
Mayer-Rokitansky-Küster-Hauser syndrome with idiopathic central precocious puberty: a case report
Highlight box
Key findings
• Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome should be considered in girls when an ultrasound examination shows uterus absence or maldevelopment, even in girls with precocious puberty.
What is known and what is new?
• MRKH syndrome concomitant with central precocious puberty (CPP) is rare but possible.
• Gonadotropin-releasing hormone analog (GnRHa) therapy could be used for the treatment of CPP in children with MRKH syndrome to inhibit the development of bone age.
What is the implication, and what should change now?
• The gonads and sexual organs of children with precocious puberty should be carefully monitored and comprehensively assessed to exclude MRKH syndrome or any sex development disorders.
• The early diagnosis of MRKH syndrome with CPP in children is important so that psychological and emotional support can be provided to the children, parents, and guardians.
Introduction
Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome is a congenital syndrome characterized by the complete absence or hypoplasia of the Müllerian ducts, including the fallopian tubes, uterus, and upper two-thirds of the vagina and has an estimated prevalence of 1 in 5,000 live female births (1). MRKH syndrome is classified into the following two subtypes: type 1, which is characterized by isolated abnormalities of the reproductive system; and type 2, which is additionally characterized by renal and skeletal malformations and a short stature (2). Most children with MRKH syndrome have a normal vulva, and normal ovarian function, develop secondary sexual characteristics in puberty and display no symptoms in childhood. The diagnosis is often made when primary amenorrhea or dyspareunia occurs. A previous study from the department of gynecology has reported that Chinese patients have a mean age of 23.24±5.37 years at the first visit of MRKH syndrome (3).
Precocious puberty (PP) is a common pediatric disorder of sexual development. MRKH syndrome accompanied by PP is extremely rare (4,5), and only one case of MRKH syndrome combined with central precocious puberty (CPP) has been reported worldwide to date. In this study, we report a case of MRKH syndrome with idiopathic CPP (ICPP) to provide evidence for the diagnosis and treatment of MRKH syndrome with CPP. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-181/rc).
Case presentation
A 7-year-old girl was admitted to the Huzhou Maternity & Child Health Care Hospital (Huzhou, China) in March 2020. She had been developing bilateral breasts for >1 year. She had no history of hormone usage and no growth spurt. The parents denied consanguineous marriage and had no history of abnormal puberty. The patient had a height of 118.9 cm [−1.0 standard deviation score (SDS)], and a bone age (BA) of 9 years (height for BA: −3.04 SDS). She was Tanner stage B2 for breast development and Tanner stage 1 for pubic hair development. Her vulva resembled that of a young girl. Gross observation of the clitoris showed no abnormality, and no labia fusion was found. No axillary hair, multiple-mole sign in the facial area, webbed neck, increased distance between breasts, or thyroid enlargement were observed.
The patient’s luteinizing hormone (LH) was 0.9 IU/L (reference range, <0.2 IU/L), follicle-stimulating hormone (FSH) was 2.2 IU/L (reference range, 0.18–3.38 IU/L), estradiol (E2) was 42.1 pmol/L (reference range, <125.5 pmol/L), and testosterone (T) was <0.087 nmol/L. After gonadotropin-releasing hormone (GnRH) stimulation, her peak LH was 12.1 IU/L, peak FSH was 12.6 IU/L, and the LH/FSH ratio was 0.96.
An ultrasound examination of the pelvic cavity showed that the size of uterus was 1.6 cm × 1.4 cm × 0.5 cm, the size of the left ovary was 1.9 cm × 1.6 cm × 1.4 cm (2.3 mL), the size of the right ovary was 2.1 cm×1.7 cm×1.0 cm (2.0 mL), and the size of the follicles were >0.4 cm in the bilateral ovaries. An ultrasound examination of the bilateral breasts showed that the size of the left and right breast was 1.7 cm × 0.9 cm, and 1.8 cm × 1.0 cm, respectively. Magnetic resonance imaging (MRI) showed that the pituitary gland was about 3.1 mm in height.
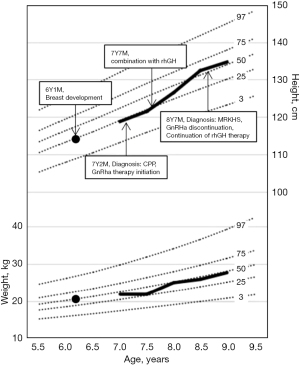
The patient was initially diagnosed with ICPP and after 5 months of gonadotropin-releasing hormone analog (GnRHa) (leuprorelin 3.75 mg, one subcutaneous injection/28 d) therapy alone, she started combined recombinant human growth hormone (rhGH; 0.15 IU/kg/d q.n.) therapy. After the treatment, her height increased from −1.0 SDS to a comparable average height, and her height for BA SDS increased from −3.04 to −1.27 (Figure 1).
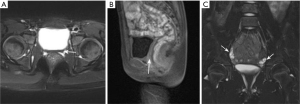
At the 17-month follow-up visit, an ultrasound showed no uterus. Pelvic MRI showed no uterus or cervix; the vaginal structure was unclear between the rectum and the bladder (Figure 2). A pediatric gynecologic examination showed no normal vaginal orifice, and a longitudinal opening of approximately 1.5 cm. Her chromosome karyotype was 46,XX. A diagnosis of MRKH syndrome with concomitant ICPP was finally confirmed. An ultrasound of the abdomen and urinary tract showed no abnormality, and echocardiography and a hearing test also returned normal results. A whole spine X-ray showed that the thoracic spine was slightly bent on the right side (at a Cobb angle of 5°), and the disease was considered type I MRKH syndrome.
Whole-exome sequencing (WES) and chromosomal microarray analysis (CMA) were performed. Samples were prepared using the IDT xGen Exome Research Panel v1.0 (IDT, Coralville, IA, USA). Final quantified libraries were seeded onto an Illumina flow cell and sequenced using paired-end 150 cycle chemistry on the NovaSeq 6000 (Illumina, San Diego, CA, USA). Initial data processing, base calling, alignments to the human genome assembly GRCh37 (hg19) and variant calls were generated by various bioinformatics tools. The mean sequence coverage per base was approximately 100X, and at least 95% of the regions were covered at no less than 20X. The interpretation of sequence variants was performed according to the American College of Medical Genetics and Genomics (ACMG). WES showed no genetic variation related to clinical phenotype, and no obvious genomic imbalance was detected in the CMA.
After confirming the diagnosis and a discussion with the parents, the GnRHa treatment was stopped based on the parents’ wishes, and rhGH treatment was continued. The patient was followed-up.
This study was approved by the Ethics Committee of the Huzhou Maternity & Child Health Care Hospital (No. 2022-J-009). All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was taken from the patient’s guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case illustrates the possibility of concomitant CPP in patients with MRKH syndrome. Once a diagnosis of MRKH syndrome is suspected, further examinations, including imaging, karyotyping/chromosome analysis, and serum hormone status tests, should be undertaken to confirm the diagnosis. MRI has an unmatched role in diagnosing MRKH syndrome (6), as its high resolution for soft tissues facilitates the clear visualization of abnormalities of the uterus, vagina, and ovaries, and enables the evaluation of any accompanying deformities (7).
An ultrasound examination is widely used for the auxiliary examination of the gonads of children with CPP, as it is non-invasive, easy to perform, and low cost (8,9). In this case, the diagnosis was initially missed due to the false negative results of the first transabdominal ultrasound. Transabdominal ultrasound examination is dependent on the experience and skill of the operator, the results may have poor accuracy and insufficient objectivity. In the present case, a pelvic ultrasound before treatment showed that the size of the “uterus” was relatively small according to the ovary volume. However, due to a limited experience of a sonologist, this image was misidentified as a uterus. A pelvic ultrasound re-examination during treatment showed no uterus, and thus pelvic MRI was suggested, but this was refused by the parents twice. A pelvic MRI examination performed after the 3rd ultrasound examination confirmed the diagnosis of MRKH syndrome.
The causes of MRKH syndrome remain unclear and might involve various environmental and genetic factors. Candidate genes mainly include mutations in LHX1, HNF-1B, TBX6, WNT9B, WNT4, HOXA, and GREB1L (10). The deletion or duplication of various chromosomal regions, including 1q21.1, 1p31-1p35, 16p11.2, 17q12, 22q11.21, and Xp22, have also been reported (11). To date, such chromosomal aberrations have only been detected in a few patients with MRKH syndrome, and these mutations are not specific to MRKH syndrome. GnRH-dependent PP is caused by the premature activation of the hypothalamic-pituitary-gonadal axis, and the activation and inactivation mutations of the KISS1, KISS1R, MKRN3, and DLK1 genes have been demonstrated to be associated with CPP (12,13). As the genetic causes of MRKH syndrome and CPP are different, these two diseases seem to be genetically unrelated. Therefore, it is speculated that in the present case, the occurrence of MRKH syndrome and CPP was a coincidence. Although a previous study (4) suggested that early stimulated FSH-related pathway may contribute to PP in MRKH syndrome, in our case, FSH was not elevated. More data is needed for further understanding the pathogenetic mechanism for CPP in MRKH syndrome.
The treatments for MRKH syndrome include psychological counseling and the correction of anatomical abnormalities. Various non-surgical vaginal dilation and Surgical vaginoplasties have been suggested for vaginal construction. In relation to the timing of the treatment, vaginal construction can only be performed after sexual maturity and if the patients are willing to be treated. Sustained-release GnRHa therapy is generally used to treat CPP, and the feasibility of GnRHa treatment for CPP children with combined MRKH syndrome remains unknown. Ai et al. (5) reported 4 cases, but only 1 patient was confirmed to have a diagnosis of CPP and type1 MRKHS, and GnRHa treatment was not prescribed. In the present case, the uterus was not found, but the development of the ovaries and the secretion of estrogen were normal. The GnRHa treatment inhibited the secretion of estrogen, which can delay the development of BA, and have a beneficial effect in increasing the final height outcome. Theoretically, GnRHa could be used for the treatment of CPP in children with MRKH syndrome to inhibit the development of BA.
Conclusions
MRKH syndrome and CPP occur together extremely rarely, MRKH syndrome should be considered in girls when an ultrasound examination shows uterus absence or maldevelopment, even in girls with PP. GnRHa therapy could be used for the treatment of CPP in children with MRKH syndrome to inhibit the development of BA. Psychological support could be more important than the treatment of anatomical abnormalities in patients with MRKH syndrome (1). Early diagnosis is important, as it could enable psychological and emotional support to be provided to the children, parents, and guardians.
Acknowledgments
Funding: This study was supported by the Zhejiang Province Natural Sciences Foundation Zhejiang Society for Mathematical Medicine (No. LSZ19H070001).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-181/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-181/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-181/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herlin M, Bjørn AM, Rasmussen M, et al. Prevalence and patient characteristics of Mayer-Rokitansky-Küster-Hauser syndrome: a nationwide registry-based study. Hum Reprod 2016;31:2384-90. [Crossref] [PubMed]
- ACOG Committee Opinion No. 728: Müllerian Agenesis: Diagnosis, Management, And Treatment. Obstet Gynecol 2018;131:e35-42. [Crossref] [PubMed]
- Chen N, Pan H, Luo G, et al. Clinical characteristics of 1,055 Chinese patients with Mayer-Rokitansky-Küster-Hauser syndrome: a nationwide multicentric study. Fertil Steril 2021;116:558-65. [Crossref] [PubMed]
- Atabek ME, Pirgon O, Sert A. Mayer-Rokitansky-Kuster-Hauser syndrome presenting as premature thelarche in a young child. Pediatr Int 2007;49:533-5. [Crossref] [PubMed]
- Ai Z, Zhu X, Chen H, et al. Precocious puberty or growth hormone deficiency as initial presentation in Mayer-Rokitansky-kuster-Hauser syndrome: a clinical report of 5 cases. BMC Pediatr 2022;22:418. [Crossref] [PubMed]
- Bhayana A, Ghasi RG. MRI evaluation of pelvis in Mayer-Rokitansky-Kuster-Hauser syndrome: interobserver agreement for surgically relevant structures. Br J Radiol 2019;92:20190045. [Crossref] [PubMed]
- Khan N, Khaliq M, Azam MM, et al. Mayer-Rokitansky-Küster-Hauser Syndrome: MR Manifestations Of Typical And Atypical Cases. J Ayub Med Coll Abbottabad 2021;33:S711-6. [PubMed]
- Talarico V, Rodio MB, Viscomi A, et al. The role of pelvic ultrasound for the diagnosis and management of central precocious puberty: An update. Acta Biomed 2021;92:e2021480. [PubMed]
- Cheuiche AV, da Silveira LG, de Paula LCP, et al. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr 2021;180:3073-87. [Crossref] [PubMed]
- Herlin MK, Le VQ, Højland AT, et al. Whole-exome sequencing identifies a GREB1L variant in a three-generation family with Müllerian and renal agenesis: a novel candidate gene in Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. A case report. Hum Reprod 2019;34:1838-46. [Crossref] [PubMed]
- Triantafyllidi VE, Mavrogianni D, Kalampalikis A, et al. Identification of Genetic Causes in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome: A Systematic Review of the Literature. Children (Basel) 2022;9:961. [Crossref] [PubMed]
- Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: Recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol (Oxf) 2021;95:542-55. [Crossref] [PubMed]
- Aguirre RS, Eugster EA. Central precocious puberty: From genetics to treatment. Best Pract Res Clin Endocrinol Metab 2018;32:343-54. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


