
Does infant sensory responsiveness explain exclusive breastfeeding 6 months after birth?—a cohort prospective study
Highlight box
Key findings
• Sensory over-responsivity in infants, (i.e., exaggerated or negative behavioral responses to non-irritating or non-painful sensory stimuli, severely interfering with everyday activities), was found to predict exclusive breastfeeding cessation prior to 6 months of age.
What is known and what is new?
• Exclusive breastfeeding is recommended for the first 6 months of life, however, breastfeeding rates in most developed countries are low. Sensory over-responsivity has been found to interfere with infant care, development, and daily functions, but has not yet been examined as a breastfeeding barrier.
• The incidence of sensory over-responsivity is twice as high among non-exclusive breastfeeding than exclusive breastfeeding infants, while also displaying more sensory over-responsivity behaviors.
What is the implication, and what should change now?
• Early identification of sensory over-responsivity in infants is paramount in the understanding of breastfeeding barriers. Development of early sensory interventions and providing sensory-tailored individualized breastfeeding supports are recommended.
Introduction
Exclusive breastfeeding (EBF) is recommended for the first 6 months of life by the World Health Organization (1) due to its well-established benefits for mother and infant health and for infant growth and development (2-10). Despite these proven advantages, breastfeeding rates in most developed countries are low (11,12). Early cessation of EBF despite the recommendations, and the reasons women stop breastfeeding earlier than they desired, are yet not entirely understood in the nursing and health care professionals community (13). The reasons that have been found so far, while extremely important, have focused on circumstantial factors, primarily maternal ones and do not address the abilities and characteristics required of infants for breastfeeding (14-18). Therefore these reasons are not sufficient to satisfactorily explain the cessation of EBF earlier than recommended (13,19). This study focuses on infant sensory responsiveness as a potential risk factor for EBF cessation prior to 6 months.
Sensory responsiveness is defined as the ability to regulate and grade behavioral responses to environmental sensations so that responses to sensory input are appropriate to the demands of daily life (20-22). Sensory over-responsivity (SOR) is characterized by exaggerated, negative behavioral responses to sensory stimuli not typically perceived as irritating, unpleasant, or painful (20,23), to an extent that severely interferes with everyday activities (24-28). The prevalence of SOR among the general population is 5–16% (23,29-31). SOR comprises all sensory systems, and among infants typically includes difficulties tolerating auditory (sudden or loud noises), visual (bright light or sunlight), tactile (bath, diaper change, texture, fabrics), vestibular and proprioception (swinging, sudden change in movement or position, hugs, massage, nesting, carrier), and olfactory and gustatory (flavors, strong smells, new foods) experiences (23,32).
An analysis of the act of breastfeeding supports an integrated view of the infant’s skills that are necessary for effective and successful breastfeeding (33). In addition to coordinated and efficient latching and sucking, adapted responses to various types and intensities of sensory stimuli are required (34). All the sensory modalities are activated during breastfeeding. The infant is attentive to the mother’s face and sustains eye contact with her (vision) (35,36); listens to the mother’s voice and her familiar, comforting heartbeat (auditory) (37); takes position skin-to-skin and feels the mother’s breast and nipple (tactile) (38); tucks in close to the mother, receiving deep and constant touch throughout the entire body (proprioception); is elevated and moved to a side-lying position (vestibular) (39); smells the mother’s unique skin and milk odors (olfactory); and tastes the breast milk, exposed to its varied flavors (gustatory) (40).
Although breastfeeding is one of the most frequent, basic activities in infant daily routine and is one of the main mutual activities shared by the infant and his mother in the first months of life, the relationship between sensory responsiveness in infants and breastfeeding has hardly been examined, with the exception of two studies. One is a case study of infants with SOR that presented how these difficulties may affect and impair their ability to breastfeed effectively (41). The second is a recently published study that found associations between infant sensory responsiveness profiles and both the duration and frequency of breastfeeding (42). However, this study used only parental-report questionnaires and not direct observational tools that examine sensory responsiveness. Moreover, the parental questionnaire was administered at 10 months of age or later, making it difficult to identify early difficulties in sensory responsiveness and to understand whether they can explain and predict exclusive and prolonged breastfeeding. The current study aimed to bridge this gap. Identifying factors associated with EBF cessation may assist health care professionals interacting with mothers and infants throughout the postpartum period, in increasing EBF rates. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-596/rc).
Methods
Aim
The aim of the study was to examine whether infant sensory responsiveness is associated with EBF and whether it can predict non-EBF (NEBF) at 6 months of age.
Design
This study is a cohort prospective study, designed to have data collected at three time points: at 2 days, 6 weeks, and 6 months after birth. It acquired relevant study factors on infants, utilizing mother’s self-reports, and observational assessments of the infants at their homes.
Participants
Mothers hospitalized in the maternity ward between June 2019 and August 2020 were recruited 2 days after birth, using a convenience sampling method. Inclusion criteria were the desire to initiate breastfeeding; older than 20 years of age; no language barriers; healthy; and having given birth to a healthy single newborn between 36–42 weeks of gestation. Exclusion criteria were undergoing chemotherapy; human immunodeficiency virus (HIV) positive; having given birth to a newborn who was born small for gestational age (below the 3rd percentile), needed to be fed partially or fully with a tube, or scored below the cut-off for typical cognitive and language development at the age of 6 months as assessed by the Bayley Scales of Infant and Toddler Development-3rd Edition (Bayley-III).
Sample size was calculated based on power analyses via G*Power 3 Software derived from a P value of 0.05 and statistical power of 0.80, yielding n=150.
Adopting an even more conservative approach, 174 mothers and their newborn infants were recruited, thus ensuring greater statistical power and a decreased risk of type II error. Ten mothers did not reach the third data collection point (6 months after birth) therefore we were unable to determine their breastfeeding status (EBF vs. NEBF). These mothers were excluded from the analyses, resulting in a sample of 164 mothers. Of these 164 mothers, 6 were unavailable to fill the questionnaires at 6 weeks after birth, however continued to participate in the study and filled the questionnaires at 6 months. Analyses which included measures from the age of 6 weeks were performed on a sample of 158 mothers. Note that only 104 out of 164 infants received at-home assessments at age 6 months due to coronavirus disease 2019 (COVID-19) restrictions.
At 6 months after birth, after completing the assessments, the participating mothers provided information about their breastfeeding status via a questionnaire and their infants were divided into two groups accordingly: (I) EBF group who exclusively breastfeed; and (II) NEBF group who did not breastfeed at all or partially breastfed (breastfeeding combined with one or more formula feedings per day). The EBF group consisted of 105 infants, and the NEBF group consisted of 59 infants.
Data collection
The study was conducted between June 2019 and January 2021 (6 months post-last participant recruitment), in a leading medical center where the hospitalization period is 48 hours following a vaginal birth and 96 hours after a cesarean birth. On the second day after birth in the maternity ward, all participating mothers provided written consent and completed self-administered paper questionnaires handed out by the main researcher. Six weeks and 6 months after birth, participating mothers completed online self-administered questionnaires. Following the submission of the 6-month questionnaires, a qualified occupational therapist conducted infant assessments at the participant’s home. In all phases of the study, the researcher collecting the data and the examiner assessing the infants at home were blinded to the breastfeeding status and duration. The examiner was not exposed to data collected at the three-time points via the questionnaires. At 6 months (the 3rd time point) the mothers were instructed to feed their infants before the home evaluation and not to reveal their infant’s breastfeeding status. The examiner did not discuss feeding methods or breastfeeding status with the mothers before completing and documenting the infant’s evaluation.
Measurements
Demographic and delivery-related information questionnaire, including information about the infant, was compiled for this study, and completed by the participating mothers at the maternity ward 2 days after birth, also indicating their intention to breastfeed. The demographic and delivery-related variables included: mother’s age, mother’s education, family status, family income, planned pregnancy (yes/no), pregnancy type, delivery type, breastfeeding in delivery room, infant’s sex, infant’s birth weight and birth order.
Infant Sensory Profile 2 (ISP2), a standardized, reliable, and valid parental report questionnaire (43), was developed to assess the sensory responsiveness as reflected in daily activities. The infant questionnaire addressed ages 0–6 months, consisting of 25 items. Parents rated the frequency of their infant’s behaviors on a five-point Likert scale from one (almost always) to five (almost never). The score for each sensory system was calculated for the auditory, visual, vestibular, tactile, and oral sections, and a total score was calculated as well. Higher scores indicate a higher frequency of over-responsivity, whereas lower scores indicate under-responsivity. The total score is interpreted relative to age norms: (I) typical performance; (II) atypical performance—more than others (SOR) or less than others (sensory under-responsivity) [1–2 standard deviation (SD)]. Participating mothers completed ISP2 6 weeks after birth. Internal consistency was demonstrated (Cronbach α=0.75), as was test re-test reliability [intraclass correlation coefficient (ICC) =0.86] and good content and structure validity (43). Cronbach’s α for the ISP2 in this study was 0.806.
The Test of Sensory Functions in Infants (TSFI) is a standardized, reliable, and valid tool (44) that was developed to asses sensory responsiveness in infants aged 4–18 months. The TSFI comprises 24 items that evaluate responses in five subdomains: tactile deep pressure, visual-tactile integration, adaptive motor function, ocular motor function, and reactivity to vestibular stimulation. Each of the subdomains produces a specific age-normed score, and a total score is calculated as the sum of all subdomains. The total score ranges from 0 to 49, a higher score indicates a more typical sensory responsiveness whereas a lower score indicates SOR behaviors. Participating infants were assessed using the TSFI at 6 months of age. The TSFI has been demonstrated to have test-retest reliability (ICC =0.88–0.99) and inter-rater reliability (ICC =0.26–0.84). Content and construct validity were established (45).
Bayley-III is a gold standard, standardized, reliable, and valid tool used to evaluate the development of infants and toddlers from 1 to 42 months of age. The tool comprises cognition, language, motor, social-emotional, and adaptive-behavior subtests (46). For this study, only the cognitive and language (receptive and expressive communication) subtests were used, solely to test the exclusion criteria. Each test item was scored as “credit” or “not credit”, and the credit scores were summed to obtain the total raw scores for each scale. The raw scores were converted into norm-based composite scores. To interpret the results, the composite score was used with an average of 100 points and SD of 15 points. Cognitive development was considered appropriate when the results of the composite score ranged from 85 to 115 points. In this study, participating infants were assessed using Bayley-III at 6 months of age. The Bayley-III has demonstrated internal consistency (Cronbach α>0.86), test-retest reliability (ICC >0.67), and concurrent validity compared to several developmental diagnostic tests (46).
Breastfeeding Status Measure is an online one-item self-report developed for this study, in which participating mothers report on their breastfeeding status: “How do you feed your infant?”. The response scale was derived from the World Health Organization (WHO) definitions [EBF (human milk only, mainly direct from the breast and not expressed, no complementary feeding); partial breastfeeding (breastfeeding combined with one or more formula feedings per day); or no breastfeeding (formula feeding only)] (47).
In the current study, the breastfeeding status measure was obtained at 6 months.
Mother working status at 6 months is an online one-item self-report developed for this study, in which participating mothers reported whether they returned to work at 6 months after birth (yes/no).
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All aspects of the study were approved by the Institutional Ethics Review Board of the Medical Center (reference number 0302-14-MMC) and the University Review Board, and written informed consent was obtained from participating mothers. All participating mothers were assured that participation was voluntary and that they could choose to withdraw from the study at any time. Mothers’ and infants’ privacy was ensured, and their details were kept confidential.
Statistical analysis
Statistical analyses were performed with SPSS® V27 (IBM Corp., Armonk, NY, USA). Data was aggregated with descriptive statistics by data type. Categorical variables were compared between the EBF and NEBF groups using a chi-square test, and the continuous variables were compared using multivariate analysis of variance (MANOVA) tests. To assess the extent to which EBF could be explained by the predictors, we employed a hierarchical logistic regression scheme using a stepwise method. The variable selection was based on t-test and MANOVA analyses, and the data was entered in chronological order: the demographic and delivery-related variables were entered at the first step of the regression. The second step included the sensory profile of the infants, assessed at 6 weeks, (typical/atypical performance). The TSFI-total score, measured at 6 months, was entered at the third step. Odds ratios (ORs) and their 95% confidence intervals are presented. All statistical tests were two-sided, and a P value of <0.050 was considered statistically significant. Nominal P values, Cohen’s d and partial eta squared effect sizes are presented. Cohen’s d values are defined as small (0.2), medium (0.5), or large (0.8); partial eta squared values are typically referred to as small (0.01), medium (0.06), and large (0.14) (48).
Results
The mothers’ age ranged from 21 to 43 years (mean age, 32.4 years) and infants were born between 36–42 weeks of gestation [mean (SD), 39.0 (1.2)]. Utilizing the Bayley-III, the mean composite score of the participating infants was found to be in the normal range for the cognitive score [mean (SD), 102.21 (9.75)] and language development score [mean (SD), 100.55 (5.04)].
Differences between groups in demographic and delivery-related characteristics
No statistically significant group differences were found for infant sex, birth order (Table 1), gestational age [mean (SD), EBF 39.1 (1.2) vs. NEBF 38.8 (1.2); t=1.84, P>0.050], or birth weight (kg) [mean (SD), EBF 3.336 (0.428) vs. NEBF 3.205 (0.369); t=1.96, P>0.050]. Furthermore, no statistically significant group differences were found for the following maternal demographic and delivery-related characteristics: mother’s age [mean (SD), EBF 32.1 (4.1) years vs. NEBF 33.0 (4.4) years; t=−1.28, P>0.050], education, family status, family income, type of delivery, and whether breastfeeding occurred in the delivery room (Table 1).
Table 1
| Characteristics | EBF (n=105) | NEBF (n=59) | P value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Mother’s education | 0.749 | |||||
| High school | 7 | 6.7 | 5 | 8.5 | ||
| Higher education | 7 | 6.7 | 5 | 8.5 | ||
| University | 91 | 86.6 | 49 | 83.0 | ||
| Family status | 0.609 | |||||
| Married | 95 | 90.5 | 53 | 89.8 | ||
| In a relationship | 8 | 7.6 | 4 | 6.8 | ||
| Single | 1 | 1.0 | 2 | 3.4 | ||
| Divorced | 1 | 1.0 | – | – | ||
| Family income | 0.832 | |||||
| Below average | 5 | 4.8 | 3 | 5.1 | ||
| Average | 10 | 9.5 | 4 | 6.8 | ||
| Above average | 90 | 85.7 | 52 | 88.1 | ||
| Planned pregnancy | 0.828 | |||||
| Yes | 92 | 87.6 | 51 | 86.4 | ||
| No | 13 | 12.4 | 8 | 13.6 | ||
| Pregnancy type | 0.445 | |||||
| Spontaneous | 98 | 93.3 | 52 | 88.1 | ||
| With fertility treatment | 4 | 3.8 | 3 | 5.1 | ||
| With IVF | 3 | 2.9 | 4 | 6.8 | ||
| Delivery type | 0.936 | |||||
| Vaginal | 79 | 75.2 | 42 | 71.2 | ||
| Vacuum | 11 | 10.5 | 7 | 11.9 | ||
| C-section (epidural) | 14 | 13.3 | 9 | 15.2 | ||
| C-section (full anesthesia) | 1 | 1.0 | 1 | 1.7 | ||
| Breastfeeding in delivery room | 0.324 | |||||
| Yes | 60 | 57.1 | 29 | 49.2 | ||
| No | 45 | 42.9 | 30 | 50.8 | ||
| Infant’s sex | 0.719 | |||||
| Boy | 60 | 57.1 | 32 | 54.2 | ||
| Girl | 45 | 42.9 | 27 | 45.8 | ||
| Birth order | 0.531 | |||||
| First | 46 | 43.8 | 19 | 32.2 | ||
| Second | 36 | 34.3 | 27 | 45.8 | ||
| Third | 17 | 16.2 | 10 | 16.9 | ||
| Fourth | 6 | 5.7 | 3 | 5.1 | ||
EBF, exclusive breastfeeding; NEBF, non-exclusive breastfeeding at 6 months after birth; IVF, in vitro fertilization; C-section, cesarean section.
Group differences in sensory responsiveness measured by the ISP2 (at 6 weeks)
Statistically significant group differences were found between EBF and NEBF infants in the ISP2 (χ2=7.41, P=0.006), indicating that 17% of infants in the EBF group vs. 36.2% of infants in the NEBF group demonstrated ‘atypical’ sensory responsiveness (mostly of the SOR type), between 1 SD and 2 SD above the mean. Figure 1 illustrates the distribution of infants according to their sensory responsiveness: typical sensory responsiveness (like the majority of others), SOR (more than others) and sensory under-responsivity (less than others). Specifically, statistically significant group differences and medium effect size were found in the ISP2 touch-processing section. Namely, the NEBF infants demonstrated more SOR behaviors than the EBF infants in the touch-related items. No group differences were found in the general, auditory, visual, movement, or oral processing sections (Table 2).
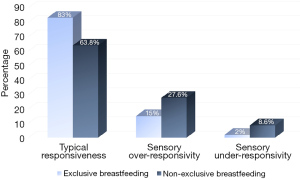
Table 2
| ISP2 sections | EBF (n=99*) | NEBF (n=57*) | F (1,154) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| General processing | 2.70 | 0.47 | 2.64 | 0.55 | 0.46 | 0.497 | – | |
| Auditory processing | 2.68 | 0.66 | 2.59 | 0.67 | 0.66 | 0.419 | – | |
| Visual processing | 1.84 | 0.87 | 1.82 | 0.69 | 0.02 | 0.854 | – | |
| Touch processing | 2.00 | 0.87 | 2.47 | 0.92 | 10.22 | 0.002 | 0.062 | |
| Movement processing | 2.22 | 0.63 | 2.32 | 0.78 | 0.84 | 0.360 | – | |
| Oral processing | 2.60 | 0.75 | 2.43 | 0.94 | 1.55 | 0.215 | – | |
*, two mothers, one from each group, were excluded automatically from this analysis due to missing values in their responses. Partial eta square values are shown only for significant differences (P<0.050). EBF, exclusive breastfeeding; NEBF, non-exclusive breastfeeding at 6 months after birth; ISP2, Infant Sensory Profile 2; SD, standard deviation; , partial eta square.
Group differences in sensory responsiveness measured by the TSFI (at 6 months)
A statistically significant group difference and medium effect size were found between EBF (n=64) and NEBF (n=40) infants in the TSFI-total score [mean (SD), EBF: 40.41 (3.22), NEBF: 37.43 (5.75), t=3.001, P=0.004, Cohen’s d=0.640]. Moreover, significant group differences as well as medium-to-large effect size were found in response to deep touch, tactile integration, and adaptive motor functions subtests (Table 3). Namely, NEBF infants demonstrated more SOR behaviors than the EBF infants and poorer motor coordination responses. No group differences were found in the ocular-motor control and reactivity to vestibular stimulation subtests (Table 3).
Table 3
| TSFI subtests | EBF (n=64) | NEBF (n=40) | F (1,102) | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Reactivity to deep pressure | 9.72 | 0.49 | 9.30 | 0.82 | 2.92 | 0.001 | 0.095 | |
| Adaptive motor functions | 8.72 | 1.81 | 7.73 | 2.14 | 2.44 | 0.013 | 0.059 | |
| Visual-tactile integration | 9.17 | 1.24 | 7.88 | 2.46 | 3.09 | <0.001 | 0.110 | |
| Ocular-motor control | 1.98 | 0.12 | 1.98 | 0.16 | 0.34 | 0.738 | – | |
| Reactivity to vestibular stimulation | 10.83 | 1.06 | 10.63 | 1.19 | 0.90 | 0.368 | – | |
Partial eta square values are shown only for significant differences (P<0.050). EBF, exclusive breastfeeding; NEBF, non-exclusive breastfeeding at 6 months after birth; TSFI, Test of Sensory Functions in Infants; SD, standard deviation; , partial eta square.
Prediction of NEBF at 6 months
Due to statistically significant differences between EBF and NEBF infants at 6 months of age in the ISP2 (typical/atypical at 6 weeks) and in the TSFI (continuous total score at 6 months), we conducted logistic regression to predict NEBF at 6 months (yes/no) (Table 4). Results revealed that the demographic and delivery-related variables (step 1) explained 11% of the variance of NEBF at 6 months (χ2=8.351, P=0.400). In step 2, we introduced the ISP2 to the model. The step was statistically significant (χ2=6.44, P=0.011) and added 8% to the explained variance of the model (χ2=14.791, P=0.097). In step 3, we introduced the TSFI-total score to the model. The step was statistically significant (χ2=8.281, P=0.040) and added 9% more to the explained variance of the model (χ2=23.072, P=0.010). In total, this model explained 28% of the variance of NEBF at 6 months. The logistic regression modeling revealed that ISP2 at 6 weeks (typical vs. atypical) and TSFI-total score at 6 months were found to predict NEBF at 6 months. Results indicate that atypical ISP2 increases the risk of NEBF by more than 4 times (OR =4.259, P=0.011). An incremental decrease of the TSFI-total score increases the risk of NEBF times 1.17 (OR =0.853, P=0.008).
Table 4
| Variables | B | SE (B) | OR (β) | Adjusted R2 |
|---|---|---|---|---|
| Step 1 | 0.110 | |||
| In a relationship (yes/no) | 1.292 | 1.358 | 3.638 | |
| Academic education (yes/no) | 0.513 | 0.594 | 1.670 | |
| Mother’s age | 0.022 | 0.069 | 1.022 | |
| C-section (yes/no) | −0.329 | 0.608 | 0.719 | |
| Gestational age | −0.066 | 0.194 | 0.937 | |
| Birth weight | 0.000 | 0.001 | 1.000 | |
| Child order | 0.264 | 0.299 | 1.302 | |
| Mother work at 6 months (yes/no) | 1.279 | 0.703 | 3.592 | |
| Step 2 | 0.188 | |||
| In a relationship (yes/no) | 1.318 | 1.363 | 3.119 | |
| Academic education (yes/no) | 0.600 | 0.614 | 1.822 | |
| Mother’s age | 0.032 | 0.073 | 1.032 | |
| C-section (yes/no) | −0.545 | 0.644 | 0.580 | |
| Gestational age | −0.042 | 0.204 | 0.959 | |
| Birth weight | 0.000 | 0.001 | 1.000 | |
| Child order | 0.238 | 0.314 | 1.268 | |
| Mother work at 6 months (yes/no) | 1.164 | 0.742 | 3.201 | |
| ISP2 (typical/atypical) | 1.335 | 0.540 | 3.799* | |
| Step 3 | 0.282* | |||
| In a relationship (yes/no) | 0.728 | 1.499 | 2.072 | |
| Academic education (yes/no) | 0.474 | 0.653 | 1.606 | |
| Mother’s age | 0.014 | 0.079 | 1.014 | |
| C-section (yes/no) | −0.785 | 0.679 | 0.456 | |
| Gestational age | −0.123 | 0.219 | 0.884 | |
| Birth weight | 0.000 | 0.001 | 1.000 | |
| Child order | 0.307 | 0.341 | 1.360 | |
| Mother work at 6 months (yes/no) | 1.170 | 0.775 | 3.222 | |
| ISP2 (typical/atypical) | 1.449 | 0.568 | 4.259* | |
| TSFI-total score | −0.159 | 0.060 | 0.853** |
*, P<0.050; **, P<0.010. NEBF, non-exclusive breastfeeding at 6 months after birth; SE, standard error; OR, odds ratio; C-section, cesarean section; ISP2, Infant Sensory Profile 2 at the age of 6 weeks; TSFI, Test of Sensory Functions in Infants at the age of 6 months.
Discussion
Our study is the first to explore infant sensory responsiveness as a significant factor in EBF cessation prior to 6 months. Specifically, we found atypical sensory responsiveness incidence, mostly of the SOR type, that was twice as high among NEBF infants than among EBF infants. Furthermore, at the age of 6 months, NEBF infants showed more SOR behaviors and achieved significantly lower scores for adaptive motor functions in the TSFI subtests compared to the EBF infants. These significant differences can be explained by the main role of the somatosensory system in breastfeeding.
The somatosensory system and breastfeeding
The somatosensory system is the first sensory system to develop during intrauterine life (49,50) and is involved in the infant’s everyday routines via tactile interactions, including human touch, textures, pressure, temperature, and even pain (32,51). During breastfeeding, infants experience extensive tactile stimulation on the whole-body skin, especially on the face and specifically the mouth area, which has a large number of tactile receptors and is therefore very sensitive (39). Modulating sensations enables the infant to adapt to lingering or repetitive stimuli (e.g., their clothing, hands, or maternal touch on the body) while attending to novel sensations (the touch of the nipple on the mouth). During breastfeeding, infants with SOR might be affected or overwhelmed by repetitive, irrelevant stimuli. They might also experience tactile input as painful (52), and therefore attempt to avoid any tactile sensation. Moreover, the nipple sensation in their mouth might trigger a gag reflex (53). Indeed, in the current study, NEBF infants showed more SOR behaviors in the ISP2 touch-processing section at the age of 6 weeks, as well as in the reactivity to deep pressure and the visual-tactile integration TSFI subtests at the age of 6 months compared to EBF infants. The constant, frequent tactile stimuli during breastfeeding may be one of the explanations as to why infants with atypical sensory responsiveness were not exclusively breastfed for the entire 6-month duration.
The somatosensory system is also responsible for the continuous flow of sensation from muscles, joints, and tendons via the proprioceptive receptors. These facilitate spatial orientation of the body, rate and timing of movements, muscle force, and speed of muscle stretching (52). These are also the foundations for body scheme formation, which are essential for the motor control of coordinated movement and posture and for planning and producing adaptive motor behavior (52,54). Thus, the somatosensory system development affects various areas during infancy, including motor-based activities and interactions with peri-personal space (20,55). Infants with difficulty processing proprioceptive information might feel disoriented, have poorly graded movement (20), and experience difficulties with the production of the coordinated fine motor acts necessary for effective breastfeeding (52).
In the somatosensory process of breastfeeding, infants must not only be able to tolerate touch but also to respond to sensory cues from the breast in order to orient to the nipple and produce an efficient motor response, thereby achieving an effective, well-organized motor function (54). Infants need to respond to the proprioceptive input of the breast in their mouth, use this information to cup and groove the tongue to form a teat and stabilize it in the mouth to achieve an efficient latch. Sequential coordinated activation of the tongue muscles is required for the wavelike tongue movements, the cheek and lip muscles must have sufficient tone to resist the intraoral negative pressure, and fine motor coordination is necessary for suck-swallow-breath coordination (34,39). Breastfeeding infants with poor motor coordination might experience each feeding as the first one and will have to plan and learn this motor task each time anew (54). These poor motor coordination skills may affect the establishment of effective, exclusive, and prolonged breastfeeding (33). Indeed, in the current study, significant differences were found between EBF and NEBF infants in their motor coordination, as demonstrated by the results of the adaptive motor functions TSFI subtest.
Sensory responsiveness, arousal, and breastfeeding
Another possible explanation for the links between NEBF and sensory responsiveness lies in the proprioceptive input relation with arousal modulation (52), and the impact of SOR on the central nervous system, as displayed in an increase in arousal (56). Increased arousal may be expressed in atypical vagal tone reactivity as seen in SOR (57,58), which is theorized to lead to poorer recovery from sensory events and lead to arousal levels outside the optimal range (59-61). When this occurs, the infant might be irritable, restless, and unable to settle down to feed, thus the self-regulation (i.e., the ability to achieve appropriate arousal for a given situation) required for sustained and prolonged breastfeeding becomes very difficult. Additionally, new sensory information is compared with previous experiences in the associative cortex, and emotional nuances are added by the limbic system. This process of association may lead infants who are frustrated with past difficulties to develop aversion, avoid latching onto the breast, and refuse to feed (39).
Prediction of EBF
In our regression model, factors that were found to predict NEBF at 6 months, beyond demographic and delivery-related variables, were the infant’s sensory profile at 6 weeks and sensory responsiveness at 6 months. Results indicate that atypical sensory responsiveness, specifically SOR, increase the risk of NEBF. These results highlight, for the first time, the infant as an active partner in breastfeeding whose skills and characteristics directly affect EBF outcomes. Moreover, these results emphasize the relevance and significance of the somatosensory system in the breastfeeding activity and sharpen the need to focus on sensory responsiveness behaviors, which might be an obstacle in achieving EBF.
Limitations and future research
This study has several limitations: assessments of infant sensory responsiveness were conducted only at 6 weeks and 6 months and not immediately after birth. Additionally, due to COVID-19 restrictions, some of the infants were not visited and assessed at home.
This study reported only infant-related factors and not maternal-related ones. Breastfeeding is a dyadic activity that involves the mother and the infant and is linked to motor coordination (33). As this is a co-occupation in which both mother and infant are active, future studies should consider the maternal factors that are linked to breastfeeding, and specifically examine sensory responsiveness.
Conclusions
The present study is the first to report that infant sensory responsiveness, both at 6 weeks and 6 months of age, is associated with EBF. Infant sensory responsiveness was found to be one of the predictors of NEBF. Breastfeeding is a frequent activity in infant daily routine that integrates all sensory systems. Increasing EBF rates is an imperative goal for physicians, nurses and health care professionals working with mothers and infants. When attempting to increase EBF rates by exploring the barriers that may be reducing these rates, it is important to consider infant sensory responsiveness, which may impair the likelihood of achieving EBF 6 months after birth. Findings may suggest identifying at-risk infants, developing early sensory intervention plans, and providing individualized breastfeeding support tailored to the infant unique sensory profile. Collaboration between breastfeeding mothers, nurses, lactation consultants and health care professionals can provide a comprehensive therapeutic approach to the breastfeeding experience, and thus may increase EBF rates.
Acknowledgments
This work was performed in partial fulfilment of the requirements of a PhD degree of Adi Freund-Azaria at The Stanley Steyer School of Health Professions, Faculty of Medicine, Tel-Aviv University, Israel. We wish to thank the participating mothers for their time and effort. We would also like to thank Ita Litmanovitz, Sofia Bauer, Janice Zausmer, and Yonat Madai and the medical and nursing staff of the maternity and neonatal ward at Meir Medical Center for their generous collaboration.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-596/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-596/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-596/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All aspects of the study were approved by the Institutional Ethics Review Board of the Medical Center (No. 0302-14-MMC) and the University Review Board, and written informed consent was obtained from participating mothers. All participating mothers were assured that participation was voluntary and that they could choose to withdraw from the study at any time. Mothers’ and infants’ privacy was ensured, and their details were kept confidential.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2012;2012:CD003517. [Crossref] [PubMed]
- Breastfeeding and the use of human milk. Pediatrics 2012;129:e827-41. [Crossref] [PubMed]
- Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr 2015;104:30-7. [Crossref] [PubMed]
- Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475-90. [Crossref] [PubMed]
- Meek JY, Noble L. Breastfeeding and the use of human milk. Pediatrics 2022;150:e2022057989. [Crossref] [PubMed]
- Kim KM, Choi JW. Associations between breastfeeding and cognitive function in children from early childhood to school age: a prospective birth cohort study. Int Breastfeed J 2020;15:83. [Crossref] [PubMed]
- Schraw JM, Bailey HD, Bonaventure A, et al. Infant feeding practices and childhood acute leukemia: Findings from the Childhood Cancer & Leukemia International Consortium. Int J Cancer 2022;151:1013-23. [Crossref] [PubMed]
- Li R, Ware J, Chen A, et al. Breastfeeding and post-perinatal infant deaths in the United States, A national prospective cohort analysis. Lancet Reg Health Am 2022;5:100094. [Crossref] [PubMed]
- Alimi R, Azmoude E, Moradi M, et al. The Association of Breastfeeding with a Reduced Risk of Postpartum Depression: A Systematic Review and Meta-Analysis. Breastfeed Med 2022;17:290-6. [Crossref] [PubMed]
- Hernández-Luengo M, Álvarez-Bueno C, Martínez-Hortelano JA, et al. The relationship between breastfeeding and motor development in children: a systematic review and meta-analysis. Nutr Rev 2022;80:1827-35. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Breastfeeding Among U.S. Children Born 2010–2017, CDC National Immunization Survey. 2022 [cited 2020 Nov 17]. Available online: https://www.cdc.gov/breastfeeding/data/nis_data/results.html
- World Health Organization. Tracking tool. Increase the rate of exclusive breastfeeding in the first 6 months up to at least 50%. 2022. Available online: https://extranet.who.int/nhdtargets/en/ExclusiveBreastfeeding
- Lau CYK, Lok KYW, Tarrant M. Breastfeeding Duration and the Theory of Planned Behavior and Breastfeeding Self-Efficacy Framework: A Systematic Review of Observational Studies. Matern Child Health J 2018;22:327-42. [Crossref] [PubMed]
- Odom EC, Li R, Scanlon KS, et al. Reasons for earlier than desired cessation of breastfeeding. Pediatrics 2013;131:e726-32. [Crossref] [PubMed]
- Shi H, Yang Y, Yin X, et al. Determinants of exclusive breastfeeding for the first six months in China: a cross-sectional study. Int Breastfeed J 2021;16:40. [Crossref] [PubMed]
- Moss KM, Dobson AJ, Tooth L, et al. Which Australian Women Do Not Exclusively Breastfeed to 6 Months, and why? J Hum Lact 2021;37:390-402. [Crossref] [PubMed]
- Brown A, Raynor P, Lee M. Healthcare professionals' and mothers' perceptions of factors that influence decisions to breastfeed or formula feed infants: a comparative study. J Adv Nurs 2011;67:1993-2003. [Crossref] [PubMed]
- Chang PC, Li SF, Yang HY, et al. Factors associated with cessation of exclusive breastfeeding at 1 and 2 months postpartum in Taiwan. Int Breastfeed J 2019;14:18. [Crossref] [PubMed]
- Brown CR, Dodds L, Legge A, et al. Factors influencing the reasons why mothers stop breastfeeding. Can J Public Health 2014;105:e179-85. [Crossref] [PubMed]
- Miller LJ, Anzalone ME, Lane SJ, et al. Concept evolution in sensory integration: a proposed nosology for diagnosis. Am J Occup Ther 2007;61:135-40. [Crossref] [PubMed]
- Schoen SA, Miller LJ, Sullivan JC. Measurement in Sensory Modulation: the Sensory Processing Scale Assessment. Am J Occup Ther 2014;68:522-30. [Crossref] [PubMed]
- Wood ET, Cummings KK, Jung J, et al. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl Psychiatry 2021;11:39. [Crossref] [PubMed]
- Schoen SA, Miller LJ, Green KE. Pilot study of the Sensory Over-Responsivity Scales: assessment and inventory. Am J Occup Ther 2008;62:393-406. [Crossref] [PubMed]
- James K, Miller LJ, Schaaf R, et al. Phenotypes within sensory modulation dysfunction. Compr Psychiatry 2011;52:715-24. [Crossref] [PubMed]
- Dean EE, Little L, Tomchek S, et al. Prevalence Models to Support Participation: Sensory Patterns as a Feature of All Children's Humanity. Front Psychol 2022;13:875972. [Crossref] [PubMed]
- Dunn W, Little L, Dean E, et al. The State of the Science on Sensory Factors and Their Impact on Daily Life for Children: A Scoping Review. OTJR (Thorofare N J) 2016;36:3S-26S. [Crossref] [PubMed]
- Bar-Shalita T, Vatine JJ, Parush S. Sensory modulation disorder: a risk factor for participation in daily life activities. Dev Med Child Neurol 2008;50:932-7. [Crossref] [PubMed]
- Kerley LJ, Meredith PJ, Harnett PH. The Relationship Between Sensory Processing and Attachment Patterns: A Scoping Review. Can J Occup Ther 2023;90:79-91. [Crossref] [PubMed]
- Bar-Shalita T, Deutsch L, Honigman L, et al. Ecological aspects of pain in sensory modulation disorder. Res Dev Disabil 2015;45-46:157-67. [Crossref] [PubMed]
- Ahn RR, Miller LJ, Milberger S, et al. Prevalence of parents' perceptions of sensory processing disorders among kindergarten children. Am J Occup Ther 2004;58:287-93. [Crossref] [PubMed]
- Ben-Sasson A, Carter AS, Briggs-Gowan MJ. Sensory over-responsivity in elementary school: prevalence and social-emotional correlates. J Abnorm Child Psychol 2009;37:705-16. [Crossref] [PubMed]
- Stallings-Sahler SA, Foley GM, Anzalone ME. Identification of Sensory Processing and Sensory-Based Movement Disorders in Infants and Young Children. Zero to Three 2022;43:39-45.
- Freund-Azaria A, Bar-Shalita T, Regev R, et al. The Role of Motor Coordination, ADHD-Related Characteristics and Temperament among Mothers and Infants in Exclusive Breastfeeding: A Cohort Prospective Study. Int J Environ Res Public Health 2022;19:5509. [Crossref] [PubMed]
- Pitonyak JS. Occupational therapy and breastfeeding promotion: our role in societal health. Am J Occup Ther 2014;68:e90-6. [Crossref] [PubMed]
- Farroni T, Csibra G, Simion F, et al. Eye contact detection in humans from birth. Proc Natl Acad Sci U S A 2002;99:9602-5. [Crossref] [PubMed]
- Niedźwiecka A, Ramotowska S, Tomalski P. Mutual Gaze During Early Mother-Infant Interactions Promotes Attention Control Development. Child Dev 2018;89:2230-44. [Crossref] [PubMed]
- Lee GY, Kisilevsky BS. Fetuses respond to father's voice but prefer mother's voice after birth. Dev Psychobiol 2014;56:1-11. [Crossref] [PubMed]
- Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry 2014;75:56-64. [Crossref] [PubMed]
- Genna CW. Supporting sucking skills in breastfeeding infants. Burlington: Jones & Bartlett Learning, 2022.
- Browne JV. Chemosensory development in the fetus and newborn. Newborn Infant Nurs Rev 2008;8:180-6. [Crossref]
- Weiss-Salinas D, Williams N. Sensory defensiveness: a theory of its effect on breastfeeding. J Hum Lact 2001;17:145-51. [Crossref] [PubMed]
- Gee BM, Aubuchon-Endsley NL, Prow A. Perinatal Maternal Mental Health and Breastfeeding Are Associated with Infant and Toddler Sensory Profiles. Children (Basel) 2021;8:766. [Crossref] [PubMed]
- Dunn W. Sensory profile 2. Bloomington: Psych Corporation, 2014.
- DeGangi GA, Greenspan SI. The development of sensory functions in infants. Phys Occup Ther Pediatr 1989;8:21-33. [Crossref]
- Eeles AL, Spittle AJ, Anderson PJ, et al. Assessments of sensory processing in infants: a systematic review. Dev Med Child Neurol 2013;55:314-26. [Crossref] [PubMed]
- Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition. APA PsycTests, 2005. doi:
10.1037/t14978-000 .10.1037/t14978-000 - World Health Organization. Indicators for assessing infant and young child feeding practices: part 2: measurement. 2010. Available online: http://apps.who.int/iris/bitstream/handle/10665/44306/9789241599290_eng.pdf?sequence=1%0Ahttp://whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf%5Cnhttp://www.unicef.org/programme/breastfeeding/innocenti.htm%5Cnhttp://innocenti15.net/declaration
- Schelly D, Ohl A. Examining Clinical Meaningfulness in Randomized Controlled Trials: Revisiting the Well Elderly II. Am J Occup Ther 2019;73:7301205120p1-7301205120p13.
- Cabral TI, da Silva LG, Martinez CM, et al. Analysis of sensory processing in preterm infants. Early Hum Dev 2016;103:77-81. [Crossref] [PubMed]
- Lecanuet JP, Schaal B. Fetal sensory competencies. Eur J Obstet Gynecol Reprod Biol 1996;68:1-23. [Crossref] [PubMed]
- Underdown A, Barlow J, Stewart‐Brown S. Tactile stimulation in physically healthy infants: results of a systematic review. J Reprod Infant Psychol 2010;28:11-29. [Crossref]
- Bundy AC, Lane SJ. Sensory integration: theory and practice. 3rd ed. Philadelphia: F. A. Davis Company, 2019.
- Thompson SD, Bruns DA, Rains KW. Picky eating habits or sensory processing issues? Exploring feeding difficulties in infants and toddlers. Young Except Child 2010;13:71-85. [Crossref]
- Koziol LF, Budding DE, Chidekel D. Sensory integration, sensory processing, and sensory modulation disorders: putative functional neuroanatomic underpinnings. Cerebellum 2011;10:770-92. [Crossref] [PubMed]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 2012;92:1651-97. [Crossref] [PubMed]
- Jung J, Zbozinek TD, Cummings KK, et al. Associations between physiological and neural measures of sensory reactivity in youth with autism. J Child Psychol Psychiatry 2021;62:1183-94. [Crossref] [PubMed]
- Schaaf RC, Benevides T, Blanche EI, et al. Parasympathetic functions in children with sensory processing disorder. Front Integr Neurosci 2010;4:4. [Crossref] [PubMed]
- Bar-Shalita T, Ben-Ziv N, Granovsky Y, et al. An Exploratory Study Testing Autonomic Reactivity to Pain in Women with Sensory Over-Responsiveness. Brain Sci 2020;10:819. [Crossref] [PubMed]
- Green SA, Hernandez L, Lawrence KE, et al. Distinct Patterns of Neural Habituation and Generalization in Children and Adolescents With Autism With Low and High Sensory Overresponsivity. Am J Psychiatry 2019;176:1010-20. [Crossref] [PubMed]
- Gavin WJ, Dotseth A, Roush KK, et al. Electroencephalography in children with and without sensory processing disorders during auditory perception. Am J Occup Ther 2011;65:370-7. [Crossref] [PubMed]
- Lane SJ, Reynolds S, Thacker L. Sensory Over-Responsivity and ADHD: Differentiating Using Electrodermal Responses, Cortisol, and Anxiety. Front Integr Neurosci 2010;4:8. [Crossref] [PubMed]


