
Tuberculous chylothorax in paediatric population—a case report and systematic review of the literature
Highlight box
Key findings
• In this systematic review of 7 articles, all reported patients presented with respiratory symptoms which were caused by pleural effusion secondary to underlying tuberculosis. In each of the cases, pleural fluids appeared milky and were confirmed biochemically to be chyle.
What is known and what is new?
• Chylothorax is a rare but recognised complication of pleural tuberculosis. This study enables clinician to be able to identify, correlate, and diagnose tuberculous chylothorax.
What is the implication, and what should change now?
• Patients with confirmed diagnosis of pleural effusion secondary to tuberculosis infection should be investigated for chylothorax; as it will influence the direction of the management.
IntroductionOther Section
Tuberculosis (TB) is a significant disease burden worldwide and it affected 1.17 million children around the globe in the year of 2021 (1). Pulmonary TB is the most common primary site of infection, ranging from 59% to 68% among children and adolescents infected with TB, while extrapulmonary TB ranges from 22–25% (2). Tuberculous pleural effusion in children ranges from 12% to 38% of cases with untreated pulmonary TB (3). Chylothorax is a rare but recognised complication of pleural TB. Unfortunately, there is no study available for the morbidity and mortality rate for paediatric pleural TB with chylothorax despite its importance. Patients may present with acute respiratory symptoms in relation to fluid accumulation in the pleural space. Further investigation will be necessary to confirm the diagnosis of chylothorax. Chylothorax is described as milky appearance of the pleural fluid and has to be confirmed biochemically by the presence of chylomicron via staining with Sudan III, or high triglyceride concentration of more than 1.1 mmol/L, and the ratio of pleural fluid to serum cholesterol of <1.0 (4,5). Clinicians need to be able to identify, correlate, and diagnose tuberculous chylothorax as it poses a significant morbidity to patients and the direction of management would be different.
We reported a patient who was under our care in Hospital Canselor Tuanku Muhriz (HCTM), Kuala Lumpur, Malaysia. The patient’s clinical records were retrospectively reviewed through information access of electronic medical records, digital laboratory system, and digital radiological images and reports.
For the systematic review, we used three large databases namely PubMed, Ovid, and Scopus to find articles with the terms “tuberculosis” AND “chylothorax” AND “children”, up to August 2022. The articles were browsed through based on the title and abstract. Selected articles were then read through, and cases included are: (I) case reports and articles within the paediatric population below the age of 18 years; (II) infected by Mycobacterium TB; and (III) written in English only. We excluded reports with patients more than 18 years, those with non-tuberculous causes of chylothorax, and articles written in other languages. We have summarised the results of the literature search using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Figure 1). A single reviewer extracted all the data independently. The data of clinical presentation, clinical findings radiology modalities and findings, laboratory results, confirmatory tests, and treatments were collected and tabulated. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-636/rc).

Case presentationOther Section
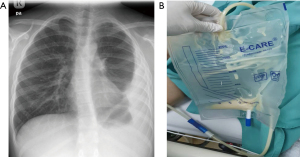
A 10-year-old boy with underlying bronchial asthma presented with a month-long history of dry cough and intermittent fever. He had a significant weight loss of 10 kg with reduced appetite and malaise. Two days prior to admission, he experienced chest discomfort which was described as sharp in nature. There was no history of contact with TB patients. During initial assessment, the patient was hemodynamically stable, not in respiratory distress with oxygen saturation in room air was 96%. There was stony dullness on percussion over the left lower zone of the chest with reduced breath sounds. Chest radiograph showed homogenous opacity at the left lateral thoracic region with blunted left costophrenic angle and meniscus sign (Figure 2A). Blood investigation showed increased inflammatory markers with serum C-reactive protein (CRP) of 2.71 mg/dL and erythrocyte sedimentation rate (ESR) of 72 mm/h. Left hemithorax ultrasound findings were suggestive of stage II parapneumonic effusion, where there was a left sided loculated heterogeneous hypoechoic pleural effusion measuring 5.9 cm in maximum depth with internal echogenic debris within. A pigtail catheter was then inserted under ultrasound guidance, and a total of 30 cc thick milky material was aspirated during the procedure (Figure 2B). Pleural fluid was characterised as exudative with high triglyceride (triglyceride 2.51 mmol/L). GENE-XPERT detected Mycobacterium TB DNA within the sample. Mantoux test was strongly positive of 25 mm. A diagnosis of chylous pleural effusion secondary to TB infection was established and the patient was treated immediately with Akurit-4 which consist of isoniazid (75 mg), rifampicin (150 mg), ethambutol (275 mg), and pyrazinamide (400 mg). He was given a low-fat diet regime for a week, and eventually the chest catheter was able to be taken off after 3 days. His subsequent follow up showed resolved respiratory symptoms and improved radiological findings. He completed the anti-TB treatment (intensive phase of 2 months, followed by a continuation phase of 7 months) and the child remains well and asymptomatic.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the participant’s legal guardian/next of kin for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
After thorough review of the articles and adhering to the inclusion and exclusion criteria, 7 articles were chosen. Ten children were reviewed from these articles (9 patients from prior studies, 1 new patient), summarised in Table 1. Two patients were female and the other 8 were male. The age range was 4 months to 15 years old. Six of the children have underlying diseases: 3 were exposed to human immunodeficiency virus (HIV), 1 was infected with HIV, 1 had idiopathic liver cirrhosis and underwent liver transplant, and the other has bronchial asthma. There was no information about the other patients’ underlying diseases, presumably they were healthy prior to their presentation with TB chylothorax. Despite socioeconomic status being one of the biggest risk factors for TB infection (13), this information was largely missing from the included articles and therefore is not reported here.
Table 1
| Parameters | Our patient | Ayuk 1 (6) | Ayuk 2 (6) | Grobbelaar 1 (7) | Grobbelaar 2 (7) | Mclaren (8) | Kant (9) | Cakir (10) | Gie (11) | Rabie (12) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patients’ demography | ||||||||||
| Age | 10 years | 12 years | 10 years | 2 years 11 months | 1 year 2 months | 1 year 8 months | 15 years | 4 months | 2 years | 3 years |
| Gender | Male | Female | Male | Male | Male | Male | Female | Male | Male | Male |
| Underlying diseases | Bronchial asthma | Liver transplant due to idiopathic liver cirrhosis | HIV exposed | N/A | HIV exposed | HIV exposed | N/A | N/A | N/A | HIV infected |
| Clinical presentations | ||||||||||
| Cough | + | + | + | + | N/A | + | N/A | N/A | N/A | N/A |
| Fever | + | + | + | N/A | N/A | + | + | N/A | N/A | + |
| Shortness of breath | − | N/A | + | N/A | N/A | + | + | + | N/A | N/A |
| Chest pain/discomfort | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Loss of weight | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Loss of appetite | + | + | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Night sweat | − | N/A | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Vomiting | − | N/A | N/A | N/A | N/A | + | N/A | N/A | N/A | N/A |
| Noisy breathing | − | N/A | N/A | N/A | N/A | + | N/A | N/A | N/A | N/A |
| Coryza | − | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lethargy | + | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Abdominal distention | − | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | + |
+, present; −, not present; N/A, information not available.
Clinical presentation varies in the reported cases. The symptoms were nonspecific and vague. Most of the patients presented with chronic symptoms namely intermittent fever (n=6) and cough (n=5). The typical symptoms of TB such as loss of appetite, loss of weight (n=3), and night sweats (n=1) were also reported. Some of the patients presented acutely with shortness of breath (n=3) as reported by Mclaren et al., Kant et al., and Cakir et al. (8-10). Chest discomfort or pain (n=3) might have been due to the accumulation of fluid over the pleural space (14). Other symptoms like vomiting (n=1), noisy breathing (n=1), coryza (n=1), and lethargy (n=1) were nonspecific (Table 1).
All 10 patients had chest radiograph performed revealing features suggestive of pleural effusion, and some had presence of lymphadenopathies. Six cases proceeded with computer tomography (CT) scan of the thorax which findings include tuberculous lymphadenopathy at various sites, as summarised in Table 2. Ayuk et al. and Kant et al. performed abdominal ultrasonography (USG), which aids to assess for presence of (I) visible lymphadenopathies; (II) other sites abscess; (III) presence of ascites; and (IV) organomegaly (6,9).
Table 2
| Parameters | Our patient | Ayuk 1 (6) | Ayuk 2 (6) | Grobbelaar 1 (7) | Grobbelaar 2 (7) | Mclaren (8) | Kant (9) | Cakir (10) | Gie (11) | Rabie (12) |
|---|---|---|---|---|---|---|---|---|---|---|
| Radiological modalities | ||||||||||
| X-ray chest | Homogenous opacity at the left lateral side obscuring left cardiophrenic border with some of the pleural border showing meniscus sign | Left pleural effusion | Right pleural effusion with left lower zone pneumonic changes |
Large right sided pleural effusion and multilobar pneumonia | Left sided pleural effusion | Large right sided pleural effusion | Bilateral pleural effusion | Right sided pleural effusion | Bilateral pleural effusion with a broad mediastinum | Large right sided pleural effusion |
| Ultrasound left hemithorax | Stage II parapneumonic effusion, where there was a left sided loculated heterogeneous hypoechoic pleural effusion measuring 5.9 cm in maximum depth with internal echogenic debris within | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Abdomen | N/A | No ascites | Multiple splenic micro abscesses | N/A | N/A | N/A | Multiple retroperitoneal and para-aortic lymphadenopathies | N/A | N/A | N/A |
| CT scan thorax | – | N/A | N/A | Right sided pleural effusion and left smaller pleural effusion. Tuberculous Lymphadenopathy over the right paratracheal, pretracheal, subcarinal, aortopulmonary window and hilar regions |
Left sided pleural effusion. Tuberculous lymphadenopathy over the right paratracheal subcarinal and both hilar regions | Right sided pleural effusion and hilar lymphadenopathy | N/A | Right sided pleural effusion. Multiple lymphadenopathies over pericardia and subcarinal | Lymphadenopathy | No lymphadenopathy intrathoracically. Abdomen: extensive lymphadenopathy intraabdominally and ascites |
| Blood investigations | ||||||||||
| Hb (g/dL) | 12.2 | N/A | N/A | N/A | N/A | N/A | Anaemia | 9.0 | N/A | N/A |
| WBC (109/L) | 7.3 | 4.7 | 14 | N/A | N/A | N/A | Normal | 7.6 | N/A | N/A |
| Lymphocytes (109/L) | 1.7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| CRP (mg/dL) | 2.71 | 18.2 | 5.7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| PCT (μg/L) | – | 0.23 | 0.68 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| ESR (mm/h) | 72 | N/A | N/A | N/A | N/A | N/A | N/A | 23 | N/A | N/A |
| Serum LDH (U/L) | – | 252 | 333 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Serum total protein (g/L) | 79 | 78 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Serum albumin (g/L) | 32 | 36 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pleural fluid analysis | ||||||||||
| Pleural fluid triglycerides (mmol/L) | 2.51 | 2.6 | 1.9 | 1.62 | 1.7 | N/A | Right side: 29.7; left side: 10.43 | 6.1 | N/A | 21.54 |
| Pleural fluid chylomicron | Not done | Present | Present | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pleural fluid LDH (U/L) | >1995 | 713 | 416 | N/A | N/A | N/A | N/A | N/A | N/A | 805 |
| Pleural fluid protein (g/L) | 90 | N/A | N/A | N/A | N/A | N/A | Right side: 42; left side: 40 | 90 | N/A | 21 |
| Fluid to serum LDH ratio | – | 0.35 | 1.24 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fluid to serum protein ratio | 1.13 | 0.62 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Pleural fluid glucose (mmol/L) | <0.3 | 4.4 | 7.4 | N/A | N/A | N/A | Right side: 2.44; left side: 2.22 | 4.8 | N/A | N/A |
| Pleural fluid cell analyses (U/L) | Predominant lymphocytes | Lymphocyte 1,750 (57%); neutrophil 1,280 (25%) | Lymphocyte 3,680 (97%); neutrophil 65 (1%) | N/A | N/A | N/A | Right side: lymphocyte 3,400 (80%); neutrophil (20%); left side: lymphocyte 3,280 (66%); neutrophil (34%) | Lymphocyte: 8,800 (67%) | N/A | Leukocyte: 13,720 |
| TB investigations | ||||||||||
| Mantoux test (mm) | 25 | 0 | 2 | N/A | N/A | N/A | N/A | 17 | N/A | N/A |
| Pleural fluid AFB | Negative | N/A | N/A | N/A | N/A | N/A | AFB: negative; culture: MTB | AFB: negative; culture: negative | N/A | N/A |
| Pleural fluid GENE-XPRET for MTB | Positive | Negative | Negative | N/A | N/A | Positive rifampicin resistant TB | N/A | N/A | N/A | N/A |
| Induced sputum AFB | Negative | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Induced sputum GENE XPERT MTB/RIF ultra-sensitive | N/A | Negative | Positive (3rd sample) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Bronchoalveolar lavage AFB | N/A | N/A | N/A | N/A | N/A | N/A | N/A | AFB: positive | N/A | N/A |
| Bronchoalveolar lavage GENE XPERT MTB/RIF ultra-sensitive | N/A | Positive | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gastric aspirate AFB | N/A | N/A | N/A | AFB: positive; culture: MTB | AFB: positive; culture: MTB | AFB: positive | N/A | AFB: negative | AFB: positive; culture: MTB | N/A |
| Gastric aspirate GENE XPERT MTB/RIF ultra-sensitive | N/A | N/A | N/A | N/A | N/A | AFB: negative | N/A | N/A | N/A | N/A |
| Other TB investigations | MTB blood culture-not isolated | N/A | N/A | N/A | N/A | N/A | Positive MTB culture of cervical lymph node specimen | N/A | N/A | MTB blood culture-isolated; bone marrow aspirate-granulomas, AFB noted. MTB culture positive |
| Miscellaneous | ||||||||||
| HIV test | N/A | Negative | Negative | N/A | N/A | N/A | N/A | N/A | N/A | Positive |
N/A, information not available; CT, computed tomography; –, not done; Hb, haemoglobin; WBC, white blood cell; CRP, C-reactive protein; PCT, procalcitonin; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; TB, tuberculosis; AFB, acid fast bacilli; MTB, Mycobacterium tuberculosis; RIF, rifampicin; HIV, human immunodeficiency virus.
Besides the radiograph findings, the laboratory investigation is important in establishing the diagnosis of chylothorax. We have assessed the investigations done by the authors to: (I) diagnose TB; (II) routine bloods with infective markers; and (III) pleural fluid analysis. All authors reported pleural drainage or tapping in the patients, which documented the classic milky appearance of pleural fluid. The pleural fluid analyses were consistent with chyle, evidenced by triglyceride level of >1.1 mmol/L in all cases. The primary cause of the chylothorax was further investigated, and the diagnosis of TB was established in all the reports by either positive Mantoux test, presence of Acid-fast bacilli in gastric aspirate or bronchoalveolar lavage, pleural fluid positive for TB culture or positive GENE-XPERT test. Kant et al. reported biopsy of the cervical lymph node which was performed and revealed caseating granuloma with positive culture for Mycobacterium TB (9). Rabie et al. sent bone marrow aspirate and blood culture, in which both grew Mycobacterium TB (12). Most cases had elevated CRP with either normal to high white blood cell (WBC) and lymphocytes count.
DiscussionOther Section
In this review, we are focusing into the paediatric population as morbidity and mortality due to TB infection are higher among the younger population (15), which is primarily due to the immaturity of their immune response (16). Apart from that, it is also difficult to diagnose TB in children as it can be mistaken for other common diseases in children like pneumonia, HIV, and malnutrition. Chylothorax is a rare complication of TB especially among paediatric patients and we managed to systemically review 10 cases including our patient to further understand patient demographics, the characteristics, and clinical nature of this rare complication of TB infection.
The pathogenesis of chylothorax and TB remains unclear (17). Nonetheless, it had been deduced that it might be caused by the disruption of thoracic duct flow due to the enlarged tuberculous lymph nodes (5,14), which subsequently leads to the accumulation of chyle in the pleural space (18). Patients can present with various symptoms particularly acute respiratory symptoms relating to the accumulation of fluid over the pleural space, which includes shortness of breath and chest discomfort. 5 out of the 10 cases reviewed presented with shortness of breath, and the severity relies on the degree of the chyle accumulation (5). Our patient and 5 other reported patients also presented with a long history of fever. Thus, the history of intermittent fever, and prolonged cough should warrant investigation for possible chronic infection like TB, particularly within an endemic area of TB and those in immunocompromised state. Nonetheless, our reported case and presumably 4 other cases reviewed were not in any significant immunocompromised state or have underlying disease that may contribute to the development of this rare TB entity. Constitutional symptoms such as loss of weight and loss of appetite provide additional clues, although not necessarily present in all cases. In our review, only 2 patients had reported to have these constitutional symptoms. Other symptoms like coryza, vomiting, lethargy, and noisy breathing may be present, although these symptoms were nonspecific.
After a complete physical examination, radiological imaging and blood investigations are important adjuncts in aiding the diagnosis of TB chylothorax. Though very limited information could be gathered from chest radiography, it is reliable as a first line radiological modalities to confirm the presence of pleural effusion. CT scans of the chest could help in evaluating the extension of the effusion, while assessing the presence of lymphadenopathies and help to narrow down the differential diagnoses and provide some clues in the primary aetiology of the pleural effusion (19). In some cases, clinicians might perform bedside USG to assess the presence of fluid, look for other sites abscess, organomegaly, aiding the process of pigtail insertion, and determine the type of fluid, whether it is free or organised (5,14). In our case, ultrasound of the chest was performed to aid the pigtail insertion for sample analysis and therapeutic draining, other than helping to monitor the progress of disease and response to treatment.
The diagnosis of chylothorax can only be ascertained when the pleural fluid is analysed biochemically. Chyle should be odourless, white, and milky in appearance (5,14,17), and should be distinguished biochemically with empyema and pseudo chylothorax. In empyema, the fluid will turn to a clear supernatant when it is centrifuged (20). As chyle contains significant amount of cholesterol, triglyceride, and chylomicron (21), therefore chylothorax is diagnosed by the presence of chylomicron or high triglyceride concentration of >1.1 mmol/L and the ratio of pleural fluid to serum cholesterol of <1.0. Other markers that may suggest chylothorax if the diagnosis is uncertain, include (I) pH of 7.4–7.8; (II) absolute cell count of >1,000 cell/L; and (III) lymphocyte count of >80% (5). All reported patients were diagnosed with chylothorax, mainly based on the triglyceride concentration of the fluid sample analysed. Additionally, the pleural fluid should also be determined to be exudative or transudative, using either Light’s or Heffner’s criteria. Light’s criteria consider fluid to be exudative if ≥1 of these is fulfilled; (I) pleural to serum protein ratio ≥0.5; (II) pleural to serum LDH ratio ≥0.6; and (III) pleural lactate dehydrogenase (LDH) >2/3 of the upper limit of the normal serum LDH. Meanwhile, Heffner’s criteria consider the pleural effusion to be exudative if it fulfills either; (I) pleural fluid protein >2.9 g/dL; (II) pleural fluid LDH level is >2/3 of the upper limit of the normal serum LDH; or (III) pleural fluid cholesterol is >45 mg/dL (22). A latest study by Gautam et al. denoted that pleural fluid classified based on pleural fluid cholesterol level alone, which exudative pleural fluid is defined as effusion cholesterol level >45 mg/dL; showed better specificity and sensitivity compared to Light’s & Heffner’s (23). Chylothorax is typically exudative in nature; however, in a small number of cases, it can be transudative especially in patients with underlying liver cirrhosis (24).
In all the reported cases, the cumulative history, physical examination, radiological findings, and lab investigations were highly suggestive of TB infection. Some of the cases, the diagnosis of TB was confirmed by GENE-XPERT of the pleural fluid. In our case, the patient had a highly positive Mantoux test of 25 mm, and the GENE-XPERT detected Mycobacterium TB DNA within the pleural fluid.
Once the diagnosis is established, the underlying cause should be treated immediately. TB treatment should be started as per local national guidelines. The fluid needs to be drained out from the chest to help reduce the accumulation. Some patients may require longer drain depending on the severity of the pleural effusion or symptoms. It is best to keep the drain only for <14 days as it poses a sequential risk of malnutrition and infection due to the loss of immunoglobulins (25). Furthermore, the patient has to be on a low-fat diet and/or total parenteral nutrition to prevent malnutrition while limiting chyle production and flow for at least 4–6 weeks (26,27). Patient needs to be followed up to assess the response to treatment and the resolution of the pleural effusion. If conservative treatment is not successful, the use of somatostatin can be considered (28). Up to the time of this review, there is still no evidence-based guideline to support the usage of somatostatin as the first line agent in managing chylothorax (29). In another report by Çakır et al., they documented a significant decrease of chylous effusion after administering Octreotide (30). Surgical intervention like duct ligation, pleuroperitoneal shunt, and pleurodesis can be considered later if chylothorax remains a significant problem despite adequate TB treatment and other management of chylothorax as discussed (5,20,21). Moreover, interventional radiology procedures like percutaneous thoracic duct embolization or the percutaneous destruction of lymphatic vessels may be done in some centres (31) for the management of chylothorax.
There are a number of limitations that we have identified for this study. The number of tuberculous chylothorax reported in the paediatric population is rather small, and some data are missing from the reports. Therefore, this study might not adequately represent the whole insight of this disease.
ConclusionsOther Section
TB is an important differential diagnosis when a patient presents with chylothorax especially in the presence of risk factors and signs to support the diagnosis of TB. Even though tuberculous chylothorax is rare, it is well recognised as one of the complications, and should be treated promptly and actively, both for the chylothorax and the underlying TB infection.
AcknowledgmentsOther Section
We would like to thank the Faculty of Medicine, The National University of Malaysia for supporting this study.
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-636/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-636/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the participant’s legal guardian/next of kin for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- World Health Organization. Global tuberculosis report 2022. 2022. Available online: https://www.who.int/publications/i/item/9789240061729
- Cowger TL, Wortham JM, Burton DC. Epidemiology of tuberculosis among children and adolescents in the USA, 2007-17: an analysis of national surveillance data. Lancet Public Health 2019;4:e506-16. [Crossref] [PubMed]
- Cruz AT, Ong LT, Starke JR. Childhood pleural tuberculosis: a review of 45 cases. Pediatr Infect Dis J 2009;28:981-4. [Crossref] [PubMed]
- Büttiker V, Fanconi S, Burger R. Chylothorax in children: guidelines for diagnosis and management. Chest 1999;116:682-7. [Crossref] [PubMed]
- Soto-Martinez M, Massie J. Chylothorax: diagnosis and management in children. Paediatr Respir Rev 2009;10:199-207. [Crossref] [PubMed]
- Ayuk AC, Gray D, Vanker A, et al. Tuberculosis in children presenting with chylothorax - Report of two cases and review of the literature. Respir Med Case Rep 2019;27:100848. [Crossref] [PubMed]
- Grobbelaar M, Andronikou S, Goussard P, et al. Chylothorax as a complication of pulmonary tuberculosis in children. Pediatr Radiol 2008;38:224-6. [Crossref] [PubMed]
- McLaren B, Song X, Mate E, et al. Chylothorax in a child with rifampicin-resistant tuberculosis. Afr J Thorac Crit Care Med 2019. doi:
10.7196/SARJ.2019.v25i3.237 .10.7196/SARJ.2019.v25i3.237 - Kant S, Verma SK, Anand SC, et al. Development of bilateral chylothorax in a younger female secondary to tuberculosis. Lung India 2011;28:56-9. [Crossref] [PubMed]
- Cakir E, Gocmen B, Uyan ZS, et al. An unusual case of chylothorax complicating childhood tuberculosis. Pediatr Pulmonol 2008;43:611-4. [Crossref] [PubMed]
- Gie RP, Goussard P, Kling S, et al. Unusual forms of intrathoracic tuberculosis in children and their management. Paediatr Respir Rev 2004;5 Suppl A:S139-41.
- Rabie H, Lomp A, Goussard P, et al. Paradoxical tuberculosis associated immune reconstitution inflammatory syndrome presenting with chylous ascites and chylothorax in a HIV-1 infected child. J Trop Pediatr 2010;56:355-8. [Crossref] [PubMed]
- Wu J, Dalal K. Tuberculosis in Asia and the pacific: the role of socioeconomic status and health system development. Int J Prev Med 2012;3:8-16. [PubMed]
- Tutor JD. Chylothorax in infants and children. Pediatrics 2014;133:722-33. [Crossref] [PubMed]
- Newton SM, Brent AJ, Anderson S, et al. Paediatric tuberculosis. Lancet Infect Dis 2008;8:498-510. [Crossref] [PubMed]
- Togun T, Kampmann B, Pai M. Diagnosis of Childhood Tuberculosis. Reference Module in Biomedical Sciences 2017. doi:
10.1016/B978-0-12-801238-3.64157-0 .10.1016/B978-0-12-801238-3.64157-0 - Vennera MC, Moreno R, Cot J, et al. Chylothorax and tuberculosis. Thorax 1983;38:694-5. [Crossref] [PubMed]
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Amar JB, Zaibi H, Dahri B, et al. Spontaneous chylothorax revealing a mediastinal and abdominal lymph node tuberculosis. Indian J Tuberc 2017;64:141-3. [Crossref] [PubMed]
- Hillerdal G. Chylothorax and pseudochylothorax. Eur Respir J 1997;10:1157-62. [Crossref] [PubMed]
- Cansiz KA, Tug T, Konuk S, et al. A Case with Tumor, Tuberculosis and Chylothorax. Arch Clin Med Case Rep 2018;2:68-74. [Crossref]
- Devkota KC, Hamal S, Panta PP. Comparison of Heffner criteria and Light criteria in differentiating exudative and transudative pleural effusion. Nepal Med Coll J 2020;22:141-5. [Crossref]
- Gautam S. Diagnostic value of pleural cholesterol in differentiating exudative and transudative pleural effusion. Ann Med Surg (Lond) 2022;82:104479. [Crossref] [PubMed]
- Maldonado F, Hawkins FJ, Daniels CE, et al. Pleural fluid characteristics of chylothorax. Mayo Clin Proc 2009;84:129-33. [Crossref] [PubMed]
- Rudrappa M, Paul M. Chylothorax. In: StatPearls. Treasure Island: StatPearls Publishing, 2023.
- Jensen GL, Mascioli EA, Meyer LP, et al. Dietary modification of chyle composition in chylothorax. Gastroenterology 1989;97:761-5. [Crossref] [PubMed]
- Marts BC, Naunheim KS, Fiore AC, et al. Conservative versus surgical management of chylothorax. Am J Surg 1992;164:532-4; discussion 534-5. [Crossref] [PubMed]
- Demos NJ, Kozel J, Scerbo JE. Somatostatin in the treatment of chylothorax. Chest 2001;119:964-6. [Crossref] [PubMed]
- William TM. Congenital chylothorax and trisomy 21 syndrome. Journal of Pediatric and Neonatal Individualized Medicine 2018;7:e070133.
- Çakır U, Kahvecioğlu D, Yıldız D, et al. Report of a case of neonatal chylothorax that responded to long-term octreotide treatment, and review of the literature. Turk J Pediatr 2015;57:195-7. [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]


