The clinical spectrum of severe acute malnutrition in children in Cameroon: a hospital-based study in Yaounde, Cameroon
Introduction
In 2009, the World Health Organization (WHO) estimated that 20 million children under 5 years suffered from severe acute malnutrition (SAM) worldwide (1), which is associated to more than half of their deaths each year in developing countries. SAM has been a real obstacle to the achievement of the fourth Millennium Goal for development (2). In Africa, severe malnutrition is responsible for 5–15% of deaths in children from 0 to 59 months, causing about 1 to 2 million deaths yearly (3). In 2011, the demographic and health multiple indicator survey revealed that Cameroon globally has 5.6% of children with acute malnutrition with 1.9% of them being severely malnourished (4), and the prevalence stayed at 1.3% in 2014 (5). Most studies on malnutrition so far assessed the nutritional status of children in certain communities of the country, notably the study of Nem in Garoua in 2010 among preschool children (6), and more recently in 2014 evaluating the nutritional status of children aged from 6 to 59 months in the localities of Mouanko (7) in the Littoral and Bafou in the West region (8).
We decided to carry out a study on malnutrition in a hospital setting precisely in Yaounde Gynaeco-Obstetric and Pediatric Hospital (YGOPH) which has a nutritional rehabilitation unit since 2003. This work aims to determine the epidemiological, clinical aspects and outcome of children with SAM managed in YGOPH.
Methods
The study was conducted in the general pediatric service of YGOPH which has a management unit for malnutrition. The YGOPH is a category one hospital located in Cameroon’s capital Yaoundé receiving patients from Yaoundé and all over the country.
This was a descriptive and retrospective cross-sectional study that was carried out from September 1st 2006 to March 31st 2015. The source of our data was the admission and medical files. The children included in our study were under 15 years old and presented with at least one of the following criteria by Golden et al. (9):
- For infants less than six months or less than 3 kg: infants too weak to suckle effectively regardless of the weight for height index (W/H), weight for age (W/A) or any other anthropometric measure; and/or infants no longer gaining weight at home after a series of weight gain during growth monitoring, and/or infant with an index of W/H <−3 Z score, and/or with bilateral edema;
- For children over six months and more than 3 kg: W/H index <−3 Z score, and/or presence of bilateral edema, and/or mid upper arm circumference (MUAC) <115 mm (6–59 months);
- For children admitted into the study we collected the following information: socio-demographic data (age and sex of patients) and characteristics of mothers (maternal age and occupation), and the father’s profession;
- Clinical data: anthropometric parameters, signs and symptoms on admission, clinical forms of SAM, complications and comorbidities on admission;
- Data on the outcome: number of days of hospitalization, weight gain, clinical evolution of those discharged alive, number of deaths and causes of death.
The data collected by the data sheets were entered and analyzed using Epi Info Version 3.5.4 software, and Microsoft Office Excel 2010. The quantitative variables were described as mean ± standard deviation and categorical variables were described in terms of proportion.
For anthropometric variables, they were interpreted using the WHO Anthro software Version 3.2.2 and WHO Anthroplus version 1.0.4 for children over 60 months. We made a description of the number of days of hospitalization with a univariate analysis. P value was calculated using the Mann Whitney/Wilcoxon test which is used to compare means. The significance level was set at P<0.05.
Results
Epidemiological features
From September 2006 to March 31, 2015, 17,981 children were admitted in the pediatric unit of the YGOPH. Among these children, 489 presented with SAM, giving a prevalence for SAM of 2.72% in our hospital setting. We excluded files that did not contain anthropometric data, that had incomplete records or that were missing. A total of 179 medical files met our eligibility criteria (Table 1) and were exploited. Among the 179 retained files, 88 (50.8%) were male and 91 (49.2%) female giving a sex ratio of 0.97. The median age on admission was 9 months with extremes of 23 days and 112 months. The most represented age group was that of 6 to 12 months, with 34.6% of cases. The average age of the mothers was 26±6.5 years, ranging from 13 to 47. About 27.7% of the mothers were within the 20–25 years age group and 48.4% of mothers were unemployed. When they were employed, it was a liberal profession in 33.3% of cases. As for fathers when their profession was known, it was mainly liberal (65.1%).

Full table
Clinical features
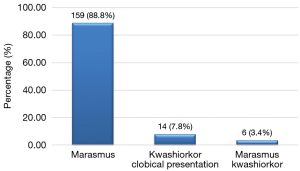
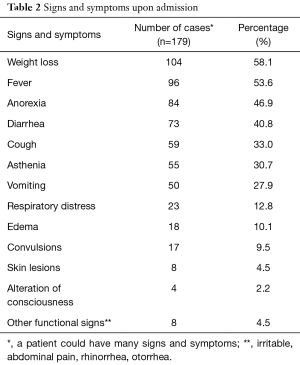
The main symptoms on admission were weight loss, fever and loss of appetite with 58.1%, 53.6% and 46.9% as respective frequencies (Table 2). The median time interval between the onset of symptoms and admission was 14 days (range, 1 to 390 days). Marasmus was the dominant clinical form with 88.8%, then kwashiorkor with 7.8% and marasmus- kwashiorkor with 3.4% (Figure 1).
Full table
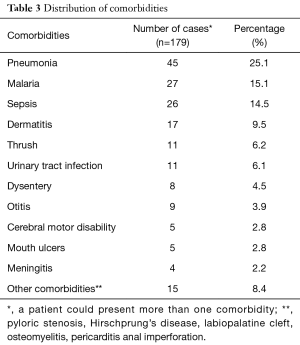
Altogether, 85.01% of the study population presented with at least one comorbidity. Respiratory tract infections were the dominant comorbidities (25.1%) followed by malaria and sepsis (Table 3). Of the 179 exploited cases, the human immuno deficiency virus (HIV) status was known for 32 children, and 14 of them tested positive (43.75%). The most common complication on admission was dehydration (29.6%). Hypoglycemia and heart failure was the least common (Table 4).
Full table
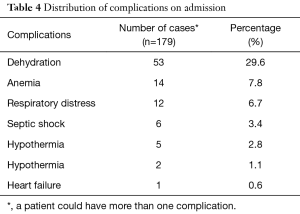
Full table
Of the 60 children in our sample whose age of introduction of foods other than breast milk was known, 26 began before 6 months, giving 43.3%. The 18 medical files of children of 6 months and above that informed us on diet from birth to 6 months showed that 10 were exclusively breastfed till 6 months, making up 8.3% of our patients.
Outcome
One hundred and five children had a favorable clinical evolution, while 27 deaths were recorded (Table 5). Septic shock (25.9%) and respiratory distress (18.5%) were the most frequently found causes of death, with 44.4% of deaths with no known cause (Table 6).
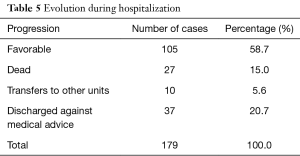
Full table
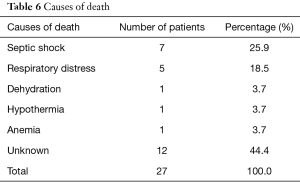
Full table
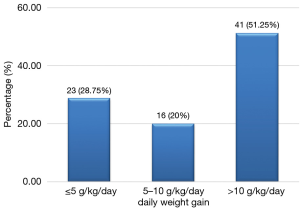
Among the 94 cases of marasmus whose discharge was authorized, 80 had gained weight; making up 85.1% while 51.25% of them had a satisfactory weight gain of greater than 10 g/kg/day (Figure 2). The average daily weight gain was 15.86±13.8 g/kg/day. Among the 10 children who had edema and were discharged, 5 had an average daily weight loss of 4.9±2.9 g/kg/day, 4 experienced a mean weight gain of 7.7±8.3 g/kg/day and 1 child had no weight gain. The overall average number of days of hospitalization was 8.25±5.6 days with a range of 1–34 days. Deaths occurred on average before the first four days of admission (3.77 days) with a range of 1 to 17 days. The death occurred almost twice earlier than the discharge date (P=0.0000) (Table 7). Children whose discharge was authorized were hospitalized for a number of days ranging from 2–34 days. Children with marasmus had on average 8.4 days of hospitalization, while those with kwashiorkor had a larger average number of days of hospitalization 14.5 days (P=0, 0116) (Table 7).
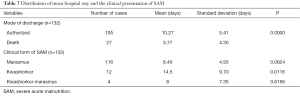
Full table
Discussion
We found a prevalence of SAM of 2.72%, the rate was similar to that found by Ubesie et al. in Nigeria which was 2.8% (10) and below the 3.75% found by Ehouzou (11) in 2014 at the Mother and Child Centre of the Chantal Biya Foundation (MCC/CBF). This difference can be explained by the fact that the MCC/CBF has a hospitalization and treatment unit for SAM with a greater capacity than the YGOPH. The sex ratio was 0.97 which indicates a virtual equality between the sexes with a slight female predominance. Mutombo et al. in Ivory Coast had found a sex ratio of 0.91 (12). Ubesie et al. in Nigeria had also found a female predominance (10) and Irena et al. in Zambia in 2014 had a male predominance (13) with no statistically significant difference. The median age of our study population was 9 months. This age was lower than the 17 months found by Irena et al. in 2011 in Zambia (13). It should also be noted that this study included children from 6 months; whereas we included children from the neonatal period. In our study, SAM was more common in the age group of 0 to 6 months and 6 to 12 months with respectively 32.4% and 34.6% of the population, while Ubesie et al. noted that children from 6 to 12 and 12 to 24 months were the majority. The age group of 0 to 6 months held the second highest proportion in our study. This can be explained by the frequency of poor feeding practices: weaning, poor dilution and poor hygiene of feeding bottles listed in our study. Those whose ages were between 6 and 12 months in our study had the highest proportion. This can be explained by the fact that it is in this age group that food diversification is done and when poorly managed, it promotes the occurrence of malnutrition.
The maternal age ranged from 13–47 years with a mean age of mothers 26.3±6.5 years, those whose ages ranged between 20 and 25 years were most represented (27.7%); this age is the usual period of maternity in Cameroon according to the 2011 Demographic Health Survey in Cameroon (4).
Weight loss and fever were the most frequent functional signs on admission representing 58.1% and 53.6% respectively. This could be due to high proportions of marasmus (88.8%) in our study population. Marasmus is reflected by severe wasting (P/T <−3 Z score). Ubesie et al. and Irena et al. had found a predominance of diarrhea in 72.7% and 59.2% respectively (10,13) in our study it was present in 40.8% of patients. The average time between the onset of symptoms and admission to the YGOPH was 30.36 days; which is relatively long. It can be explained by the habit of seeking care with self-medication and sometimes traditional medicine, which are the first means of routine care in our community. We have not found other authors who have studied this variable.
The most encountered complication was dehydration (29.6%), this is due to the large proportion of diarrhea (40.8%) in our study, although clinical signs used to assess dehydration are confused with those of SAM. The diagnosis of dehydration was made if there was recent fluid loss form diarrhea or vomiting, thirst and a change in the eyeballs, as indicated by the mother. In second place we had severe anemia (7.8%) which was considered when the hemoglobin level was less than 4 g/dL or when the hematocrit was below 12%. This rate was higher than that found by Bachou et al. in Uganda (6.5%) (14) and lower than that found by Ubesie et al. (24.2%) (10). The most predominant clinical form of SAM was marasmus (88.8%), followed by kwashiorkor (7.8%). The predominance of marasmus was found by several authors including Ubesie et al. (34.9%), and Mutombo et al. in Ivory Coast (70.5%) (10,12). In Sudan, Gabbad et al. instead found a predominance of kwashiorkor in children under 5 years (43.8%) (15).
Regarding comorbidities, 81.56% of children with SAM had at least one co morbidity that is, pneumonia (25.1%) (with 3 cases of pulmonary tuberculosis), malaria (15.1%) and sepsis of unknown focus (14.5%). These three pathologies were also the majority in the Ubesie et al. study (10), but in different proportions. In India in 2014, Kumar et al. had found a proportion of lung infections of 27.8%, relatively similar to that found in our study (16). The HIV status was known in 32 of our patients, that is 17.8%.
This rate is low compared to that found by Saloojee et al. in South Africa (39%) (17). This is due to the fact that the results of the HIV serology test were not available in the records or was not systematically requested in all cases of SAM. Among them, 14% or 43.75% had a positive status. Other studies have found a lower rate, namely in Zambia (38.9%) (13); and in Nigeria (13.6%) (10). However the HIV serology prevalence of our study was lower than that observed by Saloojee et al. in South Africa that is 87% (17). Among the 132 patients whose progression in the hospital was known, 27 died giving a mortality rate of 20.45%. Children with marasmic-kwashiorkor had a higher mortality rate of 75%. Ubesie et al. had also observed a high mortality in children with marasmic- kwashiorkor compared to other clinical forms of SAM (53.3%) (10). The mortality rate observed in our study is higher than that found by Savadogo et al. in Burkina Faso 16% (18); but significantly lower than those found in Nigeria by Ubesie et al. (10) and in Zambia by Irena et al. (13) with 40.1% and 40.5% respectively. This difference can be explained by the fact that our study focused on a more recent period and benefited from newer and improved treatment protocols.
We noted that all children who tested negative for HIV survived; while a large proportion of those who tested positive died (35.7%). It is a co-infection which forms with malnutrition a vicious circle of immune-suppression and opportunistic infections that can lead to death.
The most frequent causes of death were septic shock and respiratory distress. The high proportion of patients (20.7%) who were discharged against medical advice is due to the financial constraints of the parents. Death occurred in half of the cases in the first 2 days of hospitalization which corroborates the findings in Zambia of Irena et al. (13) this is repeated below. By the end of the first week, 78% of deaths had occurred. The average duration of hospitalization was 8.25 days with a range of 1 to 34 days, which is similar to Page et al. (8 days) (19); and to that found by Irena et al. (9 days) (13). Ubesie et al. had found a longer duration (16 days). Children who had kwashiorkor stayed longer in the hospital, 14.5 days on average in contrast to those with marasmus who spent 8 days in hospitalization on average. Ubesie et al. had also found that children with kwashiorkor spent more time in hospital than those with other clinical forms. We did not find in our study reasons that account for this difference but this can be explained by the fact that children with edema stay longer in the stabilization phase where loss of edema must occur before going to the rehabilitation phase. Therefore, their management takes longer than children with marasmus. Death occurred in half of the cases in the first 2 days of hospitalization which corroborates the findings of Irena et al. in Zambia (13).
Among the 94 cases of marasmus with favorable evolution, 80 gained weight, hence 85.1%. The mean weight gain was 15.86 (±14.80) g/kg/day. This is higher than that observed by Savadogo et al. from 1999 to 2003 in a nutritional rehabilitation center in Burkina Faso, which gave 10.18 (±7.05) g/kg/day (18). According to WHO, weight gain is considered small if it is <5 g/kg/day, medium or moderate if it is between 5 g/kg/day and 10 g/kg/day and satisfactory or good if it is >10 g/kg/day with nutritional rehabilitation (20). We recorded a satisfactory weight gain (>10 g/kg/day) of 51.25%, which is higher than that found by Hossain et al. in Bangladesh (30.90%) (21). Among children who presented with edema, half had an estimated weight loss of 4.9 (±2.99) g/kg/day, it was higher than that found by Hossain et al, which was 1.9 g/kg/day (21).
We did not address the therapeutic component because treatment protocols have been changed several times during the study period.
Conclusions
SAM remains a frequent severe public health problem in developing countries such as Cameroon and is associated with high morbidity and mortality. It mainly affects infants under 24 months. The causes are dominated by poor feeding practices. SAM is grafted to significant comorbidities and complications responsible for the death of affected children. Information, education and communication to parents on the causes and prevention means of this condition must be strengthened by medical staff to reduce morbidity and mortality related to malnutrition.
Acknowledgements
We are grateful to all the staff of the pediatric unit for taking good care of the children during hospitalization and Mr. Tchouine Frédéric for the statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: The study was approved by the Institutional Committee for Ethics in Research in Human Health (No: 136/CIERSH/DM/2015) of the Yaounde Gynaeco-Obstetric and Pediatric Hospital. Since it was a retrospective study on patients’ file informed consent from the patients was not necessary, but confidentiality of the information collected was ensured.
References
- OMS, UNICEF. Prise en charge communautaire de la malnutrition aiguë sévère. Genève: Déclaration commune de l’Organisation mondiale de la Santé, du Programme alimentaire mondial, du Comité permanent de la nutrition du Système des Nations Unies et du Fonds des Nations Unies pour l’enfance. Available online: http://www.who.int/nutrition/publications/severemalnutrition/978-92-806-4148-6_fre.pdf
- UNO. Millenium Development Goals. United Nations Organisation, New York; 2012.
- Collins S, Dent N, Binns P, et al. Management of severe acute malnutrition in children. Lancet 2006;368:1992-2000. [Crossref] [PubMed]
- INS, MINPAT, MINSANTE. Enquête Démographique et de Santé et à Indicateurs Multiples 2011. Yaoundé: ICF International MINSANTE Cameroun; 2011.
- Institut National de la Statistique. Enquête par grappes à indicateurs multiples (MICS5) 2014: Rapport de résultats clés. Yaoundé, Cameroun: Institut National de la Statistique; 2015.
- Nem TD. Données anthropométriques des enfants d’âge préscolaire à Garoua, Cameroun. Mémoire FMSB 2009. Université de Yaoundé I; 2009. Available online: http://www.memoireonline.com/03/12/5476/m_Donnees-anthropometriques-des-enfants-d-age-prescolaire--Garoua-Cameroun5.html
- Kana D. Evaluation de l’état nutritionnel des enfants de 6 à 59 mois dans la localité de Mouanko. Thèse FMSB Yaoundé; 2014.
- Nyaga F. Evaluation de l’état nutritionnel des enfants de 6 à 59 mois dans la localité de Bafou. Thesis, Faculty of Medicine and Biomedical Sciences, University of Yaounde I; 2014.
- Golden M, Grellety Y. Protocole prise en charge intégrée de la malnutrition aiguë. Afrique l'ouest: 2012.
- Ubesie AC, Ibeziako NS, Ndiokwelu CI, et al. Under-five protein energy malnutrition admitted at the University of Nigeria Teaching Hospital, Enugu: a 10 year retrospective review. Nutr J 2012;11:43. [Crossref] [PubMed]
- Ehouzou M. Malnutrition aigüe sévère: profil épidémiologique, clinique et évolutif des cas infectés par le VIH au Centre Mère et Enfant de la Fondation Chantal Biya. Mémoire FMSB Yaoundé; 2013.
- Mutombo T, Keusse J, Sangare A. Sida et malnutrition en milieu pediatrique semirural - Experience de l’hôpital protestant de Dabou en Cote d’Ivoire. Méd Afr Noire 1996;43:72-7.
- Irena AH, Mwambazi M, Mulenga V. Diarrhea is a major killer of children with severe acute malnutrition admitted to inpatient set-up in Lusaka, Zambia. Nutr J 2011;10:110. [Crossref] [PubMed]
- Bachou H, Tumwine JK, Mwadime RK, et al. Risk factors in hospital deaths in severely malnourished children in Kampala, Uganda. BMC Pediatr 2006;6:7. [Crossref] [PubMed]
- Gabbad AA, Adam A, Elawad MA. Epidemiological aspects of malnutrition in children less than five years admitted to Gaafar Ibn oaf paediatric hospital, Khartoum, Soudan. Asian Journal of Natural & Applied Sciences 2014;3:67-72.
- Kumar R, Singh J, Joshi K, et al. Co-morbidities in hospitalized children with severe acute malnutrition. Indian Pediatr 2014;51:125-7. [Crossref] [PubMed]
- Saloojee H, De Maayer T, Garenne ML, et al. What's new? Investigating risk factors for severe childhood malnutrition in a high HIV prevalence South African setting. Scand J Public Health Suppl 2007;69:96-106. [Crossref] [PubMed]
- Savadogo L, Zoetaba I, Donnen P, et al. Management of severe acute malnutrition in an urban nutritional rehabilitation center in Burkina Faso. Rev Epidemiol Sante Publique 2007;55:265-74. [Crossref] [PubMed]
- Page AL, de Rekeneire N, Sayadi S, et al. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One 2013;8:e68699. [Crossref] [PubMed]
- Sultana K, Ann A, Alan J, et al. Directives pour le traitement hospitalier des enfants sévèrement malnutris. OMS: 2004.
- Hossain MI, Dodd NS, Ahmed T, et al. Experience in managing severe malnutrition in a government tertiary treatment facility in Bangladesh. J Health Popul Nutr 2009;27:72-9. [Crossref] [PubMed]