
Diagnostic challenges of hypoganglionosis based on immunohistochemical method
Highlight box
Key findings
• Each segment of intestine in hypoganglionosis had different numbers of ICCs, sizes, and distributions of ganglia, as well as patterns of musculature, which may range from severely abnormal to nearly normal.
What is known and what is new?
• Smooth muscle layers in hypoganglionosis may appear without hyper- or hypotrophy.
• Reduced number of ICCs was observed in almost all segments of the intestine in hypoganglionosis.
What is the implication, and what should change now?
• International consensus regarding diagnostic criteria for hypoganglionosis is urgently needed.
Introduction
Hypoganglionosis is one of the rarest entities among other variants of Hirschsprung’s disease (1). This disease accounts for only 5% of all classified congenital innervation defects of the intestine (2). Clinically, hypoganglionosis resembles Hirschsprung’s disease as in both diseases, patients may present with severe constipation or pseudo-obstruction. However, unlike Hirschsprung’s disease, hypoganglionosis is characterized by the presence of myenteric and submucosal ganglia along the gastrointestinal tract, but with sparse distribution and low number of myenteric ganglia (3). As such, diagnosis of this disease requires a different diagnostic approach from that of Hirschsprung’s disease.
The first five cases of hypoganglionosis were first described in 22 cases of megasigmoid and megarectum patients by Bentley in 1964 (4). However, until now, diagnosis of hypoganglionosis is still difficult to be established due to lack of international consensus regarding diagnostic criteria (1). One consensus from 1990, recommended at least three full-thickness biopsies to assess characteristic features of hypoganglionosis disease: very low mucosal activity of acetylcholinesterase, significant deficiency of nerve cells in the myenteric plexus and hypertrophic circular muscle (CM) and longitudinal muscle (LM) layer (5). The use of immunohistochemical staining may aid in the understanding of these morphological features. Nevertheless, due to its rarity, there are few studies available regarding the morphological and histological features of hypoganglionosis.
The motility of the gastrointestinal tract is regulated and coordinated by smooth muscles, the enteric nervous system, and interstitial cells of Cajal (ICCs) (6). Disruption of these systems, particularly in ganglia within the ENS and ICCs, is suggested to be the etiology of many gastrointestinal motility problems such as hypoganglionosis (7).
Therefore, this study aims to evaluate the use of immunohistochemistry to provide objective support for our initial subjective impression of hypoganglionosis. Furthermore, we also aim to describe the morphological features of the distribution of the enteric nervous system, muscular layer thickness, and the presence of ICCs in various sections of the intestines from patients with hypoganglionosis as well as to match those data with patients’ clinical features. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-592/rc).
Methods
This is a cross-sectional study aimed to evaluate the use of immunohistochemistry as well as to describe the morphological features of different segments of the intestines from patients with hypoganglionosis. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written ethical approval and informed consent were waived by the Kyushu University Ethics Committee because this research was conducted on preexisting tissue samples. Parents from each patient provided verbal consent for inclusion in this study.
Tissue specimens
Three resected intestinal samples from patients with hypoganglionosis who underwent surgical resection at Kyushu University Hospital, Fukuoka, Japan, were included in this study. Hypoganglionosis was diagnosed subjectively based on morphological full-thickness biopsies of several parts of resected intestine (paucity and immature ganglia on myenteric plexus) in combination with clinical manifestations of the patients, as there is still no international consensus to specify this disease (3). Hirschsprung’s disease was already excluded through rectal suction biopsy and subsequent acetylcholine esterase staining. All patients presented with clinical features of functional obstruction and were unintentionally male, with the youngest being 1 day old and the oldest being 4 days old when the first operation was performed. Data regarding age at first surgery, procedure performed, length of the remaining intestine, post-surgical problems, and outcome were also recorded. Samples for full-thickness biopsies were obtained during surgery from each patient, which included several segments of jejunum, ileum, appendix, and colon, depending on the extent of the obstruction (Table S1). Intestinal sample (jejunum, ileum, appendix, and colon) from an autopsy of a 30-day-old boy without gastrointestinal disease was used as control. All samples were then formalin-fixed, embedded into paraffin and cut into 4 µm thickness in preparation for immunohistochemical staining.
Immunohistochemistry
Prior to immunohistochemistry analysis, hematoxylin and eosin staining was used to visualize the submucosal, myenteric plexus and smooth muscle layers of the intestinal specimens. All specimens were then immunohistochemically stained by using standard avidin-biotin complex method against polyclonal antibody S-100 protein (code No. 422091 Nichirei Co., Ltd., Tokyo, Japan; as a general neuronal marker), antibody to c-kit protein (CD-117, diluted 1:100; DakoCytomation, Carpinteria, CA, USA; as marker of ICCs), and monoclonal antibody to α-smooth muscle actin (α-SMA, clone IA4, diluted 1:200, Sigma Immunochemicals, St. Louis, MO, USA; general muscle marker). The entire slides of intestinal samples of each patient were stained in this study. Immunohistochemical staining was performed according to the method described in our previous study (8).
Evaluation and analysis
Morphological evaluation was performed by comparing resected intestinal tissue samples of patients with hypoganglionosis to those of normal healthy intestinal tissue with the aid of immunohistochemical staining. The differences between those groups were also analyzed quantitatively to provide more objective data. Random slides of resected intestinal samples of each patient were quantified in this study to avoid observer bias. All tissue samples were photographed using a light microscope at 4×, 10×, 20×, and 40× magnifications. Quantitative measurement to assess immunoreactivity towards the polyclonal S-100 antibody were performed by measuring the area stained from each slide. The thickness of LM layer and CM layer were calculated based on α-SMA antibody immunoreactivity. C-kit staining evaluation was performed by calculating the amount of c-kit positive area from each individual slide. All measurements were done by using the ImageJ version 1.43s software program (National Institutes of Health, Bethesda, MD, USA) in an area of 4,080×3,072 pixels wide and converted to micrometre according to each magnification.
Statistical analysis
Differences between the area of the nervous system, c-kit-positive area, and width of the muscle layers of the intestinal segments were compared using the Mann-Whitney U test. Statistical significance was set at P<0.05. All statistical analyses were performed using the SPSS statistical software program (SPSS Inc., Chicago, IL, USA).
Results
Three resected intestinal samples from patients with hypoganglionosis who underwent surgical resection at Kyushu University Hospital, Fukuoka, Japan, were included in this study. All patients were unintentionally male, with the youngest being 1 day old and the oldest being 4 days old when the first surgery was performed. Patient characteristics, surgical procedures, post-surgical problems, and outcomes were described in Table 1.
Table 1
| Case | Age during study | Age at first surgery | Surgical procedure | Remaining intestine after resection | Post-surgical problems | Ganglion cells | Outcome |
|---|---|---|---|---|---|---|---|
| 2 | 15 y, 9 m | 4 days | Ileostomy; jejunostomy; intestinal resection | 106 cm | Intestinal dysfunction; micronutrient imbalance; recurrence enterocolitis; growth retardation | Few-moderate | Died (15 y, 9 m) |
| 3 | 8 y, 2 m | 2 days | Jejunostomy; jejunostomy reconstruction; trans-anal anastomosis (pull-through); jejunal resection; serial central venous catheter insertion for parenteral nutrition | 75 cm small intestine; no colon | Intestinal dysfunction; recurrence enterocolitis; septicemia; frequent dehydration; micronutrient & electrolyte imbalance; growth retardation; require long-term parenteral nutrition | Few-moderate | Alive |
| 4 | 3 y, 7 m | 1 day | Ileostomy (2×); subtotal ileum-colon resection; serial central venous catheter insertion of parenteral nutrition | 134 cm | Recurrence enterocolitis; intestinal dysfunction; micro intestine; poor feeding; growth retardation; require long-term parenteral nutrition; septicemia; multi organ failure | Very few-none | Died (3 y, 7 m) |
y, years; m, months.
S-100 protein staining
Polyclonal S-100 protein staining was used to provide a better depiction of the enteric nervous system (ganglion cells and nerve fiber) distribution in intestinal tissue samples (8). In healthy control sample, the nervous system was found to be equally distributed in myenteric (Auerbach) and submucosal (Meissner) plexuses throughout the bowel segment. This was shown by positive immunoreactivity towards S-100 antibody at these particular locations. Several nerve fibers in the muscularis mucosa and villus of the lamina propria were also labeled with S-100 antibody. In contrast, tissue samples from all hypoganglionosis patients showed varying smaller ganglia sizes as well as reduced quantity and density of ganglion cells compared to those in the control sample (Figure 1). Hypoplasia of the myenteric ganglia and marked reduction of intramuscular nerve fibres were also observed in several segments of the intestine. Nerve fibres between the CM and LM layers were not as prominent as those in control sample and consisted of smaller and thinner nerve fibres. In addition, some segments were indistinguishable from control sample especially on submucosal plexus; suggesting the presence of a normal distribution of nervous system in some parts of the intestinal segments in hypoganglionosis. Quantitative study compared the area of nervous system distribution between the normal and hypoganglionosis samples as shown in Table 2. All hypoganglionosis samples showed significantly less area of nervous system compared to those in a normal sample (P<0.001), with the sample from patient number 4 showing the least area {case 4: 2,305.63 [interquartile range (IQR), 1,430.06–4,161.93] µm2/mm2}. This indicated that a lower amount of nervous system was present in a given area in patients with hypoganglionosis.

Table 2
| Parameters | Case 2 | Case 3 | Case 4 | Normal |
|---|---|---|---|---|
| Nervous system (μm2/mm2) | 4,036.42* (SD: 1,380.93) |
15,381.33* (IQR: 9,201.17–21,300.85) |
2,305.63* (IQR: 1,430.06–4,161.93) |
74,725.04 (IQR: 60,844.84–418,945.03) |
| CM layer area (mm2/cm2) | 13.37 (SD: 6.54) |
27.58 (IQR: 22.94-31.12) |
40.19 (IQR: 33.64–52.37) |
21.68 (SD: 2.87) |
| LM layer area (mm2/cm2) | 13.42 (SD: 6.59) |
15.32 (SD: 6.95) |
17.04 (SD: 9.44) |
16.89 (IQR: 7.11–21.85) |
| ICCs positive area (μm2/mm2) | 1,561.70* (SD: 1,114.51) |
1,345.90* (IQR: 804.07–4,403.70) |
3,701.00* (IQR: 2,255.16–4,908.21) |
15,090.55 (SD: 7,294.12) |
*, significant difference compared to normal sample (P<0.001). SD, standard deviation; IQR, interquartile range; CM, circular muscle; LM, longitudinal muscle; ICCs, interstitial cells of Cajal.
α-SMA staining
Staining with α-SMA antibody was used to demonstrate muscular thickness in intestinal tissue samples. Immunoreactivity against α-SMA antibody was observed uniformly in all muscular layers (muscularis mucosa, CM layer, and LM layer) in healthy intestinal sample; suggesting the absence of hypotrophy or hypertrophy under normal conditions. Interestingly, the same pattern was also observed in almost all intestinal segments in hypoganglionosis patients’ sample, although slight hypotrophy of CM layer and hypertrophy of LM layer were observed on some segments. In addition, the innermost of CM layer was remarkably thin in some segments. However, there was no reduction in immunoreactivity against α-SMA antibody in all intestinal segments among samples from patients with hypoganglionosis (Figure 2). Quantitative analysis was also in accordance with these findings, as there was no significant difference of CM and LM layer thickness between hypoganglionosis samples and control as shown in Table 2.
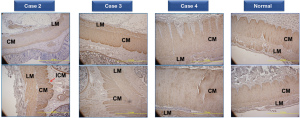
C-kit staining
C-kit immunostaining was performed on intestinal tissue samples to detect the presence of ICCs. Healthy intestinal tissue sample from control patient was observed to contain c-kit-positive cells, presumably ICCs, around myenteric (Auerbach) plexus located in between CM and LM layers. Additionally, a small number of c-kit-positive cells were also found within CM and LM layer as well as at the innermost part of CM layer. In contrast, intestinal samples from patients with hypoganglionosis presented with reduced immunoreactivity and a reduced number of c-kit-positive cells in almost all segments of the resected intestine, even around the myenteric plexus (Figure 3). Nevertheless, some parts were similar to those of the control sample, suggesting a normal distribution in some areas. Quantitative analysis showed a significantly lower c-kit-positive area in the resected intestinal samples of all patients with hypoganglionosis compared to those of a normal sample (P<0.001) (Table 2).

Discussion
Hypoganglionosis is a rare disease that mimics the clinical features of Hirschsprung’s disease. Patients may present with chronic constipation, intestinal obstruction, and enterocolitis. This disease accounts for only 5% of all classified congenital innervation defects of the colon (2). The first five cases of hypoganglionosis were first described in 22 cases of megasigmoid and megarectum by Bentley in 1964 (4). Even until now, diagnosis of hypoganglionosis is still difficult to make due to lack of international consensus regarding diagnostic criteria (1). One systematic review of 92 cases presented in 11 publications revealed that only 32% cases were diagnosed during newborn period (9). Overall reported male to female ratio of patients with hypoganglionosis varies, ranging from 6:1 to 1.25:1 (10,11). This is in accordance with our study, which suggest that hypoganglionosis is more predominant on male gender. The median age at diagnosis for these patients was reported at 4.85 years, based on a systematic review (9). This differed significantly from our study in which most patients were diagnosed and operated during neonatal period.
Immunohistochemical evaluation was performed by using three different stains to evaluate various aspects of hypoganglionosis. Staining with S-100 antibody in hypoganglionosis was previously performed in one study (12). This study concluded that myenteric plexus ratio from ileum segments of patients with hypoganglionosis was significantly lower than that of normal controls. Apparently, this number also correlated with clinical severity of each patient. Our study also observed the same pattern, in which intestinal tissue samples from all patients with hypoganglionosis showed smaller ganglia size as well reduced quantity and density of ganglia. Study by Kapur et al. (3) also reported that histopathological changes of hypoganglionosis are only restricted to the myenteric plexus with normal or minimal change of submucosal plexus, which are similar to our study. This study demonstrated a high proportion of very small ganglia with or without excessive immature ganglion cells as well as sparse neuropil.
In addition, hypoplasia of the myenteric ganglia and a marked reduction of intramuscular nerve fibres around the myenteric plexus were also observed in several segments of the intestine. Nerve fibres between CM and LM were also not as prominent as those in control and consisted of smaller and thinner nerve fibres, which are confirmed by Kapur et al. that demonstrated the absence of myenteric neurons after being stained by calretinin and NeuN immunostaining (3). This was significantly different in oligoganglionic segments of Hirschsprung’s disease, which were characterized by thick nerve strands when stained with S-100 antibody (13). Our study also observed the presence of normal intestinal tissue in some intestinal segments among patients with hypoganglionosis. Thus, unlike in Hirschsprung’s disease, rectal suction biopsy may result in normal histologic morphology in hypoganglionosis as this procedure does not sample the myenteric plexus (3).
One consensus recommended at least three full-thickness biopsies to assess characteristic features of this disease: very low mucosal activity of acetylcholinesterase, significant deficiency of nerve cells in the myenteric plexus and hypertrophic CM and LM layers. However, this consensus did not mention any cut off number and size of ganglia and nerve cells, the distance between ganglia, and the acetylcholinesterase activity to diagnose hypoganglionosis (5). Recent study suggested that full-thickness biopsies should include at least 1 cm of myenteric plexus and from more than 1 intestinal site to adequately diagnose hypoganglionosis.
However, biopsy sample should not be obtained from the appendix as it has irregular arrangement of ganglion cells and smooth muscle that may complicate the assessment of the myenteric ganglia as shown in our study as well as a study by Kapur et al. (3) Furthermore, even though appendix samples from all our patients were aganglionosis, the appendix by itself should not be used as a sole specimen to diagnose hypoganglionosis as this finding can also be found in other conditions such as total colonic aganglionosis and long segment Hirschsprung’s disease (14).
Quantitative analysis of nervous system distribution in our study reported that patients number 4, who had the lowest nervous system area, also had severe clinical manifestations and urgently needed operation at 1-day of age. This indicated that the ratio of nervous system may correlate well with clinical severity as mentioned in a study by Kubota et al. (12).
Immunoreactivity against α-SMA antibody was observed uniformly on all muscular layers in both healthy intestinal sample and most intestinal segments of patients with hypoganglionosis. However, we also observed some hypotrophy of CM layer and hypertrophy of LM layer in some intestinal segments of hypoganglionosis patients. Nevertheless, these findings were not supported by quantitative analysis, which suggested that there was no significant difference of CM and LM layer thickness between hypoganglionosis samples and control. This may be due to the fact that in our study, most biopsies were performed at early age and hence, hypertrophy of muscular layers was yet to develop. Classical mechanical obstruction commonly presents with severely hypertrophic CM and LM layer proximal to the obstruction, whereas the distal part remains intact. This is particularly true for Hirschsprung’s disease. However, studies regarding intestinal muscular layer thickness of patients with hypoganglionosis are currently limited. One consensus described the characteristics features to diagnose this disease included the findings of hypertrophic CM and LM layers (5). Still, this consensus did not specify on the cut-off limit to define hypertrophy or the exact part of muscular hypertrophy that should be present to adequately diagnose hypoganglionosis. Moreover, the time frame should biopsy be performed was also not elaborated in the consensus.
Intestinal samples from patients with hypoganglionosis presented with reduced number of ICCs, in almost all segments of resected intestine, even around the myenteric plexus. Similar results were also found in several studies, emphasizing the reduction of ICCs in patients with hypoganglionosis (15,16). Study from Rolle et al. described that the reduction of ICCs did not only occur in myenteric plexus, but also within CM and LM layer as well as on the innermost part of CM (16). Interestingly, ICCs within myenteric plexus and within smooth muscle layers, are prominently known to act as intestinal pacemaker activity. These cells are capable of generating spontaneous electrical pacemaker activity that is propagated throughout ICCs network as well as regulating smooth muscle membrane potential and mediating neurotransmitters (17). Hence, reduction in ICCs number may be a contributing factor to motility problems in hypoganglionosis (15). However, our study also observed some intestinal segments with normal ICCs distribution in patients with hypoganglionosis. This finding has not been reported in any previous study.
Kobayashi et al. suggested that isolated hypoganglionosis in children can be classified into two patterns (7). Type A is a mild condition involving a short area in older children and have good prognosis when treated conservatively or by surgical intervention (sphincterotomy or pull-through) (7). The other type termed as type B, is a severe condition present from birth, affects the small intestine, and has poor a prognosis even after surgical intervention (7). All three of our patients whom we described in this report have type B. Systematic review of hypoganglionosis reported the overall mortality rate for this disease was 8% (9). Most patients that died were newborns suffering from severe enterocolitis (9). Typical post-surgery complications included recurrent enterocolitis, chronic constipation, overflow encoporesis and need to re-do pull through (9). Our study also reported similar complications.
Limitations of this study include the lack of evaluation regarding acetylcholinesterase activity and the low number of samples due to the rarity of the disease. In addition, our study included only 1 control patient which may affect the validity of our quantitative analysis. Therefore, more control samples from various age groups should be included in order to match the intestinal samples with each specific age group as well as to increase the validity of the study.
Conclusions
In conclusion, hypoganglionosis is characterized by reduced distribution of nervous system and ICCs. Each segment of intestine in hypoganglionosis had different numbers of ICCs, sizes, and distributions of ganglia, as well as patterns of musculature, which may range from severely abnormal to nearly normal. This disease may clinically resembles Hirschsprung’s disease, however, morphologic features of these diseases differ significantly. Therefore, diagnostic and treatment approaches for Hirschsprung’s disease should not be implemented for hypoganglionosis. Further large-scale investigations regarding the definition, etiology, diagnosis, and treatment of this disease should be performed to improve the prognosis of this disease.
Acknowledgments
We would like to acknowledge all parents who had provided their verbal consent for this study. The authors would also like to thank Lukito Ongko MD for his assistance in preparing the manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-592/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-592/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-592/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-592/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written ethical approval and informed consent were waived by the Kyushu University Ethics Committee because this research was conducted on preexisting tissue samples. Parents from each patient provided verbal consent for inclusion in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Friedmacher F, Puri P. Classification and diagnostic criteria of variants of Hirschsprung's disease. Pediatr Surg Int 2013;29:855-72. [Crossref] [PubMed]
- Meier-Ruge W. Epidemiology of congenital innervation defects of the distal colon. Virchows Arch A Pathol Anat Histopathol 1992;420:171-7. [Crossref] [PubMed]
- Kapur RP, Bellizzi AM, Bond S, et al. Congenital Myenteric Hypoganglionosis. Am J Surg Pathol 2021;45:1047-60. [Crossref] [PubMed]
- Bentley JF. Some new observations on magacolon in infancy and childhood with special reference to the management of megasigmoid and megarectum. Dis Colon Rectum 1964;7:462-70. [Crossref] [PubMed]
- Borchard F, Meier-Ruge W, Wiebecke B, et al. Disorders of the innervation of the large intestine--classification and diagnosis. Results of a consensus conference of the Society of Gastroenteropathology 1 December 1990 in Frankfurt/Main. Pathologe 1991;12:171-4. [PubMed]
- Obermayr F, Hotta R, Enomoto H, et al. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol 2013;10:43-57. [Crossref] [PubMed]
- Kobayashi H, Yamataka A, Lane GJ, et al. Pathophysiology of hypoganglionosis. J Pediatr Gastroenterol Nutr 2002;34:231-5. [Crossref] [PubMed]
- Alatas FS, Masumoto K, Esumi G, et al. Significance of abnormalities in systems proximal and distal to the obstructed site of duodenal atresia. J Pediatr Gastroenterol Nutr 2012;54:242-7. [Crossref] [PubMed]
- Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int 2010;26:1111-5. [Crossref] [PubMed]
- Ure BM, Holschneider AM, Schulten D, et al. Clinical impact of intestinal neuronal malformations: a prospective study in 141 patients. Pediatr Surg Int 1997;12:377-82. [Crossref] [PubMed]
- Meier-Ruge WA, Brunner LA, Engert J, et al. A correlative morphometric and clinical investigation of hypoganglionosis of the colon in children. Eur J Pediatr Surg 1999;9:67-74. [Crossref] [PubMed]
- Kubota A, Yamauchi K, Yonekura T, et al. Clinicopathologic relationship of hypoganglionosis. J Pediatr Surg 2001;36:898-900. [Crossref] [PubMed]
- Watanabe Y, Ito F, Ando H, et al. Morphological investigation of the enteric nervous system in Hirschsprung's disease and hypoganglionosis using whole-mount colon preparation. J Pediatr Surg 1999;34:445-9. [Crossref] [PubMed]
- Mohanty S, Kini U, Das K, et al. Appendicular Biopsy in Total Colonic Aganglionosis: A Histologically Challenging and Inadvisable Practice. Pediatr Dev Pathol 2017;20:277-87. [Crossref] [PubMed]
- Yamataka A, Ohshiro K, Kobayashi H, et al. Intestinal pacemaker C-KIT+ cells and synapses in allied Hirschsprung's disorders. J Pediatr Surg 1997;32:1069-74. [Crossref] [PubMed]
- Rolle U, Yoneda A, Solari V, et al. Abnormalities of C-Kit-positive cellular network in isolated hypoganglionosis. J Pediatr Surg 2002;37:709-14. [Crossref] [PubMed]
- Al-Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol 2013;19:3-15. [Crossref] [PubMed]


