
The comparisons of vitamin D3 levels in IgA vasculitis across different subgroups and healthy children: a comparative study
Highlight box
Key findings
• Vitamin D3 levels were significantly lower in the IgA vasculitis children than the healthy children and were even more reduced when the children also suffered from renal or gastrointestinal symptoms.
What is known and what is new?
• Vitamin D deficiency is prevalent in autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, autoimmune thyroid disorders, and antiphospholipid syndrome.
• Vitamin D deficiency is also prevalent in IgA vasculitis patients.
What is the implication, and what should change now?
• Reduced vitamin D levels may be involved in the development of IgA vasculitis.
Introduction
Immunoglobulin A (IgA) vasculitis (also known as Henoch-Schönlein purpura) is the most frequent form of vasculitis in children (1). The main pathological feature of IgA vasculitis is acute leukocytoclastic vasculitis, which is characterized by non-thrombocytopenic cutaneous purpura and often presents with a combination of gastrointestinal bleeding or pain, arthralgia, and renal impairment (2,3). IgA vasculitis frequently occurs in children and has a varied prognosis depending on the complications. The incidence rate of IgA vasculitis is estimated to be between 3–27 per 100,000 cases a year (1).
Generally, IgA vasculitis patients have excellent outcomes; however, approximately 20–70% of cases have renal involvement, which predominantly presents as glomerulonephritis with mesangial IgA deposits (4-6). The extent of renal damage determines the long-term prognosis of IgA vasculitis patients. Most cases of IgA vasculitis occur in autumn and winter, and boys tend to be affected more frequently than girls (7). The seasonal tendency of IgA vasculitis indicates a correlation between IgA vasculitis and viral infection (8). The etiology and pathogenesis of this disease are not yet fully understood (9); however, a wide variety of pathogens, infections, immune statuses, stimulation of allergic reactions, and gene polymorphisms have been associated with the occurrence of the disease (10).
As a fat-soluble vitamin, vitamin D presents subepidermally as an inactive cholecalciferol and is activated by ultraviolet irradiation, after being hydroxylated twice in the liver and kidney to form the biologically active 1,25-dihydroxyvitamin D3. In addition to its well-known functions of regulating calcium and phosphorus metabolism, and maintaining bone health, vitamin D has a broad regulatory effect on the immune system (11,12). Studies have shown that vitamin D deficiency is prevalent in autoimmune diseases, such as systemic lupus erythematosus (SLE) (13), multiple sclerosis (14), autoimmune thyroid disorders (15), and antiphospholipid syndrome (16). A systematic review showed that SLE patients had significantly lower vitamin D levels than healthy controls (13), and lower vitamin D levels were associated with SLE disease pathogenesis (17).
The main mechanisms underlying the role of vitamin D in immunity include the upregulation of T helper cell type 2 (Th2) cell activity, the inhibition of T helper cell type 1 (Th1) and T helper cell type 17 (Th17) cells, the enhancement of regulatory T cell (Treg) function, damage to the development and function of B cells, and the reduction of monocyte activation (11,12). Roy et al. (18) found that vitamin D has a profound long-term affect on the immune system and can help the immune system reach a new steady state. Roy et al. also reported that optimal vitamin D levels range from 50–100 mol/L with both the pathogen and effector T-cell levels kept within reasonable limits (18). In conclusion, while vitamin D has been widely studied in autoimmune diseases, few studies have investigated the role of vitamin D in IgA vasculitis patients. At present, only a few studies with small sample sizes have shown that IgA vasculitis patients have lower vitamin D levels than healthy controls (19-21). The distribution of vitamin D levels in renal involvement, gastrointestinal involvement, and joint involvement has not been thoroughly studied. Thus, we conducted a large retrospective study to examine the relationship between vitamin D levels and IgA vasculitis. This article is presented in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-176/rc).
Methods
Study design and participants
A retrospective study was performed between February 2017 and October 2019 to compare serum 25-hydroxyvitamin D3 (25(OH)D) levels between children with IgA vasculitis and healthy controls. The study was carried out in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of Ningbo Women and Children’s Hospital (Registry No. EC2022-044). Given the retrospective nature of this study, the requirement of informed consent was waived.
A total of 663 children (aged under 16 years) who had been diagnosed with IgA vasculitis at the Ningbo Women and Children’s Hospital were enrolled in this study. IgA vasculitis was diagnosed according to the 2005 European League against Rheumatism (22) and the Chinese Society of Pediatric Rheumatology diagnostic criteria for IgA vasculitis (23). The diagnostic criteria were as follows: a palpable rash (required) predominantly on the lower limbs with any one of the following: diffused abdominal pain; arthritis/arthralgia, kidney damage (hematuria/proteinuria), or IgA deposition on biopsies from any site. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) be a new-onset patient; (II) be an inpatient; and (III) be aged ≤16 years. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had another immune system disease; (II) had a history of hematuria or proteinuria; and/or (III) had a negative nutcracker sign; (IV) lost to follow-up within 6 months (Figure 1).
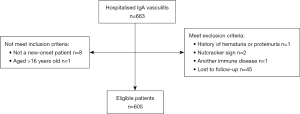
The renal involvement diagnosis was based on the revised diagnostic criteria of the Nephrology Group, Society of Pediatrics, Chinese Medical Association (24). Hematuria and/or proteinuria within 6 months of developing IgA vasculitis was recorded. The diagnostic criteria for proteinuria were as follows: (I) routine urine results showing positive urine protein 3 times within 1 week; (II) 24-h urinary protein quantification >150 mg or urinary protein/creatinine (mg/mg) >0.2; or (III) urinary microalbumin levels higher than the normal value 3 times in 1 week. The diagnostic criteria for hematuria were as follows: gross hematuria or 3 microscopic hematuria samples within 1 week with >3 red blood cells/high power field. Children who met the diagnostic criteria for IgA vasculitis nephritis (IgA vasculitisN) at any time were classified as having renal damage. An antistreptolysin O value of more than 400 U/L was considered positive.
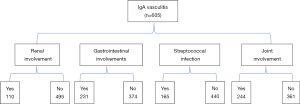
During the 6-month follow-up period, 12 patients never checked their routine urine results, the urine of 18 patients was not regularly monitored, and 15 patients could not be contacted by telephone. The remaining 605 IgA vasculitis patients were divided into the joint-involvement (Figure 2) and no-joint-involvement groups, the gastrointestinal-involvement and no-gastrointestinal-involvement groups, the IgA vasculitis-nephritis and no-renal-involvement (no-IgA vasculitis-nephritis) groups, and the streptococcal-infection and no-streptococcal-infection groups. The clinical data of the included patients were collected between February 2017 and October 2019, and the patients were followed up for 6 months (in some instances, by telephone). Data from the group of 400 healthy outpatient children examined at the same hospital were also retrospectively collected to confirm the following: (I) no immune system diseases, such as IgA vasculitis, and no recent infectious diseases; (II) normal growth and development; and (III) no history of chronic diseases or other notable diseases.
Serum vitamin D level measurements
The serum 25(OH)D levels were measured using automated chemiluminescence immunoassays (ADVIA Centaur). The kit was purchased from Siemens Medical Diagnostics Co., Ltd. (Shanghai) (product No. 10699533).
Season determination
According to the onset season, the IgA vasculitis patients were divided into the following 4 groups: spring, summer, autumn, and winter. Spring occurs from March to May, summer from June to August, autumn from September to November, and winter from December to February.
Statistical analysis
All the data processing and statistical analyses were performed using SPSS (statistical product and service solutions) version 20.0 (SPSS Inc., IBM Company Headquarters, 233 S. Wacker Drive, 11th floor Chicago, Illinois 60606). The data measurements are expressed as the mean ± standard deviation. A t-test was used to compare age and vitamin D levels between the different groups of IgA vasculitis patients. A 1-way analysis of variance (followed by a Tukey test) was used to compare vitamin D levels between different seasons. For differences between sexes, the χ2 test was used for comparisons between groups. Results with a P value <0.05 were considered statistically significant.
Results
Participants
A total of 663 IgA vasculitis patients and 400 healthy children participated in this study between February 2017 and October 2019. In the IgA vasculitis group, 45 patients, were lost to follow-up and 605 patients completed the study, of whom 231 had gastrointestinal symptoms, 244 had joint swelling and pain, and 110 had compromised renal function. In IgA vasculitis participants, 27.3% (165/605) of the patients had streptococcal infection. Approximately half of the patients were male (52.7%), which was similar to the sex distribution in the healthy children group (55.5%). The median age of the patients was 7.23±2.63 years. There were no significant differences in terms of age and sex between the IgA vasculitis and control groups (Table 1).
Table 1
| Characteristic | IgA vasculitis patients | Healthy children | P |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 319 (52.7) | 222 (55.5) | 0.388 |
| Female | 286 (55.5) | 178 (44.5) | 0.388 |
| Age (years) (mean ± SD) | 7.23±2.63 | 7.33±2.26 | 0.513 |
Distribution of vitamin D levels of each group
As Table 2 shows, our results indicate that the levels of serum 25(OH)D in the IgA vasculitis group were significantly decreased (P<0.001), indicating that there may be an association between serum 25(OH)D and IgA vasculitis. Further, the serum 25(OH)D levels of the IgA vasculitisN patients were lower than those of the NIgA vasculitisN patients. The serum 25(OH)D levels were also lower in the streptococcal-infection group (14.20±6.06 ng/mL) than the no-streptococcal-infection group (15.93±6.71 ng/mL) (P=0.004). The vitamin D levels of the children with IgA vasculitis combined with gastrointestinal symptoms were significantly decreased, compared with no gastrointestinal symptoms (P=0.002), but there was no significant difference (P=0.565) in the vitamin D levels of children with or without joint symptoms.
Table 2
| Grouping [N] | Levels of 25-hydroxyvitamin D3 [mean ± SD] [ng/mL] | P |
|---|---|---|
| IgA vasculitis patients [605] | 15.47±6.58 | 0.00 |
| Healthy children [400] | 22.48±6.24 | |
| Renal involvement [110] | 12.99±4.92 | 0.00 |
| No renal involvement [495] | 16.02±6.78 | |
| Streptococcal infection [165] | 14.20±6.06 | 0.004 |
| No streptococcal infection [440] | 15.93±6.71 | |
| Gastrointestinal symptoms [231] | 14.43±6.33 | 0.002 |
| No gastrointestinal symptoms [374] | 16.10±6.59 | |
| Joints involved [244] | 15.27±6.88 | 0.565 |
| No joints involved [361] | 15.58±6.37 |
Correlation between IgA vasculitis onset season and vitamin D levels
The number of IgA vasculitis patients was highest in winter, and the vitamin D levels of these patients were low. In summer, the number of IgA vasculitis cases was the lowest, while the vitamin D levels were the highest. The vitamin D levels of the patients were significantly lower (P<0.001) in winter and spring than summer and autumn (Table 3).
Table 3
| Onset season | Number of cases (%) | Levels of 25-hydroxyvitamin D3 (ng/mL) | F value | P |
|---|---|---|---|---|
| Spring | 125 (20.66%) | 13.374* | 5.807 | 0.000 |
| Summer | 109 (18.17%) | 17.625 | ||
| Autumn | 181 (29.92%) | 16.740 | ||
| Winter | 190 (31.40%) | 14.393* |
The Tukey test was used for comparisons between groups; *, indicates P<0.05, compared to the summer and autumn groups. The population variances for each group were equal.
Discussion
In this study, the serum vitamin D levels of children with IgA vasculitis and healthy children were compared to explore the relationship between IgA vasculitis and vitamin D levels. We found that the vitamin D levels of the IgA vasculitis children were significantly lower than those of the healthy children. To our knowledge, this is the largest study to date on IgA vasculitis and vitamin D levels.
As is widely known, vitamin D is a fat-soluble vitamin, and 25(OH)D is its active form. In fact, 25(OH)D is the main circulating form of vitamin D with a half-life of approximately 3 weeks; thus, measuring serum 25(OH)D levels facilitates the determination of vitamin D status (25,26). For a long time, vitamin D deficiency has been known to play a vital role in the pathogenesis of autoimmune diseases (27). Reports have shown that patients with SLE have significantly insufficient vitamin D levels (13), as do those with other immune diseases, such as multiple sclerosis (14), autoimmune thyroid disorders (15), and antiphospholipid syndrome (16). There is increasing evidence that IgA vasculitis patients also have lower serum levels of vitamin D than healthy children, but most of these previous studies had small sample sizes (19-21). Thus, an observational retrospective study was conducted with a large sample size to compare vitamin D levels between children with IgA vasculitis and healthy children.
In this study, we showed that compared to the healthy control population, children with IgA vasculitis have decreased 25(OH)D levels, which is in line with the findings of previous trials (19-21). The immune effects of vitamin D have been studied extensively. Research has shown that vitamin D regulates the growth, metabolism, differentiation, death, and reproduction of immune cells through vitamin D receptors (28). Vitamin D response elements have been identified in multiple sclerosis (29), which can also occur in IgA vasculitis. Metabolically active vitamin D can be produced by dendritic cells, which indicates that vitamin D is an immunomodulator (30). The main mechanisms underlying the role of vitamin D in immunity are the upregulation of Th2 cell activity, the inhibition of Th1 and Th17 cells, the enhancement of Treg cell function, damage to the development and functioning of B cells, and the reduction of monocyte activation (11,12). In addition, vitamin D also plays a role in regulating inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) (31). The levels of inflammatory cytokines, such as TNF-α and IL-6, can be changed in IgA vasculitis, as can the polyclonal activation of B cells, and the changes in their levels are related to the degree of IgA vasculitis disease activity (32).
Vitamin D deficiency may be involved in autoimmune mechanisms due to its effects on intestinal barrier function and microbiome composition (33). Given the immunological effects of vitamin D, high serum concentrations may have a preventive effect on autoimmune diseases. A prospective cohort study by Hahn J et al. (34) found that vitamin D supplementation for 5 years reduced the development of autoimmune disease by 22%. The target population of Hahn et al.’s cohort study was people aged >65 years, but it made substantial progress in elucidating the relationship between vitamin D deficiency and autoimmune diseases. It has been reported that vitamin D, combined with cimetidine, increases the Toll-like receptor 2 protein expression rate and improves cellular immunity in the monocytes of children with IgA vasculitis (35). Piantoni et al. (36) found that vitamin D had a profound long-term effect, and helped the immune system to reach a new steady state. It has also been shown that the production of Tregs and Th2 cytokines was enhanced after 2 years of vitamin D administration in patients with SLE (36).
In the present study, we identified a correlation between vitamin D deficiency and renal involvement, which indicates that 25(OH)D may be involved in the pathogenesis of renal injury in IgA vasculitis. The mechanisms of pathogenesis are unclear; however, a previous study (37) speculated that the reduced synthesis of 1,25-dihydroxyvitamin D3 leads to the release of downstream inflammatory mediators (IL-8 and TNF-α), which in turn causes the inflammatory destruction of the vessel wall, and ultimately leads to purpura development. Other studies have shown that vitamin D is central to the regulation of a wide range of inflammatory immune responses, and it can act on B lymphocytes, inhibit the production of inflammatory cytokines by B lymphocytes, and inhibit the renin-angiotensin system, thereby protecting the kidney (38,39). Interestingly, Kim et al. found that vitamin D supplementation reduced proteinuria (40). These results suggested that vitamin D plays an important role in preventing renal damage.
We also found that children with IgA vasculitis with streptococcal infections and gastrointestinal symptoms had significantly lower vitamin D levels than no streptococcal infections and no gastrointestinal symptoms, which is also a strong risk factor for kidney involvement. However, the vitamin D levels were not significantly decreased in children with IgA vasculitis and joint symptoms.
The relationship between season and vitamin D levels was also considered in this study. A previous study (41) found that the 25(OH)D levels of the human body reach their lowest values in winter and spring, and their highest values in summer and autumn. This is in line with the findings of our study, which showed that the lowest number of IgA vasculitis cases occurred in summer and the highest number in winter. This seasonal variation is not only related to vitamin D levels but may also be related to viral infections (42), as the epidemic patterns of influenza and rotaviruses are similar to that of IgA vasculitis (42).
Limitations of this study
This study had a few limitations. The sample size was large, but it was a single-center study, the follow-up time was only 6 months, and the long-term prognosis of IgA vasculitisN was not determined. Further, some parents refused to allow their children to undergo renal biopsy, as it is an invasive test. However, analyze 25(OH)D levels combined with renal biopsy may be more accurate. In this retrospective study, the number of renal biopsies, skin biopsies, and gastrointestinal endoscopic intestinal biopsy was not counted, which will be the main topic in our next study.
Conclusions
In summary, we found that compared to healthy children, the IgA vasculitis patients had lower vitamin D levels. The IgA vasculitis patients with kidney damage, gastrointestinal tract involvement, and streptococcal infection also had lower vitamin D levels than no streptococcal infection group. Thus, vitamin D supplementation may reduce the incidence of IgA vasculitis, and maintaining high vitamin D levels in IgA vasculitis patients may prevent renal damage.
Acknowledgments
Funding: This study was sponsored by the Ningbo Clinical Research Center for Children’s Health and Diseases (No. 2019A21002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-176/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-176/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-176/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-176/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was carried out in accordance with the principles of the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Ningbo Women and Children’s Hospital (Registry No. EC2022-044). Given the retrospective nature of this study, the requirement of informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Piram M, Maldini C, Biscardi S, et al. Incidence of IgA vasculitis in children estimated by four-source capture-recapture analysis: a population-based study. Rheumatology (Oxford) 2017;56:1358-66. [Crossref] [PubMed]
- Mossberg M, Segelmark M, Kahn R, et al. Epidemiology of primary systemic vasculitis in children: a population-based study from southern Sweden. Scand J Rheumatol 2018;47:295-302. [Crossref] [PubMed]
- Marzano AV, Genovese G, Tavecchio S, et al. Clinical and immunopathologic features of idiopathic cutaneous immunoglobulin M/G vasculitis versus idiopathic skin-limited immunoglobulin A vasculitis. J Am Acad Dermatol 2021;84:175-8. [Crossref] [PubMed]
- Stewart M, Savage JM, Bell B, et al. Long term renal prognosis of Henoch-Schönlein purpura in an unselected childhood population. Eur J Pediatr 1988;147:113-5. [Crossref] [PubMed]
- Koskimies O, Mir S, Rapola J, et al. Henoch-Schönlein nephritis: long-term prognosis of unselected patients. Arch Dis Child 1981;56:482-4. [Crossref] [PubMed]
- Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child 2005;90:916-20. [Crossref] [PubMed]
- Pillebout E, Sunderkötter C. IgA vasculitis. Semin Immunopathol 2021;43:729-38. [Crossref] [PubMed]
- Oni L, Sampath S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Front Pediatr 2019;7:257. [Crossref] [PubMed]
- Pan YX, Ye Q, Shao WX, et al. Relationship between immune parameters and organ involvement in children with Henoch-Schonlein purpura. PLoS One 2014;9:e115261. [Crossref] [PubMed]
- Shin JI, Lee JS. Familial clusturing of Henoch-Schönlein purpura or IgA nephropathy: genetic background or environmental triggers? Pediatr Dermatol 2008;25:651. [Crossref] [PubMed]
- Aslam MM, John P, Bhatti A, et al. Vitamin D as a Principal Factor in Mediating Rheumatoid Arthritis-Derived Immune Response. Biomed Res Int 2019;2019:3494937. [Crossref] [PubMed]
- Yamamoto E, Jørgensen TN. Immunological effects of vitamin D and their relations to autoimmunity. J Autoimmun 2019;100:7-16. [Crossref] [PubMed]
- Athanassiou L, Kostoglou-Athanassiou I, Tsakiridis P, et al. Vitamin D levels in Greek patients with systemic lupus erythematosus. Lupus 2022;31:125-32. [Crossref] [PubMed]
- Bivona G, Gambino CM, Lo Sasso B, et al. Serum Vitamin D as a Biomarker in Autoimmune, Psychiatric and Neurodegenerative Diseases. Diagnostics (Basel) 2022;12:130. [Crossref] [PubMed]
- Galușca D, Popoviciu MS, Babeș EE, et al. Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto's Disease and Grave's Disease), Diabetes Mellitus and Obesity. Medicina (Kaunas) 2022;58:194. [Crossref] [PubMed]
- Riancho-Zarrabeitia L, Cubería M, Muñoz P, et al. Vitamin D and antiphospholipid syndrome: A retrospective cohort study and meta-analysis. Semin Arthritis Rheum 2018;47:877-82. [Crossref] [PubMed]
- Stagi S, Rigante D. Vitamin D and juvenile systemic lupus erythematosus: Lights, shadows and still unresolved issues. Autoimmun Rev 2018;17:290-300. [Crossref] [PubMed]
- Roy S, Shrinivas K, Bagchi B. A stochastic chemical dynamic approach to correlate autoimmunity and optimal vitamin-D range. PLoS One 2014;9:e100635. [Crossref] [PubMed]
- Korkmaz FN, Ozen G, Unal AU, et al. Vitamin D levels in patients with small and medium vessel vasculitis. Reumatol Clin 2022;18:141-6. (Engl Ed). [Crossref] [PubMed]
- Fu Q, Shi MF, Chen Y. Clinical effect of alfacalcidol in children with Henoch-Schönlein purpura: a prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi 2021;23:797-801. [PubMed]
- Zhu L, Zhang C, Xiang R, et al. Correlations of Leukotriene B4 and 25-Hydroxyvitamin D3 Levels with Disease Severity in Children with Henoch-Schonlein Purpura. Clin Lab 2022;68. [Crossref] [PubMed]
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 2006;65:936-41. [Crossref] [PubMed]
- Wu XC. Interpretation of evidence-based diagnosis and treatment recommendations for children with Henoch-Schonlein purpura. Chin J Pediatr 23. Journal of Science and Technology 2013;51:508-11.
- Subspecialty Group of Nephrology. Chinese Medical Association. Evidence-based guidelines on diagnosis and treatment of childhood common renal diseases (II): evidence-based guideline on diagnosis and treatment of Henoch-Schonlein purpura nephritis. Zhonghua Er Ke Za Zhi 2009;47:911-3. [PubMed]
- Holick MF, Vitamin D. Deficiency—NEJM(J). New England Journal of Medicine. 2007;
- Charoenngam N, Shirvani A, Holick MF. Vitamin D for skeletal and non-skeletal health: What we should know. J Clin Orthop Trauma 2019;10:1082-93. [Crossref] [PubMed]
- Franco AS, Freitas TQ, Bernardo WM, et al. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7024. [Crossref] [PubMed]
- Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev 2016;96:365-408. [Crossref] [PubMed]
- Cocco E, Meloni A, Murru MR, et al. Vitamin D responsive elements within the HLA-DRB1 promoter region in Sardinian multiple sclerosis associated alleles. PLoS One 2012;7:e41678. [Crossref] [PubMed]
- Bizzaro G, Shoenfeld Y. Vitamin D: a panacea for autoimmune diseases? Can J Physiol Pharmacol 2015;93:395-7. [Crossref] [PubMed]
- Calton EK, Keane KN, Newsholme P, et al. The Impact of Vitamin D Levels on Inflammatory Status: A Systematic Review of Immune Cell Studies. PLoS One 2015;10:e0141770. [Crossref] [PubMed]
- Xu H, Pan Y, Li W, et al. Association between IL17A and IL17F polymorphisms and risk of Henoch-Schonlein purpura in Chinese children. Rheumatol Int 2016;36:829-35. [Crossref] [PubMed]
- Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol 2019;10:3141. [Crossref] [PubMed]
- Hahn J, Cook NR, Alexander EK, et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022;376:e066452. [Crossref] [PubMed]
- Qiong T, Peng T, Biao S. Effects of vitamin D combined with cimetidine on Toll-like receptor 2, T cell subsets and NK cells in peripheral blood mononuclear cells of children with Henoch-Schonlein purpura. Chin J Med Frontier: Electronic Edition 2019;11:4.
- Piantoni S, Andreoli L, Scarsi M, et al. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015;24:490-8. [Crossref] [PubMed]
- Guo GM, Wang J, Xia M, et al. The levels of plasma 1,25(OH)2D3 and vitamin D receptor in children with Henoch-Schonlein purpura Expression and significance of 24-hydroxylase. Chin J Practical Clinical Pediatrics 2013;28:1640-2.
- Kanai H, Sawanobori E, Kobayashi A, et al. Early treatment with methylprednisolone pulse therapy combined with tonsillectomy for heavy proteinuric henoch-schönlein purpura nephritis in children. Nephron Extra 2011;1:101-11. [Crossref] [PubMed]
- Lee JK, Lee JH, Lee H, et al. Clonal Expansion of Macrolide-Resistant Sequence Type 3 Mycoplasma pneumoniae, South Korea. Emerg Infect Dis 2018;24:1465-71. [Crossref] [PubMed]
- Kim MJ, Frankel AH, Donaldson M, et al. Oral cholecalciferol decreases albuminuria and urinary TGF-β1 in patients with type 2 diabetic nephropathy on established renin-angiotensin-aldosterone system inhibition. Kidney Int 2011;80:851-60. [Crossref] [PubMed]
- Schramm S, Lahner H, Jöckel KH, et al. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts-a note of caution. Endocrine 2017;56:658-66. [Crossref] [PubMed]
- Hwang HH, Lim IS, Choi BS, et al. Analysis of seasonal tendencies in pediatric Henoch-Schönlein purpura and comparison with outbreak of infectious diseases. Medicine (Baltimore) 2018;97:e12217. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


