
Decreased placental TLR3 is associated with hepatitis B virus vaccine responsiveness in infants born to HBsAg-positive mothers
Highlight box
Key findings
• HBsAg-positive maternal microenvironment may affect placental toll-like receptor 3 (TLR3) protein expression. The decreased placental TLR3 expression is associated with impaired responsiveness to HBV vaccination in babies born to the HBsAg-positive mothers.
What is known and what is new?
• It is known that babies born to HBsAg-positive mothers bear a high risk of being poor responsive to the HBV vaccine with unilluminated mechanism. The placenta, as a special organ that connects mother and child, has a profound effect on babies’ immune function, but its role in responsiveness to HBV vaccination in the babies born to the HBsAg-positive mothers is unknown.
• At present study, we found that TLR3 plays a vital role in placental immunity and affects the immune response of the babies born to HBsAg-positive mothers to HBV vaccine.
What is the implication, and what should change now?
• Placental TLR3 may be a potential target for improving hepatitis B vaccine response in babies born to HBsAg-positive mothers.
Introduction
Hepatitis B virus (HBV) infection is a highly prevalent global public health issue (1). According to the World Health Organization (WHO), approximately 296 million people are living with chronic HBV infection and approximately 887,000 HBV-related deaths worldwide each year (2,3). Although HBV infection can be safely and effectively prevented with the available vaccines (4), epidemiological studies demonstrate that 5–10% of the population exhibits poor immune responses to the vaccines (5). Babies born to hepatitis B surface antigen (HBsAg)-positive mothers experience a high rate of non- or hypo-responsiveness to the vaccine (approximately 10–40%) (6-8) and therefore remain vulnerable to HBV. Moreover, nearly 90% of infant infections remain chronic (9), and 15–25% of infected infants die from HBV-related liver disease in their lifetime (10). Consequently, improving the immune response rate of babies born to HBsAg-positive mothers could be key to preventing HBV infection. Identifying the related causes of their poor immune response to the vaccine may facilitate to achieving this aim.
Numerous factors, such as mutations in the HBV genome, vaccine type, and host immunity, contribute to the reaction to vaccination in infants born to HBsAg-positive mothers (11-14). However, the mechanisms through which these factors, which partly are associated with HBsAg-positive mothers, influence infant immunity and are involved in HBV vaccine responsiveness remain largely understudied. It has recently been reported that sustaining low levels of pathogen stimulation alters neonatal cytokine responses by causing a defense response in the placenta (15), which might help understanding the response to the HBV vaccine in infants born to HBsAg-positive mothers. The fetal-placental unit, as an actively developing immune system, can be modified by the maternal immune response and is closely related to the neonatal immune response (16,17). Throughout pregnancy, persistent exposure of HBsAg-positive mothers’ placentas to maternal HBV-associated intrauterine microenvironments may influence placental immune function and consequently alter infant immunity, affecting the HBV vaccine response.
Toll-like receptors (TLRs), which are important pattern recognition receptors, play vital roles in innate immunity. The placenta performs pregnancy-specific immune functions through the expression of TLRs (18-20). In principle, placental immune mechanisms are intended to protect pregnant mothers and fetuses from pathogenic microorganisms, while the mothers need to be kept immunologically tolerant to the semi-allogenic fetus (21). HBV infects trophoblasts in vivo and in vitro (22) and suppresses the expression of placental TLRs to disrupt the placental immune response in HBV-positive mothers (23). TLR3 plays a vital role in the response to HBV (24). The placenta expresses a high level of TLR3 (25-27). Our previous study found that both the mRNA and protein expressions of placental TLR3 decreased in HBsAg-positive mothers suggesting that TLR3 may be a protective factor against HBV infection in the placenta (28). However, the role of placental TLR3 in the immune response to the HBV vaccine in infants born to HBsAg-positive mothers has not been analyzed in detail.
Therefore, in the present study, we aimed to first detect the expression of TLR3 in the placentas of HBsAg-positive mothers with different HBV serological markers and then analyze the infant HBV serological markers and cytokines involved in the response to the HBV vaccine. Finally, we investigated the potential role of placental TLR3 in the immune response to HBV vaccination in infants born to HBsAg-positive mothers under these multiple factors. We present this article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-266/rc).
Methods
Participants and study design
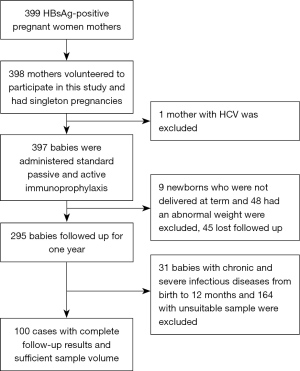
HBsAg-positive mothers and their children were recruited from the Third People Hospital of Taiyuan City, Shanxi Province, China, from June 2011 to July 2013. All neonates were administered hepatitis B immune globulin (HBIG) within 24 h after birth and HBV vaccine at 0, 1 and 6 months of age. Follow-up was performed until the infants reached the age of 1. All mothers who volunteered to participate in this study had singleton pregnancies and no history of infection with human immunodeficiency virus (HIV), hepatitis C virus (HCV), or other viruses at enrollment. All newborns were delivered at full term (gestational weeks 37–42) and had an average weight of 2.5–4.0 kg. Infants with chronic and severe infectious diseases from birth to 12 months were excluded. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Shanxi Medical University (No. 2010032). All mothers and legal guardians of the children signed informed consent forms for participation in this study. The study flowchart is shown in Figure 1.
Specimen collection
After delivery, face-to-face interviews were conducted by well-trained interviewers with standardized questionnaires to collect general demographic information about the mothers and their children. Before delivery, peripheral blood samples were collected from the elbow veins of all mothers. The placentas were collected within 30 min after delivery. Then, tissue samples were fixed in 4% paraformaldehyde, dehydrated with an alcohol gradient, cleared with xylene, and embedded in paraffin. We collected femoral venous blood samples from the infants. All blood samples were pretreated and stored at −80 ℃ until use in subsequent experiments.
Determination of HBV serological markers
The HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc levels of the mothers and their babies were measured by electrochemiluminescence immunoassay (ECLIA) (Roche Diagnostics GmbH, Germany) according to the manufacturer’s instructions. Six months after completing the active-passive hepatitis B vaccination regimen, children with anti-HBs titers <10 mIU/mL were defined as non-responders, those with anti-HBs titers ≥10 and <100 mIU/mL were defined as hypo-responders, and those with anti-HBs levels ≥100 mIU/mL were defined as high responders (11). Since HBs titers ≥100 mIU/mL are associated with prolonged and effective seroprotection (29), we grouped cases with anti-HBs titers <100 mIU/mL into a non- or hypo-responsiveness group and cases with anti-HBs levels ≥100 mIU/mL into a highly responsiveness group.
Quantification of serum HBV-DNA
A fluorescence quantitative polymerase chain reaction (FQ-PCR) kit was used to detect the serum HBV DNA load. The procedure and evaluation of the results were carried out according to the instruction manual (DAAN Gene Co. Ltd., Sun Yat-sen University, Guangdong, China).
Detection of TLR3 in placental tissue
For immunostaining, the placental tissue was sectioned into 4-µm-thick samples and deparaffinized. Thereafter, the slides were subjected to a heat-induced epitope retrieval step for 2 min before incubation with primary antibodies. The antibodies specific for TLR3 (ab62556) were rabbit polyclonal antibodies obtained from Abcam (USA), which were used at a dilution of 1:400. We used EnVision Plus to detect these antibodies, which were developed in 1:1,000 DAB for 5 minutes and then counterstained with hematoxylin. Appropriate positive and negative control cases were stained in parallel. Blinded evaluator performed the evaluation of immunohistochemistry.
The results were scored according to the integral composed of staining intensity (1, weak; 2, moderate; and 3, strong) and positive cell percentage (0, negative; 1, <25%; 2, 25–50%; 3, >50%) and graded into the following categories: 0 [0], 1 [1–2], 2 [3–4], and 3 [>4]. The expression of TLR3 was divided into increase and decrease relative to the integral of the median in the control group.
Measurement of cytokines
Serum cytokines, including IL-6, IL-12, TNF-α, IFN-α, and IFN-γ, were assessed by enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, USA) according to the manufacturer’s instructions.
Statistical analysis
Database creation was performed using Epidata 3.1. Statistical analysis was performed using the SAS version 9.4 software package. Descriptive data are presented as the mean ± standard deviation (SD) or absolute numbers (n) with percentages based on the data type and distribution. Statistical significance was tested by a t-test or nonparametric test for continuous data and by the chi-square test for categorical data. All statistical tests were two-tailed, with P<0.05 considered indicative of statistically significant results. A logistic regression model was utilized to examine the association between placental TLR3 expression and responsiveness to the HBV vaccine in babies born to HBsAg-positive mothers.
Results
General information on HBsAg-positive mothers and children
All 100 pairs of HBsAg-positive mothers and their children were collected at the end of the 1-year follow-up. Among these children, there were 14 cases of non-response, 36 cases of hypo-response, and 50 cases of high response. The average age of the 100 mothers was 27.71±4.33 years, and the average gestational time was 39.09±1.13 weeks. On average, the 100 infants were 11.20±3.39 kg in weight and 76.84±4.12 cm in height. There were no significant differences between the non- or hypo-response and high-response HBsAg-positive mothers in age, gestational time, mode of delivery, history of antiviral drug use, HBV serological markers, and height and weight of the infants. Table 1 shows the general information of the 100 HbsAg-positive mothers and children in the non- or hypo-responsiveness and high-responsiveness groups.
Table 1
| Characteristics | Total (n=100) | Non- or hypo-responsiveness (n=50) | High responsiveness (n=50) | t/χ2/Z | P |
|---|---|---|---|---|---|
| Mothers | |||||
| Age (year) | 27.71±4.33 | 28.16±4.74 | 27.24±3.85 | −0.99 | 0.32† |
| Gestational week | 39.09±1.13 | 39.24±1.14 | 38.94±1.11 | 1.11 | 0.27† |
| Mode of delivery | 1.02 | 0.31‡ | |||
| Vaginal delivery | 43 (43.00) | 24 (48.00) | 19 (38.00) | ||
| Cesarean section | 57 (57.00) | 26 (52.00) | 31 (62.00) | ||
| Taking antiviral drugs | 1.78 | 0.18‡ | |||
| Yes | 17 (17.00) | 6 (12.00) | 11 (22.00) | ||
| No | 83 (83.00) | 44 (88.00) | 39 (78.00) | ||
| Serum HBeAg | 0.65 | 0.42‡ | |||
| Positive | 44 (44.00) | 20 (40.00) | 24 (48.00) | ||
| Negative | 56 (56.00) | 30 (60.00) | 26 (52.00) | ||
| Serum HBV DNA | 3.35 | 0.07‡ | |||
| Positive | 41 (41.00) | 16 (32.00) | 25 (50.00) | ||
| Negative | 59 (59.00) | 34 (64.00) | 25 (50.00) | ||
| Serum HBV DNA (IU/mL) | 0.75 | 0.69‡ | |||
| <200 | 63 (63.00) | 32 (62.00) | 31 (64.00) | ||
| 200–200,000 | 10 (10.00) | 6 (8.00) | 4 (12.00) | ||
| >200,000 | 27 (27.00) | 12 (30.00) | 15 (24.00) | ||
| Placental TLR3 protein | 10.39 | 0.001‡ | |||
| Decrease expression | 56 (56.00) | 36 (72.00) | 20 (40.00) | ||
| Increase expression | 44 (44.00) | 14 (28.00) | 30 (60.00) | ||
| Infants | |||||
| Gender | 0.36 | 0.55‡ | |||
| Male | 54 (54.00) | 28 (56.00) | 26 (52.00) | ||
| Female | 46 (46.00) | 22 (44.00) | 24 (48.00) | ||
| Height (cm) | 76.84±4.12 | 77.18±4.43 | 76.48±3.79 | −0.84 | 0.40† |
| Weight (kg) | 11.20±3.39 | 11.55±4.54 | 10.84±1.48 | −1.03 | 0.30† |
| Cytokines (pg/mL) | |||||
| IL-6 | 30.14 (246.99) | 26.09 (178.18) | 30.23 (637.08) | 0.61 | 0.54§ |
| IL-12 | 0.92 (0.44) | 0.92 (0.45) | 0.92 (0.45) | 0.08 | 0.94§ |
| TNF-α | 2.51 (7.14) | 2.51 (7.21) | 2.89 (7.08) | −0.19 | 0.85§ |
| IFN-α | 0.65 (0.31) | 0.58 (0.17) | 0.65 (0.69) | 0.56 | 0.57§ |
| IFN-γ | 0.78 (1.24) | 0.71 (1.22) | 0.93 (1.41) | 0.71 | 0.48§ |
†, one-way ANOVA (the one-way analysis of variance), described by ; ‡, chi-square test, described by n (%); §. Wilcoxon rank-sum test, described by median (interquartile range). HBeAg, hepatitis B virus e antigen; HBV, hepatitis B virus; TLR3, toll-like receptor 3; IL-6, interleukin-6; IL-12, interleukin-12; TNF-α, tumor necrosis factor-α; IFN-α, interferon-α; IFN-γ, interferon-γ.
Expression of the placental TLR3 protein in HBV vaccine non- or hypo-responders and high responders
Immunohistochemistry was performed to investigate the TLR3 protein expression in the placenta to analyze the role of the placental TLR3 protein in HBV vaccine non- or hypo-responsiveness and high responsiveness. The TLR3 protein was detected in all samples. Cytoplasmic expression of the TLR3 protein was mainly found in trophoblastic cells but was also detectable in part decidual cells, villous mesenchymal cells, and villous capillary endothelial cells (as shown in Figure S1). TLR3 expression was decreased in 72% of non- or hypo-responsive cases. However, reduced expression of TLR3 accounted for 40% of cases in the high-responsiveness group. The chi-square test showed that the decreased expression of placental TLR3 was significantly more common in the non- or hypo-responsiveness group, as compared to the high-responsiveness group.
Expression of inflammatory cytokines in HBV vaccine non- or hypo-responsive and highly responsive infants
We assessed infant serum cytokines related to the immune response to the hepatitis B vaccine, including IL-6, IL-12, TNF-α, IFN-α, and IFN-γ, in non- or hypo-responders and high responders. Although there was no significant difference between the two responses, the expressions of IL-6, TNF-α, IFN-α, and IFN-γ showed a trend towards lower levels in the non- or hypo-responders relative to that in the high responders (as shown in Table 1).
Association between placental TLR3 expression and HBV vaccine non- or hypo-responsiveness
Potentially relevant factors determining non- or hypo-response, such as mother HBeAg, mother HBV DNA, and infant cytokines, were included in a logistic regression model. As shown in Table 2, placental TLR3 was introduced into the model. Increased expression of the placental TLR3 protein decreased the odds of HBV vaccine non- or hypo-responsiveness in the babies of HBsAg-positive mothers [OR =0.25 (95% CI: 0.11–0.58)], and this association remained after accounting for maternal factors such as HBeAg and HBV DNA, and infant cytokines including IL-6, IL-12, TNF-α, IFN-α, and IFN-γ [OR =0.15 (95% CI: 0.05–0.44); Table 2].
Table 2
| Factors | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| TLR3 | 0.25 (0.11–0.58) | 0.001 | 0.15 (0.05–0.44) | 0.001 | |
TLR3, toll-like receptor 3; HBsAg, hepatitis B surface antigen; OR, odds ratio; CI, confidence interval.
Discussion
Infants of HBsAg-positive mothers suffer high rates of non- or hypo-responsiveness to HBV vaccination, which predisposes them to poor outcomes after HBV infection. Therefore, it is imperative to clarify the possible mechanism of non- or hypo-responsiveness in this population. By comprehensively analyzing data from HBsAg-positive mothers, placentas, and babies, we found that decreased placental TLR3 protein expression was associated with HBV vaccine non- or hypo-responsiveness.
Placental structure and function can be affected by viral factors in the mother (20-32). TLR3 is a vital molecule for the placenta to perform pregnancy-specific immune functions but can be impaired by HBV (33). Our previous study found that both the mRNA and protein expressions of placental TLR3 decreased in HBsAg-positive mothers relative to the normal controls (28). Furthermore, we also found that the expression of TLR3 was significantly decreased in the placentas of HBsAg-positive mothers who delivered infants with non- or hypo-responsiveness to the HBV vaccine. Given the crucial role of the placenta in protecting fetal growth and influencing the long-term health of the offspring (34), these results indicated that long-term exposure to a HBsAg-positive maternal intrauterine microenvironment may significantly affect placental TLR3 expression and impair fetal-placental unit immune function, possibly influencing the baby’s immune response to the vaccine. This may be related to HBV polymerase, a key enzyme related to HBV replication, in HBsAg-positive mothers. Studies have shown that HBV polymerase has an inhibitory effect on TLR3 signal (33).
As a pregnancy-specific component of the immune system, placental immunity is not only sensitive to maternal factors but also affects the baby’s immune system development (35), which is closely related to non- or hypo-responsiveness (36). Although the differences observed in the present study were not significant, serum cytokines indicating an immune response such as IL-6, TNF-α, IFN-α, and IFN-γ showed numerically lower levels in the non- or hypo-responders. These results might suggest to some extent that the immune response of babies with non- or hypo-responsiveness might be somewhat inadequate. This is consistent with current research showing that decreased levels of cytokines such as IL-6 and TNF are involved in insufficient responses to the HBV vaccine (37,38). TLR3 signals induce the activation of NF-κB and IRF3 in the nucleus, which bind to the sites of relevant gene promoters to induce I-IFN production (39) and enhance the expressions of inflammatory cytokines such as IL-6, IL-8, IL-12, and TNF-α (39,40). Nevertheless, the mechanism underlying the effect of placental TLR3 on infant cytokine expression needs to be elucidated further.
Our study was constrained by a relatively small sample size due to ethical concerns about performing invasive sampling techniques on babies, which resulted in sample collection difficulties and an inability to perform more extensive analysis on the small sample volume. Further studies are needed to confirm our findings and to explore the molecular mechanism of placental TLR3 in the HBV vaccine response.
In summary, the non- or hypo-responsiveness to HBV vaccination in babies born to HBsAg-positive mothers involves many factors. Our current study observed a striking association between reduced placental TLR3 expression and reduced response to HBV vaccination in these babies, indicating that the HBsAg-positive maternal microenvironment may affect trophoblast cell TLR3, which may subsequently impair the baby’s immune response to the HBV vaccine. The underlying molecular mechanisms as well as implications for strategies overcoming hypo-responsiveness of these babies to HBV vaccination require further studies.
Conclusions
The findings presented in this study indicate that the microenvironment of HBsAg-positive mothers could affect the placental TLR3 expression, leading to impaired baby immune response to HBV vaccine and ultimately contributing to an inadequate response to the vaccine.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (Nos. 81573212 and 81072341).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-266/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-266/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-266/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-266/coif). FT’s lab has received research funding from Allergan, Bristol-Myers Squibb, Gilead and Inventiva. FT has received honoraria for consulting or lectures from Astra Zeneca, Gilead, AbbVie, BMS, Boehringer, Madrigal, Intercept, Falk, Ionis, Inventiva, Merz, Pfizer, Alnylam, NGM, CSL Behring, Novo Nordisk, Novartis. FT has participated on a data safety monitoring board for Pfizer. and has received support for attending scientific meetings from Gilead. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Shanxi Medical University (No. 2010032). All mothers and legal guardians of the children signed informed consent forms for participation in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022;7:796-829. [Crossref] [PubMed]
- WHO Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. (Accessed on 28 January 2023). Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- WHO. Global hepatitis report, 2017. (Accessed on 18 February 2023). Available online: https://www.who.int/publications/i/item/9789241565455
- Organization WLC-i-PDWH. Preventing Perinatal Hepatitis B Virus Transmission: A Guide for Introducing and Strengthening Hepatitis B Birth Dose Vaccination 2015.
- Peeridogaheh H, Meshkat Z, Habibzadeh S, et al. Current concepts on immunopathogenesis of hepatitis B virus infection. Virus Res 2018;245:29-43. [Crossref] [PubMed]
- Zhuge S, Ge C, Yang Y, et al. The prevalence of occult HBV infection in immunized children with HBsAg-positive parents: a hospital-based analysis. Hepatol Int 2020;14:503-12. [Crossref] [PubMed]
- Gu H, Yao J, Zhu W, et al. The effects of booster vaccination on hepatitis B vaccine in anti-HBs negative infants of HBsAg-positive mothers after primary vaccination. Hum Vaccin Immunother 2013;9:1292-5. [Crossref] [PubMed]
- Wang F, Zhang G, Zheng H, et al. Post-vaccination serologic testing of infants born to hepatitis B surface antigen positive mothers in 4 provinces of China. Vaccine 2017;35:4229-35. [Crossref] [PubMed]
- Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48:335-52. [Crossref] [PubMed]
- EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. [Crossref] [PubMed]
- Bracciale L, Fabbiani M, Sansoni A, et al. Impact of hepatitis B vaccination in children born to HBsAg-positive mothers: a 20-year retrospective study. Infection 2009;37:340-3. [Crossref] [PubMed]
- Roh EY, Song EY, Yoon JH, et al. Effects of interleukin-4 and interleukin-12B gene polymorphisms on hepatitis B virus vaccination. Ann Hepatol 2017;16:63-70. [Crossref] [PubMed]
- Ghaziasadi A, Alavian SM, Norouzi M, et al. Mutational analysis of HBs Ag-positive mothers and their infected children despite immunoprophylaxis. Iran J Allergy Asthma Immunol 2013;12:352-60. [PubMed]
- Wang C, Wang C, Jia ZF, et al. Protective effect of an improved immunization practice of mother-to-infant transmission of hepatitis B virus and risk factors associated with immunoprophylaxis failure. Medicine (Baltimore) 2016;95:e4390. [Crossref] [PubMed]
- Stinson LF, Payne MS, Keelan JA. Placental and intra-amniotic inflammation are associated with altered fetal immune responses at birth. Placenta 2019;85:15-23. [Crossref] [PubMed]
- Zenclussen ML, Thuere C, Ahmad N, et al. The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol 2010;63:200-8. [Crossref] [PubMed]
- Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017;17:469-82. [Crossref] [PubMed]
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782-7. [Crossref] [PubMed]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol 2005;560:11-8. [Crossref] [PubMed]
- Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta 2005;26:540-7. [Crossref] [PubMed]
- Motomura K, Hara M, Ito I, et al. Roles of human trophoblasts' pattern recognition receptors in host defense and pregnancy complications. J Reprod Immunol 2023;156:103811. [Crossref] [PubMed]
- Bhat P, Anderson DA. Hepatitis B virus translocates across a trophoblastic barrier. J Virol 2007;81:7200-7. [Crossref] [PubMed]
- Tian T, Sun D, Wang P, et al. Roles of Toll-like Receptor 7 and 8 in Prevention of Intrauterine Transmission of Hepatitis B Virus. Cell Physiol Biochem 2015;37:445-53. [Crossref] [PubMed]
- Karimi-Googheri M, Arababadi MK. TLR3 plays significant roles against hepatitis B virus. Mol Biol Rep 2014;41:3279-86. [Crossref] [PubMed]
- Tangerås LH, Stødle GS, Olsen GD, et al. Functional Toll-like receptors in primary first-trimester trophoblasts. J Reprod Immunol 2014;106:89-99. [Crossref] [PubMed]
- Patni S, Wynen LP, Seager AL, et al. Expression and activity of Toll-like receptors 1-9 in the human term placenta and changes associated with labor at term. Biol Reprod 2009;80:243-8. [Crossref] [PubMed]
- Gierman LM, Stødle GS, Tangerås LH, et al. Toll-like receptor profiling of seven trophoblast cell lines warrants caution for translation to primary trophoblasts. Placenta 2015;36:1246-53. [Crossref] [PubMed]
- Li SZ, Wang SP, Yuan CL, et al. Relationship between TLR3 expression in placenta and hepatitis B virus infection in pregnant women. Chinese Journal of Public Health 2008;24:129-31.
- He P, Xia J, Zhang P, et al. Durability of Antibody Response Against Hepatitis B Virus for a Decreased Crowd: A Retrospective Polycentric Cohort Study from a 10-Year Follow-Up Clinical Study. Infect Drug Resist 2022;15:7389-99. [Crossref] [PubMed]
- Moro L, Bardají A, Macete E, et al. Placental Microparticles and MicroRNAs in Pregnant Women with Plasmodium falciparum or HIV Infection. PLoS One 2016;11:e0146361. [Crossref] [PubMed]
- Baergen RN, Heller DS. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr Dev Pathol 2020;23:177-80. [Crossref] [PubMed]
- Molás RB, Ribeiro MR, Ramalho Dos Santos MJC, et al. The involvement of annexin A1 in human placental response to maternal Zika virus infection. Antiviral Res 2020;179:104809. [Crossref] [PubMed]
- Yu S, Chen J, Wu M, et al. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol 2010;91:2080-90. [Crossref] [PubMed]
- Gaccioli F, Lager S, Powell TL, et al. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 2013;4:101-15. [Crossref] [PubMed]
- Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296-302. [Crossref] [PubMed]
- Wang XF, Shi XH, Xu XX, et al. Effect of interleukin-6 and interleukin-12 on immune response to hepatitis B vaccination in infants of HBsAg-positive mothers. Zhonghua Liu Xing Bing Xue Za Zhi 2017;38:950-3. [PubMed]
- Xu XX, Wang B, Wang XF, et al. Effect of telbivudine on infants born to HBsAg-positive mothers with non-/hypo-response to hepatitis B vaccine during their second and third trimesters of pregnancy. Zhonghua Liu Xing Bing Xue Za Zhi 2017;38:168-72. [PubMed]
- Singh AK, Jena A, Mahajan G, et al. Meta-analysis: hepatitis B vaccination in inflammatory bowel disease. Aliment Pharmacol Ther 2022;55:908-20. [Crossref] [PubMed]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006;124:783-801. [Crossref] [PubMed]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007;7:353-64. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


