
The effect of probiotics in the prevention of atopic dermatitis in children: a systematic review and meta-analysis
Highlight box
Key findings
• In this study, the meta-analysis showed that probiotics can effectively prevent the occurrence of atopic dermatitis (AD). Further subgroup analysis showed that taking rhamnose-lactobacillus probiotics and mixed bacteria before and after delivery could significantly prevent the incidence of AD.
What is known and what is new?
• When given in adequate amounts, probiotics can exert beneficial effects not only in the gastrointestinal tract but also in the gut-brain-skin axis. Previous studies have reported that probiotics have anti-inflammatory effects and may reduce gastrointestinal inflammation and the clinical symptoms of AD;
• The prevention effect of Lactobacillus rhamnosus and probiotics with mixed flora on AD is superior, which may be related to the reduction of intestinal microorganisms such as bifidobacterium and Lactobacillus in the intestines of children with AD.
What is the implication, and what should change now?
• AD in children can be prevented in clinical practice using probiotics, especially when given pre- and postnatally;
• Further subgroup analysis of probiotic dosage and supplementation time was not performed in this study, which can be explored later.
Introduction
Atopic dermatitis (AD) is a relapsing, chronic, non-infectious, inflammatory skin disease characterized by persistent itching of the skin, with an incidence of up to 20% in children (1,2). In recent decades, the incidence of pediatric AD has been increasing every year in both developed and developing countries (3). The clinical manifestations of AD in children include eczema-like rashes, such as erythema, papules, and exudative lesions at specific sites (4). As a non-fatal skin disease, pediatric AD imposes a significant psychosocial burden on patients and their families. A previous study reported that children with AD are more likely to have allergies, asthma, and mental health problems (5). Infants and children with AD are often treated with topical corticosteroids, antihistamines, and even antibiotics (6). However, these drugs have some side effects, and AD symptoms may recur quickly after the treatment is stopped. Therefore, it is necessary to carry out relevant research on the prevention and treatment of AD in children.
A previous study found that the gut microbiota is closely related to the occurrence and development of various human diseases. The gut microbiota of newborns comes from customized meconium, which is the result of maternal intestinal translocation. The gut microbiota in the early stages of the body’s life may play an important role in the occurrence of allergic diseases in the subsequent life cycle (7). Changing the microbiome of the body can effectively prevent and treat allergic diseases. According to the definition of the World Health Organization, probiotics are living microorganisms that have a beneficial effect on the host organism and help protect the host from harmful bacteria (7). When given in sufficient amounts, probiotics can exert beneficial effects not only in the gastrointestinal tract but also in the gut-brain-skin axis (8-10). Previous studies have reported that probiotics exert anti-inflammatory effects and can alleviate gastrointestinal inflammation and the clinical symptoms of AD (11). The levels of bifidobacteria and lactobacilli in the intestines of AD patients decreased, while the levels of Clostridium increased (11). However, previous research on probiotics for preventing AD has not yielded consistent results. Ou et al. found in a study of 191 pregnant women and newborns that oral probiotics can reduce the risk of AD in pregnant women, but there is no significant correlation with the risk of AD in newborns (12). However, Schmidt et al. conducted a randomized controlled experiment on 290 study subjects and found that oral probiotics can reduce the risk of AD in newborns (13). The heterogeneity of different research results may be related to differences in oral probiotic strains, probiotic combinations, study population, and treatment duration. In this study, we performed a meta-analysis to evaluate the efficacy of probiotics at home and abroad compared with placebo in the prevention of AD in children, aiming to provide a certain theoretical basis for the prevention of AD in children. We present the following article in accordance with the PRISMA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-200/rc).
Methods
Literature search strategy and inclusion and exclusion criteria
The search terms were based on the objectives of this study. The English and Chinese search terms were as follows: “infant”, “children”, “Atopic dermatitis”, and “Probiotic”. Based on the above search terms, a systematic search was carried out in the China National Knowledge Infrastructure (CNKI), Wanfang database, PubMed, and Web of Science databases, and the retrieval time was from the establishment of the database to November 10, 2022. A manual search was also performed of all relevant literature, including published reviews and meta-analyses.
The inclusion criteria were based on the Population, Intervention, Comparison, Outcomes and Study (PICOS) principle: (I) Study: the article is clearly indicated as a randomized controlled trial (RCT); (II) Participants: the age of the observation object is ≤18 years old; the observation object is clinically diagnosed with AD; (III) Intervention: the experimental group was treated with probiotics (including Lactobacillus, Bifidobacterium, Lactobacillus rhamnosus, or mixed microbial communities).; (IV) Comparison: the control group was treated with a placebo. (V) Outcome: the clinical diagnosis is AD. However, if multiple reports assessed the same group of patients, we only selected the latest complete report.
The exclusion criteria were as follows: (I) the subject of the study was not probiotics and pediatric AD; (II) there was no control group, the baseline balance of the components was poor, or the two groups were not comparable; (III) outcome: the evaluation indicators were not clear; (IV) duplicate or incomplete literature, such as literature with only an abstract but no full text and no contact with the author, or literature with missing specific data (the number of people in both groups and the occurrence of AD were not reported); (V) reviews or case reports.
Data extraction and quality assessment
Two researchers independently conducted a quality assessment and extracted the data, and any disagreements were resolved through third-party discussion. The following data were extracted from the studies included in this article: author, year, recipients of intervention, the timing of probiotic intervention, duration of probiotic intervention, type of probiotic, follow-up time, study region, and other information.
The risk of bias in the included studies was assessed using the Cochrane systematic review methods tool (Cochrane ROB Tool v1). The assessments included sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias (14). Each quality item was categorized as low risk of bias, high risk of bias, or unclear risk of bias. The evaluation for each entry involved assessing the risk of bias as ‘Yes’ (low risk), ‘No’ (high risk), or ‘Not available’ (unclear risk) (15). Differences were resolved by consensus.
Statistical analysis
In this study, statistical analysis was performed using STATA 14.0 (Computer Resource Center, USA). Each effect size was represented by the risk ratio (RR) and its 95% confidence interval (95% CI). Heterogeneity testing was evaluated using I2 (the proportion of heterogeneity variation to overall variation). When I2=0, it indicates homogeneity and no heterogeneity between studies; I2<50%, with low heterogeneity. For cases where heterogeneity does not exist or is low, a fixed effects model is used for meta-analysis; I2≥50%, with significant heterogeneity between studies, a random effects model was used for meta-analysis. Conduct subgroup analysis on the intervention target (mother and/or infant), intervention timing (pre/post-natal), type of probiotics (Lactobacillus/Bifidobacterium, or Lactobacillus rhamnosus and mixed microbiota), intervention duration (<2 years/≥2 years), and experimental region (Europe, Oceania, or Asia). The Harbor method is based on the statistics and variance of integral tests to correct linear regression, which can avoid the risk of adding Class 1 errors to the traditional publication bias detection method Egger test. The Harbord method was used to determine whether there was publication bias among the included studies. Heterogeneity was assessed using Rabe plots. If the circles on the Rabe diagram are distributed in a straight line, it indicates that the heterogeneity in meta-analysis is relatively small. Statistical analysis with P<0.05 indicates statistical significance.
Results
Literature search results and the basic characteristics of the included articles
A total of 786 studies were initially obtained, including 341 articles retrieved from PubMed, 258 from Web of Science, 18 from CNKI, and 169 from the Wanfang database. Of these, 118 studies were excluded by eliminating duplicate articles; 423 studies that do not align with the research topic were excluded by reading the titles, abstracts, and full texts and 128 studies, including comments, reviews, and case reports, were excluded. After reading the full texts and re-screening, excluding 31 studies that did not find the full text and 49 studies with incomplete clinical data (without reporting the occurrence of AD in both groups). Finally, 37 articles were included in this meta-analysis, with 2,986 patients in the experimental group receiving probiotics and 3,145 patients in the control group. The literature screening process and results are shown in Figure 1.
The basic characteristics of the included studies are listed in Table 1. Among them, there were 19 studies involving mothers and infants as the intervention objects, seven studies with mothers as the intervention objects, and 11 studies with infants as the intervention objects. The intervention time was prenatal in one study, postpartum in 11 studies, and prenatal and postpartum in 25 studies. Moreover, 12 studies used Lactobacillus rhamnosus alone, eight studies used Lactobacillus alone, one study used Bifidobacterium as probiotics, and 16 studies utilized mixed probiotics. The study area included 24 studies in Europe, six studies in Asia, and seven studies in Oceania. The research conducted by Kalliomäki [2003] (25) and Kalliomäki [2007] (26) were follow-up studies of Kalliomäki [2001] (24); studies by Kuitunen [2009] (29) and Peldan [2017] (33) were follow-up studies of Kukkonen [2007] (30); studies by Wickens [2012] (46), Wickens [2013] (48), and Wickens [2018] (44) were follow-up studies of Wickens [2008] (47). Therefore, Kalliomäki [2003] (25), Kalliomäki [2007] (26), Kuitunen [2009] (29), Peldan [2017] (33), Wickens [2012] (46), Wickens [2013] (48), and Wickens [2018] (44) were excluded. Thirty studies were finally included in further analysis.
Table 1
| First author | Year | Intervention participants | Intervention timing | Intervention duration | Types of probiotics | Follow-up period | Region | Remarks |
|---|---|---|---|---|---|---|---|---|
| Abrahammson (16) | 2013 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 12 months after delivery | Lactobacillus | 7 years | Europe | – |
| Allen (17) | 2014 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 6 months after delivery | Mixed probiotics: Lactobacillus salivarius, Lactobacillus paracasei, Bifidobacterium animalis subsp Lactobacillus and Bifidobacterium bifidum | 2 years | Europe | – |
| Böttcher (18) | 2008 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 12 months after delivery | Lactobacillus | 2 years | Europe | – |
| Boyle (19) | 2011 | Mothers | Prenatal | From 4 weeks before the expected date of delivery to the delivery | Lactobacillus rhamnosus | 1 years | Europe | – |
| Cabana (20) | 2017 | Infants | Postpartum | From birth to 6 months | Lactobacillus rhamnosus | 2 years | Europe | – |
| Dotterud (21) | 2010 | Mothers | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 3 months after delivery | Mixed probiotics | 2 years | Europe | – |
| Huurre (22) | 2008 | Mothers and their infants | Prenatal and postpartum | From 3 months before the expected date of delivery to the end of breastfeeding | Mixed probiotics | 1 year | Europe | – |
| Jensen (23) | 2012 | Infants | Postpartum | From birth to 6 months | Lactobacillus | 1 years | Asia | – |
| Kalliomaki (24) | 2001 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 2 years | Europe | – |
| Kalliomäki (25) | 2003 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 4 years | Europe | A follow-up study by Kalliomaki [2001] |
| Kalliomäki (26) | 2007 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 7 years | Europe | A follow-up study by Kalliomaki [2001] |
| Kim (27) | 2010 | Mothers and their infants | Prenatal and postpartum | From 8 weeks before the expected date of delivery to 6 months after delivery | Mixed probiotics | 1 year | Asia | – |
| Kopp (28) | 2008 | Mothers and their infants | Prenatal and postpartum | From 6 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus | 2 years | Europe | – |
| Kuitunen (29) | 2009 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 5 years | Europe | A follow-up study by Kukkonen [2007] |
| Kukkonen (30) | 2007 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 2 years | Europe | – |
| Loo (31) | 2014 | Infants | Postpartum | From birth to 6 months | Mixed probiotics | 5 years | Asia | – |
| Niers (32) | 2009 | Mothers and their infants | Prenatal and postpartum | From 6 weeks before the expected date of delivery to 12 months after delivery | Mixed probiotics | 2 years | Europe | – |
| Ou (12) | 2012 | Mothers and their infants | Prenatal and postpartum | From 16 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 18 months | Asia | – |
| Peldan (33) | 2017 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 10 years | Europe | A follow-up study by Kukkonen [2007] |
| Plummer (34) | 2020 | Infants | Postpartum | From birth to 1months after delivery | Mixed probiotics: Lactobacillus rhamnosus, Lactobacillus, Streptococcus thermophilus | 1 years | Oceania | – |
| Prescott (35) | 2008 | Infants | Postpartum | From birth to 6 months | Lactobacillus | 2.5 years | Oceania | – |
| Rautava (36) | 2012 | Mothers | Prenatal and postpartum | From 8 weeks before the expected date of delivery to 2 months after delivery | Lactobacillus rhamnosus | 2 years | Europe | – |
| Rautava (37) | 2002 | Mothers and their infants | Prenatal and postpartum | From 2–4 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 2 years | Europe | – |
| Rø (38) | 2017 | Mothers | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 3 months after delivery | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium | 2 years | Europe | – |
| Rozé (39) | 2012 | Infants | Postpartum | From birth to 6 months | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium | 6 months | Europe | – |
| Schmidt (13) | 2019 | Infants | Postpartum | 8–14 months | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium | 6 months | Europe | – |
| Simpson (40) | 2015 | Mothers | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 3 months after delivery | Mixed probiotics: Lactobacillus rhamnosus, Lactobacillus acidophilus and Bifidobacterium animalis | 6 years | Europe | – |
| Taylor (41) | 2007 | Infants | Postpartum | From birth to 6 months | Lactobacillus | 5 years | Europe | – |
| West (42) | 2009 | Infants | Postpartum | 4–13 months | Lactobacillus | 4–13 months | Europe | – |
| West (43) | 2013 | Infants | Postpartum | 4–13 months | Lactobacillus | 9 years | Europe | – |
| Wickens (44) | 2018 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 2 years after delivery | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium | 11 years | Oceania | A follow-up study by Wickens [2008] |
| Wickens (45) | 2018 | Mothers | Prenatal and postpartum | From 24 weeks before the expected date of delivery to 6 months after delivery | Lactobacillus rhamnosus | 1 years | Oceania | – |
| Wickens (46) | 2012 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 2 years after delivery | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium animalis | 4 years | Oceania | A follow-up study by Wickens [2008] |
| Wickens (47) | 2008 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 2 years after delivery | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium animalis | 2 years | Oceania | – |
| Wickens (48) | 2013 | Mothers and their infants | Prenatal and postpartum | From 4 weeks before the expected date of delivery to 2 years after delivery | Mixed probiotics: Lactobacillus rhamnosus and Bifidobacterium animalis | 6 years | Oceania | A follow-up study by Wickens [2008] |
| Han (49) | 2019 | Infants | Postpartum | 3 days to 10 days after birth | Mixed probiotics: Bifidobacterium, Lactobacillus, Streptococcus thermophilus | 1 months | Asia | – |
| Wu (50) | 2010 | Mothers | Prenatal and postpartum | From 4 weeks before the expected date of delivery to the end of breastfeeding | Bifidobacterium | 2 years | Asia | – |
Quality assessment of the included studies
Table 2 shows that all of the studies had a low risk of bias in terms of the blinding of investigators and patients, and 23 studies had a low risk of bias in terms of the blinding of outcome measures. Only one of the 30 studies was unclear about the random method bias; the remaining 29 studies had a low risk of random method bias. Additionally, 17 studies had low risk of bias in terms of allocation concealment, 17 studies had a low risk of bias for completeness of outcome data, 15 studies had a low risk of bias in terms of the reported outcomes of selective studies, and 12 studies had a low risk of other bias.
Table 2
| First author | Year | Sequence generation | Allocation sequence concealment | Blinding methods | Incomplete outcome data | Selective outcome reporting | Other potential sources of bias | |
|---|---|---|---|---|---|---|---|---|
| Researchers and patients | Outcome measurers | |||||||
| Kalliomaki | 2001 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Rautava | 2002 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Kukkonen | 2007 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Taylor | 2007 | Yes | Yes | Yes | Yes | Not available | No | Not available |
| Böttcher | 2008 | Yes | Yes | Yes | Not available | Yes | No | Not available |
| Huurre | 2008 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Kopp | 2008 | Yes | Not available | Yes | Yes | Yes | Yes | Yes |
| Prescott | 2008 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Wickens | 2008 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Niers | 2009 | Not available | Yes | Yes | Yes | Not available | No | Not available |
| West | 2009 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Dotterud | 2010 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Kim | 2010 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Wu | 2010 | Yes | Yes | Yes | Not available | Yes | No | Not available |
| Boyle | 2011 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Jensen | 2012 | Yes | Yes | Yes | Not available | Not available | No | Not available |
| Ou | 2012 | Yes | Not available | Yes | Not available | No | Yes | Yes |
| Rautava | 2012 | Yes | Not available | Yes | Yes | No | Yes | Yes |
| Roze | 2012 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Abrahammson | 2013 | Yes | Yes | Yes | Not available | Not available | No | Not available |
| West | 2013 | Yes | Yes | Yes | Not available | Yes | No | Not available |
| Allen | 2014 | Yes | Yes | Yes | Yes | Yes | No | Not available |
| Loo | 2014 | Yes | Not available | Yes | Not available | Yes | No | Not available |
| Simpson | 2015 | Yes | Not available | Yes | Yes | No | Yes | No |
| Cabana | 2017 | Yes | Not available | Yes | Yes | Yes | No | Not available |
| Rø | 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wickens | 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Schmidt | 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Not available |
| Han | 2019 | Yes | Not available | Yes | Yes | Yes | Yes | Not available |
| Plummer | 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Effect of probiotics on AD prevention
As shown in Figure 2, the heterogeneity test results showed that P<0.001, I2=65.2% and Pheterogeneity<0.001, indicating that there is heterogeneity among these studies, which may originate from 49 studies with different intervention subjects, intervention timing, and probiotic dosage. Using the random effect model, the pooled effect size results showed that probiotic intervention has a significant effect on the prevention of AD [RR (95% CI) =0.83 (0.73, 0.94)].
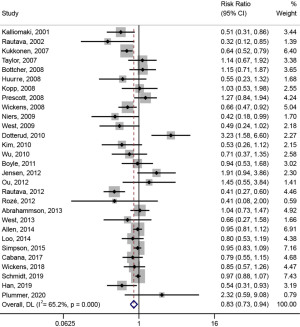
Subgroup analysis
Subgroup analysis was carried out for the intervention object, follow-up time, intervention probiotic species, intervention duration and trial region.
In the subgroup analysis on the intervention objects (shown in Figure 3), probiotic intervention had a significant effect on the prevention of AD [RR (95% CI) =0.65 (0.49, 0.86), I2=49.3%, Pheterogeneity=0.027] when the intervention recipients were mothers and infants. When the patients were mothers or infants, probiotic intervention had no significant effect on preventing AD [RR (95% CI) =0.69 (0.38, 1.26), I2=79.7%, Pheterogeneity<0.001; RR (95% CI) =0.85 (0.62, 1.17), I2=44.7%, Pheterogeneity=0.054].
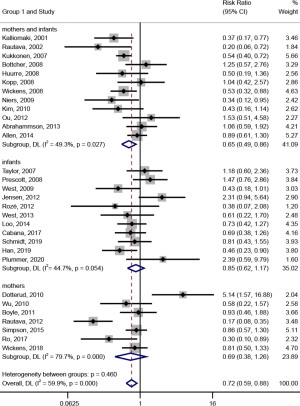
In the subgroup analysis on the timing of the intervention (shown in Figure 4), the effect of probiotic intervention on the prevention of AD was significant [RR (95% CI) =0.64 (0.49, 0.85), I2=66.6%, Pheterogeneity<0.001] when the timing of the intervention was prenatal and postpartum. However, when it was prenatal or postpartum, probiotic intervention had no significant effect on the prevention of AD [RR (95% CI) =0.93 (0.46, 1.88), I2=0.0%; RR (95% CI) =0.85 (0.62, 1.17), I2=44.7%, Pheterogeneity=0.054].
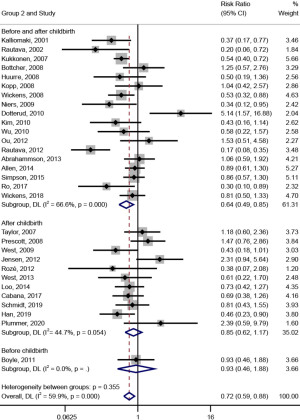
In subgroup analysis on the types of probiotics (shown in Figure 5), the effect of probiotic intervention on the prevention of AD was significant [RR (95% CI) =0.54 (0.36, 0.80), I2=68.2%, Pheterogeneity=0.003; RR (95% CI) =0.70 (0.52, 0.93), I2=52.4%, Pheterogeneity=0.014] when the types of probiotics were Lactobacillus rhamnosus and mixed flora; however, when the probiotics were Lactobacillus or Bifidobacterium, the effect of probiotic intervention on the prevention of AD was not significant [RR (95% CI) =1.09 (0.79, 1.49), I2=25.7%, Pheterogeneity=0.224; RR (95% CI) =0.58 (0.22, 1.57), I2=0.0%].
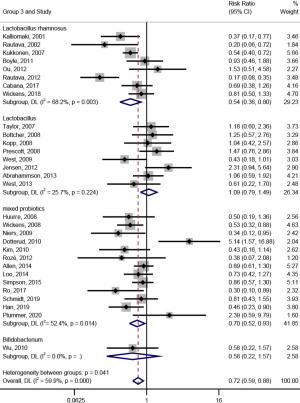
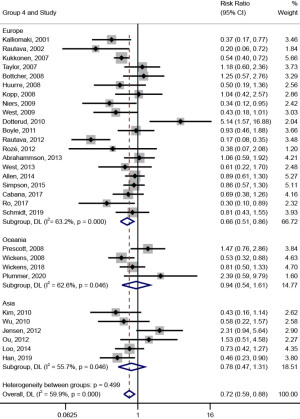
In subgroup analysis on the trial region (shown in Figure 6), probiotic intervention had a significant effect on the prevention of AD [RR (95% CI) =0.66 (0.51, 0.86), I2=63.2%, Pheterogeneity<0.001] when the trial region was Europe; meanwhile, when the trial region were Oceania and Asia, the effect of probiotic intervention on the prevention of AD was not significant [RR (95% CI) =0.94 (0.54, 1.61), I2=62.6%, Pheterogeneity=0.046; RR (95% CI) =0.78 (0.47, 1.31), I2=55.7%, Pheterogeneity=0.046].
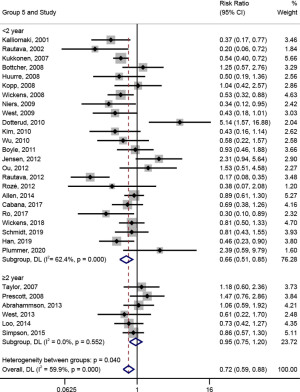
In subgroup analysis on the follow-up time (shown in Figure 7), probiotic intervention had a significant effect on the prevention of AD [RR (95% CI) =0.66 (0.51, 0.85)] when the follow-up time was less than 2 years; meanwhile, when the follow-up time was longer than 2 years, the effect of probiotic intervention on the prevention of AD was not significant [RR (95% CI) =0.95 (0.75, 1.20), I2=0.0%, Pheterogeneity=0.552].
Evaluation of publication bias and heterogeneity
As shown in Figure 8, the publication bias analysis results indicated that the included studies had no obvious publication bias (P=0.433). A Rabe diagram was used to evaluate the heterogeneity of the literature included in the analysis, and the results are shown in Figure 9. The literature was basically distributed according to a straight line, indicating that the heterogeneity was small. However, it is also important to note that there may be errors in the intuitive evaluation through the use of a Rabe diagram. The results of this meta-analysis are generally reliable.
Discussion
In this meta-analysis, we systematically reviewed 37 double-blind RCTs on the preventive effects of oral probiotics in pregnant women and/or their infants on AD. The results of the analysis showed that probiotics could effectively prevent the incidence of AD. Furthermore, the subgroup analysis results showed that the occurrence of AD could be significantly prevented when mothers and infants took Lactobacillus rhamnosus and mixed flora probiotics before and after delivery. Also, studies with follow-up times of less than 2 years and those conducted in Europe found that probiotics were more effective in preventing AD. These findings provide evidence for the efficacy of probiotics in preventing AD in children.
In recent years, researchers have gained a better understanding of the physiological functions of human gut microbiota, and gut microbiota have become a research hotspot (51,52). Newborns’ first exposure to microbes is provided by the maternal microbiota, and the newborn’s gut microbes are influenced by the mode of delivery, type of feeding, and use of antibiotics (53). Colonized gut microbiota is indispensable for intestinal physiological regulation and immune function, and changes in their types and levels may affect the risk of related diseases.
According to the definition by the World Health Organization, probiotics are a type of living microorganism, and a certain amount of probiotics can provide benefits to the health of the host (54,55). Probiotics can promote and regulate immune maturation and prevent allergic diseases by regulating the structure of the intestinal microbiota as well as the function of immune cells (56). However, there is still no consensus on the efficacy of probiotics for the clinical prevention and treatment of allergic diseases, and further research is needed. Previous studies have analyzed the efficacy of probiotic intervention in the prevention of AD (1,38,45). However, the sample size of a single study is limited, and confounding factors such as intervention objects, types of probiotics, and appropriate intervention timing were not adjusted. Therefore, this study conducted a meta-analysis of previous studies on the prevention of AD with probiotics, aiming to provide evidence for probiotic intervention in the prevention of AD.
This study found that probiotics could effectively prevent AD [RR (95% CI) =0.83 (0.73, 0.94)], which was basically consistent with previous research results both at home and abroad. Sun et al. conducted a meta-analysis of 17 RCTs involving a total of 4,011 children and found that probiotic intervention had a significant benefit in preventing eczema in children [RR (95% CI) =0.59 (0.45, 0.78)] (57). Pan et al. conducted a meta-analysis of eight RCTs involving a total of 2,575 newborns and found that probiotics can effectively prevent AD [RR (95% CI) =0.86 (0.78, 0.95)] (58). Probiotics can stimulate intestinal immunoglobulin A (IgA), reduce the adhesion of pathogenic bacteria, and form tight junctions with intestinal epithelial cells to decrease intestinal permeability (59). On the other hand, probiotics can mediate allergic skin inflammation by promoting T helper type 1 (Th1) cytokines, reducing the production of IgE, and decreasing the secretion of Th2 cytokines such as interleukin (IL)-5 and IL-10 (60).
Our subgroup analysis found that probiotic interventions for both mothers and infants had a significant effect on AD prevention when taken at the prenatal and postpartum stages; however, probiotic interventions only for mothers or infants, and only prenatal or postnatal interventions had no significant effect. Maternal microbes are transferred to offspring during pregnancy and can affect immune development and susceptibility to allergic diseases in the offspring (61). Prenatal and postnatal probiotic interventions are beneficial for the prevention of AD in infants. Lactobacillus rhamnosus and probiotics with mixed flora have better preventive effects on AD, which may be related to the reduction of intestinal microorganisms such as Bifidobacterium and Lactobacillus in the intestines of children with AD.
This meta-analysis accounted for the differences in intervention objects, intervention timing, types of probiotics, follow-up time, and trial regions, and carried out subgroup analyses to ensure that the results were detailed and reliable. Yet, there were still some limitations that should be noted. Firstly, we cannot confirm whether there are unpublished relevant data, and there may be some bias in the selection of literature and data extraction. Second, there may be differences in the criteria for AD diagnosis in different studies. In addition, this study did not conduct further subgroup analyses on the dosage of probiotics and the duration of supplementing probiotics.
In conclusion, our study found that probiotics can be an effective means of preventing AD and provide a basis for the clinical prevention of AD. Finally, it cannot be ignored that there is heterogeneity in this study, which may stem from differences in the general characteristics (age, gender, family factors, etc.) of different research subjects, as well as differences in the dosage of probiotics used. Therefore, further research is needed to explore the preventive effect of probiotic intervention on pediatric specific dermatitis in the future.
Conclusions
This study found through meta-analysis that probiotics may be an effective means of preventing AD, and this study can provide a certain degree of basis for the prevention of AD in clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-200/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-200/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-200/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guimarães P, Batista A, Zieger M, et al. Artificial Intelligence in Multiphoton Tomography: Atopic Dermatitis Diagnosis. Sci Rep 2020;10:7968. [Crossref] [PubMed]
- Tokura Y, Hayano S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol Int 2022;71:14-24. [Crossref] [PubMed]
- Johnson H, Yu J. Current and Emerging Therapies in Pediatric Atopic Dermatitis. Dermatol Ther (Heidelb) 2022;12:2691-703. [Crossref] [PubMed]
- Lockhart MK, Siegfried EC. Evolving Landscape of Systemic Therapy for Pediatric Atopic Dermatitis. Dermatol Clin 2022;40:137-43. [Crossref] [PubMed]
- Mubanga M, Lundholm C, D'Onofrio BM, et al. Association of Early Life Exposure to Antibiotics With Risk of Atopic Dermatitis in Sweden. JAMA Netw Open 2021;4:e215245. [Crossref] [PubMed]
- Naik PP. Recent insights into the management of treatment-resistant pediatric atopic dermatitis. Int J Womens Dermatol 2022;8:e023. [Crossref] [PubMed]
- Khoder G, Al-Yassir F, Al Menhali A, et al. Probiotics Upregulate Trefoil Factors and Downregulate Pepsinogen in the Mouse Stomach. Int J Mol Sci 2019;20:3901. [Crossref] [PubMed]
- Snigdha S, Ha K, Tsai P, et al. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol Ther 2022;231:107978. [Crossref] [PubMed]
- Giron M, Thomas M, Dardevet D, et al. Gut microbes and muscle function: can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle 2022;13:1460-76. [Crossref] [PubMed]
- Compare D, Sgamato C, Nardone OM, et al. Probiotics in Gastrointestinal Diseases: All that Glitters Is Not Gold. Dig Dis 2022;40:123-32. [Crossref] [PubMed]
- Håkansson Å, Andrén Aronsson C, Brundin C, et al. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019;11:1925. [Crossref] [PubMed]
- Ou CY, Kuo HC, Wang L, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy 2012;42:1386-96. [Crossref] [PubMed]
- Schmidt RM, Pilmann Laursen R, Bruun S, et al. Probiotics in late infancy reduce the incidence of eczema: A randomized controlled trial. Pediatr Allergy Immunol 2019;30:335-40. [Crossref] [PubMed]
- Voigt J, Lele M. Lactobacillus rhamnosus Used in the Perinatal Period for the Prevention of Atopic Dermatitis in Infants: A Systematic Review and Meta-Analysis of Randomized Trials. Am J Clin Dermatol 2022;23:801-11. [Crossref] [PubMed]
- Zhou Q, Chen ZH, Zhang JX, et al. A systematic review of the quality of reporting and risk of bias for randomized crossover trials in digestive disease journals. Therap Adv Gastroenterol 2022;15:17562848211067874. [Crossref] [PubMed]
- Abrahamsson TR, Jakobsson T, Björkstén B, et al. No effect of probiotics on respiratory allergies: a seven-year follow-up of a randomized controlled trial in infancy. Pediatr Allergy Immunol 2013;24:556-61. [Crossref] [PubMed]
- Allen SJ, Jordan S, Storey M, et al. Probiotics in the prevention of eczema: a randomised controlled trial. Arch Dis Child 2014;99:1014-9. [Crossref] [PubMed]
- Böttcher MF, Abrahamsson TR, Fredriksson M, et al. Low breast milk TGF-beta2 is induced by Lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy. Pediatr Allergy Immunol 2008;19:497-504. [Crossref] [PubMed]
- Boyle RJ, Ismail IH, Kivivuori S, et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 2011;66:509-16. [Crossref] [PubMed]
- Cabana MD, McKean M, Caughey AB, et al. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017;140:e20163000. [Crossref] [PubMed]
- Dotterud CK, Storrø O, Johnsen R, et al. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol 2010;163:616-23. [Crossref] [PubMed]
- Huurre A, Laitinen K, Rautava S, et al. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy 2008;38:1342-8. [Crossref] [PubMed]
- Jensen MP, Meldrum S, Taylor AL, et al. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol 2012;130:1209-1211.e5. [Crossref] [PubMed]
- Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076-9. [Crossref] [PubMed]
- Kalliomäki M, Salminen S, Poussa T, et al. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003;361:1869-71. [Crossref] [PubMed]
- Kalliomäki M, Salminen S, Poussa T, et al. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2007;119:1019-21. [Crossref] [PubMed]
- Kim JY, Kwon JH, Ahn SH, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol 2010;21:e386-93. [Crossref] [PubMed]
- Kopp MV, Hennemuth I, Heinzmann A, et al. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 2008;121:e850-6. [Crossref] [PubMed]
- Kuitunen M, Kukkonen K, Juntunen-Backman K, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol 2009;123:335-41. [Crossref] [PubMed]
- Kukkonen K, Savilahti E, Haahtela T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007;119:192-8. [Crossref] [PubMed]
- Loo EX, Llanora GV, Lu Q, et al. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. Int Arch Allergy Immunol 2014;163:25-8. [Crossref] [PubMed]
- Niers L, Martín R, Rijkers G, et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 2009;64:1349-58. [Crossref] [PubMed]
- Peldan P, Kukkonen AK, Savilahti E, et al. Perinatal probiotics decreased eczema up to 10 years of age, but at 5-10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy 2017;47:975-9. [Crossref] [PubMed]
- Plummer EL, Chebar Lozinsky A, Tobin JM, et al. Postnatal probiotics and allergic disease in very preterm infants: Sub-study to the ProPrems randomized trial. Allergy 2020;75:127-36. [Crossref] [PubMed]
- Prescott SL, Wiltschut J, Taylor A, et al. Early markers of allergic disease in a primary prevention study using probiotics: 2.5-year follow-up phase. Allergy 2008;63:1481-90. [Crossref] [PubMed]
- Rautava S, Kainonen E, Salminen S, et al. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol 2012;130:1355-60. [Crossref] [PubMed]
- Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol 2002;109:119-21. [Crossref] [PubMed]
- Rø ADB, Simpson MR, Rø TB, et al. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy 2017;47:1014-21. [Crossref] [PubMed]
- Rozé JC, Barbarot S, Butel MJ, et al. An α-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: a multicentre, double-blind, randomised trial. Br J Nutr 2012;107:1616-22. [Crossref] [PubMed]
- Simpson MR, Dotterud CK, Storrø O, et al. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol 2015;15:13. [Crossref] [PubMed]
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2007;119:184-91. [Crossref] [PubMed]
- West CE, Hammarström ML, Hernell O. Probiotics during weaning reduce the incidence of eczema. Pediatr Allergy Immunol 2009;20:430-7. [Crossref] [PubMed]
- West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease--follow-up at 8-9 years of age. Allergy 2013;68:1015-20. [Crossref] [PubMed]
- Wickens K, Barthow C, Mitchell EA, et al. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol 2018;29:808-14. [Crossref] [PubMed]
- Wickens K, Barthow C, Mitchell EA, et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr Allergy Immunol 2018;29:296-302. [Crossref] [PubMed]
- Wickens K, Black P, Stanley TV, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy 2012;42:1071-9. [Crossref] [PubMed]
- Wickens K, Black PN, Stanley TV, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2008;122:788-94. [Crossref] [PubMed]
- Wickens K, Stanley TV, Mitchell EA, et al. Early supplementation with Lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: does it also reduce atopic sensitization? Clin Exp Allergy 2013;43:1048-57. [Crossref] [PubMed]
- Han C. Early application of probiotics in the prevention of neonatal eczema [Master]. Beijing: North China University of Science and Technology, 2019.
- Wu F, Feng X, Liu X, et al. The effect of bifidobacterium on the composition of human milk and its relationship with infant allergic diseases. Journal of Clinical Pediatrics 2010;28:260-3.
- Collado MC, Cernada M, Baüerl C, et al. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012;3:352-65. [Crossref] [PubMed]
- Sorbara MT, Pamer EG. Microbiome-based therapeutics. Nat Rev Microbiol 2022;20:365-80. [Crossref] [PubMed]
- Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016;375:2369-79. [Crossref] [PubMed]
- Di Mauro A, Neu J, Riezzo G, et al. Gastrointestinal function development and microbiota. Ital J Pediatr 2013;39:15. [Crossref] [PubMed]
- Yadav MK, Kumari I, Singh B, et al. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol 2022;106:505-21. [Crossref] [PubMed]
- Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am 2017;46:77-89. [Crossref] [PubMed]
- Sun S, Chang G, Zhang L. The prevention effect of probiotics against eczema in children: an update systematic review and meta-analysis. J Dermatolog Treat 2022;33:1844-54. [Crossref] [PubMed]
- Pan H, Su J. Association of Probiotics with Atopic Dermatitis among Infant: A Meta-analysis of Randomized Controlled Trials. Oxid Med Cell Longev 2022;2022:5080190. [Crossref] [PubMed]
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017;14:491-502. [Crossref] [PubMed]
- Flinterman AE, Knol EF, van Ieperen-van Dijk AG, et al. Probiotics have a different immunomodulatory potential in vitro versus ex vivo upon oral administration in children with food allergy. Int Arch Allergy Immunol 2007;143:237-44. [Crossref] [PubMed]
- Shen X, Wang M, Zhang X, et al. Dynamic construction of gut microbiota may influence allergic diseases of infants in Southwest China. BMC Microbiol 2019;19:123. [Crossref] [PubMed]





