064±5.683 | 36.728±0.645 | 0.271 |
| CD16+56+, mean ± SD | 13.525±0.661 | 13.986±0.668 | 0.634 |
| CD19+, mean ± SD | 21.167±0.744 | 22.729±0.638 | 0.113 |
| CD4/CD8, mean ± SD | 1.821±0.086 | 1.940±0.070 | 0.281 |
*, statistically significant values (P<0.05). SD, standard deviation.
Patients’ clinical parameters by age group
Since age is one of the main factors affecting the severity of HFMD, we conducted the baseline survey in the early stage. It was found that there was no significant difference in the age stratified by 3 years, so we divided the age into groups. There was no significant difference in the age of disease onset between the two groups after adjusting this factor (Table 3). According to our study, in patients ≤3 years of age, the PCT levels were higher in the severe group than in the mild group (0.556 vs. 0.225 mg/dL, respectively; P=0.001). However, there was no statistically significant difference in lymphocyte subsets, BNP, WBC, CRP, ALT, AST, TBIL, and CKMB levels between the two groups (P>0.05, Tables 3,4). In patients >3 years of age, none of the evaluated parameters showed a statistically significant difference between the mild and severe groups (P>0.05, Table 3).
Table 3
| Characteristics | ≤3 years | >3 years | |||||
|---|---|---|---|---|---|---|---|
| Mild group (N=54) | Severe group (N=98) | P value | Mild group (N=22) | Severe group (N=9) | P value | ||
| WBC count (×109/L), mean ± SD | 14.66±0.833 | 13.87±0.542 | 0.411 | 15.86±1.42 | 14.83±1.74 | 0.683 | |
| CRP (mg/L), mean ± SD | 22.71±4.28 | 20.51±2.25 | 0.616 | 17.28±4.31 | 12.30±3.66 | 0.493 | |
| BNP, mean ± SD | 445.17±54.39 | 572.17±57.27 | 0.147 | 252.18±39.73 | 263.84±76.38 | 0.883 | |
| PCT (ng/mL), mean ± SD | 0.225±0.030 | 0.556±0.067 | 0.001* | 0.642±0.18 | 0.694±0.31 | 0.882 | |
| ALT (IU/L), mean ± SD | 20.68±2.07 | 19.19±0.79 | 0.426 | 14.04±2.54 | 11.88±0.71 | 0.599 | |
| AST (IU/L), mean ± SD | 43.33±2.33 | 40.86±1.36 | 0.323 | 29.68±1.54 | 30.44±3.11 | 0.809 | |
| TBIL (mmol/L), mean ± SD | 6.39±0.38 | 7.13±0.31 | 0.147 | 7.09±0.58 | 6.88±1.33 | 0.871 | |
| CKMB (µg/mL), mean ± SD | 2.82±0.21 | 2.83±0.14 | 0.965 | 1.46±0.14 | 2.02±0.28 | 0.050 | |
*, statistically significant values (P<0.05). WBC, white blood cell; SD, standard deviation; CRP, hypersensitive C-reactive protein; BNP, brain natriuretic peptide; PCT, procalcitonin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, serum total bilirubin; CKMB, creatine kinase isoenzyme.
Table 4
| Lymphocyte subsets (%) | ≤3 years | >3 years | |||||
|---|---|---|---|---|---|---|---|
| Mild group (N=54) | Severe group (N=98) | P value | Mild group (N=22) | Severe group (N=9) | P value | ||
| CD3+CD8+, mean ± SD | 21.935±0.88 | 20.488±0.59 | 0.161 | 24.004±1.23 | 22.822±2.00 | 0.614 | |
| CD3+, mean ± SD | 62.616±1.45 | 60.708±0.77 | 0.203 | 63.095±1.84 | 66.155±2.09 | 0.345 | |
| CD3+CD4+, mean ± SD | 45.968±7.95 | 36.601±0.65 | 0.245 | 35.936±2.02 | 38.111±1.79 | 0.525 | |
| CD16+56+, mean ± SD | 13.790±0.80 | 14.427±0.71 | 0.571 | 12.872±1.17 | 9.188±1.25 | 0.078 | |
| CD19+, mean ± SD | 20.988±0.92 | 22.734±0.67 | 0.126 | 21.604±1.26 | 22.677±2.08 | 0.655 | |
| CD4/CD8, mean ± SD | 1.942±0.11 | 1.952±0.74 | 0.942 | 1.522±0.098 | 1.811±0.211 | 0.168 | |
SD, standard deviation.
Discussion
HFMD is a common pediatric infectious disease transmitted by human enteroviruses. Although most of the symptoms are mild, rare cases can rapidly progress and develop critical manifestations such as encephalitis, meningitis, pulmonary edema, circulation disorders, and even death. Generally, children under 10 years of age are at a high risk of developing HFMD (13). However, more than 90 % of the cases are diagnosed under 5 years. An epidemiological study on HFMD cases in mainland China reported a higher mortality rate among children ≤2 years of age compared to older children, indicating that the susceptibility and severity of HFMD are associated with age (14). Moreover, other study has demonstrated that BNP and PCT levels can be an indicator of severe fatal HFMD. Although the pathogenesis of HFMD has not yet been fully clarified (15), it is related to the inhibition of immune function, especially cellular inhibition of immune function. Therefore, we conducted a retrospective study to analyze the relationship between the severity of HFMD and cellular immunity, age, and PCT and BNP levels.
In our retrospective study, among the 183 included patients, 97.3% were under 5 years of age [similar to that in a previous study (13)], and 83% were younger than 3 years of age. Consistent with other studies (2,16), we found that age is one of the factors affecting the degree of severity, with severe clinical presentations occurring in younger children. Meanwhile, we also found significant differences in PCT levels between the two groups. Our results indicate that PCT is a good predictor of severe cases and a valuable tool for their early identification. PCT is a precursor of calcitonin that is released from all tissues during bacterial infections. It is secreted at 4 hours and reaches its peak at 8 hours after stimulation (17), and its levels can increase nearly 1,000 times after highly invasive bacterial infections (17). Therefore, PCT is a biochemical marker of bacterial sepsis with excellent diagnostic and prognostic value for its early identification, which can alert clinicians to the disease’s severity. It can also be used to monitor the response to antimicrobial treatment (18). Our study found a higher PCT level in the severe group compared to the mild group, indicating that severely affected patients will likely develop complications from bacterial infection. For children with increased PCT levels, early use of antibiotics can promote the recovery of the disease. Notably, PCT is not the only indicator of antibiotic use in HFMD; it is also associated with pneumonia, as well as high BNP and CRP levels. Our study showed similar rates of antibiotic use in the severe and mild groups (20.5% and 17%, respectively). Additionally, BNP levels between the two groups were not significantly different in our study after adjusting for age, which suggests that BNP can only predict critical HFMD, which was also confirmed by other studies (6-8).
A study has also suggested that cellular and humoral immunity are activated after enterovirus infection, contributing to immune surveillance, response, self-stabilization, and defense against viral infection (19). T-lymphocyte subsets are an important part of the cellular immune function. Numerous studies have shown that compared with healthy children, the proportions of CD3+, CD4+, and CD8+ in patients with HFMD are significantly reduced (20-22), and in patients with COVID-19, the total, CD8+, and CD4+ T-cell counts were also markedly decreased (23). Therefore, we sought to compare the proportion of T-lymphocyte subsets between children with mild and severe HFMD. Our study detected no statistically significant difference in lymphocyte subsets between patients with severe and mild HFMD, which indicates that lymphocyte subsets may not be an early indicator of HFMD severity between stages 1 and 2.
There are several limitations in this study that should be noted. Firstly, although our patients were continuously screened within the 48 hours after hospital admission, we did not monitor the lymphocyte subsets after this period due to the need for repeated venous blood sampling and the associated anxiety for patients and their parents. However, we can conclude that in the early stage (48 hours), no significant change in lymphocyte subsets existed between patients in stages 1 and 2. Secondly, owing to the widespread enterovirus 71 (EV71) vaccination in mainland China, there are very few patients with HFMD stages 3 to 4 (critically severe), and many of them are hospitalized more than 48 hours after the disease onset. Therefore, it is difficult to count the lymphocyte subsets of these patients at an early stage. Lastly, this was a retrospective study, and due to selection bias, there were more patients with stage 2 disease than stage 1, large sample and multi-center studies are needed in the future.
Conclusions
Collectively, our results indicate that PCT plays an important role in the early identification of severe HFMD. Further well-designed and multicenter studies are needed to confirm the potential of lymphocyte subset count in the early identification of severe and critical HFMD cases.
Acknowledgments
Funding: This study was sponsored by the Ningbo Clinical Research Center for Children’s Health and Diseases (No. 2019A21002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-146/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-146/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-146/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-146/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Ningbo Women and Children’s Hospital (approval No. EC2022-M005). Given the retrospective nature of this case-control study, the requirement for informed consent was waived by the Ethics Committee of Ningbo Women and Children’s Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HJ, Hyeon JY, Hwang S, et al. Epidemiology and virologic investigation of human enterovirus 71 infection in the Republic of Korea from 2007 to 2012: a nationwide cross-sectional study. BMC Infect Dis 2016;16:425. [Crossref] [PubMed]
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr 2022;11:1502-9. [Crossref] [PubMed]
- Hu L, Maimaiti H, Zhou L, et al. Changing serotypes of hand, foot and mouth disease in Shanghai, 2017-2019. Gut Pathog 2022;14:12. [Crossref] [PubMed]
- Hong J, Liu F, Qi H, et al. Changing epidemiology of hand, foot, and mouth disease in China, 2013-2019: a population-based study. Lancet Reg Health West Pac 2022;20:100370. [Crossref] [PubMed]
- Cao RY, Dong DY, Liu RJ, et al. Human IgG subclasses against enterovirus Type 71: neutralization versus antibody dependent enhancement of infection. PLoS One 2013;8:e64024. [Crossref] [PubMed]
- Song C, Yibing C, Guo Y, et al. Risk factors of severe hand, foot and mouth disease complicated with cardiopulmonary collapse. Infect Dis (Lond) 2015;47:453-7. [Crossref] [PubMed]
- Jan SL, Lin SJ, Fu YC, et al. Plasma B-type natriuretic peptide study in children with severe enterovirus 71 infection: a pilot study. Int J Infect Dis 2013;17:e1166-71. [Crossref] [PubMed]
- Deng HL, Zhang YF, Li YP, et al. N-terminal pro-brain natriuretic peptide levels associated with severe hand, foot and mouth disease. BMC Infect Dis 2016;16:585. [Crossref] [PubMed]
- Tong-Minh K, van der Does Y, Engelen S, et al. High procalcitonin levels associated with increased intensive care unit admission and mortality in patients with a COVID-19 infection in the emergency department. BMC Infect Dis 2022;22:165. [Crossref] [PubMed]
- Park M, Hur M, Kim H, et al. Prognostic Utility of Procalcitonin, Presepsin, and the VACO Index for Predicting 30-day Mortality in Hospitalized COVID-19 Patients. Ann Lab Med 2022;42:406-14. [Crossref] [PubMed]
- Rajaniemi SM, Hautala N, Sironen T, et al. Plasma B-type natriuretic peptide (BNP) in acute Puumala hantavirus infection. Ann Med 2014;46:38-43. [Crossref] [PubMed]
- National Health Commission of the People’s Republic of China. Hand-foot-mouth disease diagnosis and treatment guidelines (2018 Edition). Chinese Journal of Clinical Infectious Diseases 2018;11:161-6.
- Esposito S, Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur J Clin Microbiol Infect Dis 2018;37:391-8. [Crossref] [PubMed]
- Liu SL, Pan H, Liu P, et al. Comparative epidemiology and virology of fatal and nonfatal cases of hand, foot and mouth disease in mainland China from 2008 to 2014. Rev Med Virol 2015;25:115-28. [Crossref] [PubMed]
- Liu J, Yan X, Xie Z, et al. Study on etiology and infection influencing factors of children with hand, foot and mouth disease in a hospital from 2012 to 2017. Chinese Journal of Nosocomiology 2019;29:2037-40.
- Choi CS, Choi YJ, Choi UY, et al. Clinical manifestations of CNS infections caused by enterovirus type 71. Korean J Pediatr 2011;54:11-6. [Crossref] [PubMed]
- Nijsten MW, Olinga P. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med 2000;28:458-61. [Crossref] [PubMed]
- Delèvaux I, André M, Colombier M, et al. Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis 2003;62:337-40. [Crossref] [PubMed]
- Zhang Z, Zheng Y, Jiang L, et al. Analysis of etiology and complications of hand, foot and mouth disease in sentinel surveillance in China from 2015 to 2016. Chinese Journal of Epidemiology 2019;40:627-32. [PubMed]
- Guo FF, Xie LS. Meta analysis of changes of peripheral blood T cell subsets in children with hand, foot and mouth disease. Chinese Journal of Experimental and Clinical Virology 2018;32:543-7.
- Wang J, Deng H, Yuan J, et al. Correlation between imbalance of lymphocyte subsets and severity of EV71 hand, foot and mouth disease. Chinese Journal of Experimental and Clinical Infectious Diseases 2017;11:156-61. (Electronic Edition).
- Bai ZJ, Li YP, Huang J, et al. The significance of Notch ligand expression in the peripheral blood of children with hand, foot and mouth disease (HFMD). BMC Infect Dis 2014;14:337. [Crossref] [PubMed]
- Zhang W, Li L, Liu J, et al. The characteristics and predictive role of lymphocyte subsets in COVID-19 patients. Int J Infect Dis 2020;99:92-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


