
Novel dominant-negative FOXJ1 mutation in a family with heterotaxy plus mouse model
Highlight box
Key findings
• Identification of a novel heterozygous deletion variant, c.1129delC, in FOXJ1 (p.Leu377Trpfs*76) in a Chinese family with type 43 primary ciliary dyskinesia (CILD43).
What is known and what is new?
• FOXJ1 is a master regulator of the ciliogenic program, and loss-of-function mutations in FOXJ1 have been related to human primary ciliary dyskinesia.
• In this study, the first Foxj1 knock-in mice were generated and recapitulated the ciliopathies of our patients. Our research revealed that c.1129delC variant exerts a dominant-negative effect and provided some ideas for the role of FOXJ1 in heart disease.
What is the implication, and what should change now?
• Previous report supposed that haploinsufficiency of FOXJ1 may be the cause of CILD43. Thus, more research is needed to understand the action mechanism of FOXJ1 variants. In addition, CILD43 patients should be aware of the cardiac abnormality and CHD individuals could test for FOXJ1 variants.
Introduction
Primary ciliary dyskinesia (PCD, OMIM#244400) is a rare inherited motile ciliopathy, characterized by upper and lower respiratory tract disease, organ laterality defects, subfertility, and hydrocephalus due to the defective ciliary apparatus (1). Over 40 genes have been reported to cause PCD, an autosomal recessive or, less frequently, X-chromosomal recessive inheritance pattern disorder (2). In 2019, type 43 PCD (CILD43, OMIM#618699) was created by de novo autosomal-dominant loss-of-function mutations identified in Forkhead box J1 (FOXJ1) from six sporadic cases (3). Subsequently, six unrelated individuals harboring heterozygous variants in FOXJ1 were described in two separate studies (4,5). The phenotypic presentation of these patients encompasses the expected symptoms of a multisystem motile ciliary defect, but exhibits a remarkably variability and expressivity, involving the central nervous system, the respiratory system and other systems (6). So far, only eight FOXJ1 variants have been reported in individuals with PCD or hydrocephalus, and the functional roles of these mutants have rarely been studied. Moreover, additional patients carrying mutations in FOXJ1 with unusual phenotypic presentation could promote a deeper understanding of the functional basis of this gene.
FOXJ1, also known as HFH-4, a member of the forkhead family of transcription factors, is mainly expressed in motile ciliated cells, where as a master regulator of the ciliogenic program (7). Numerous researches have revealed a pivotal role of FOXJ1 in many tissues that possess highly ciliated cells, such as conducting airways, choroid plexus, ependyma of the brain, testis, and oviduct (8-10) as well as motile mono-cilia in the embryonic left/right organizer (LRO) (11,12). Consistently, gene modifications in animal models and mutations identified in humans have demonstrated that FOXJ1 dysfunction impairs ciliary action in specific zones causing a variety of genetic disorders (3,13) including hydrocephalus (14), laterality phenotypes (11) and mucociliary clearance disorder (15) due to a dramatic number reduction of motile cilia and axoneme structure severe defects (11,13). Yet, Foxj1 knock-in mouse model corresponding to human mutation identified in CILD43 cases has not been reported and limited information is currently known about the underlying molecular mechanisms.
In this study, we characterized a family with heterotaxy and the proband with complex congenital heart disease (CHD). A heterozygous variant c.1129delC (p.Leu377Trpfs*76) in FOXJ1 was identified. We found that this mutant exhibited a dominant-negative effect on the transcriptional activity of RFX3, downstream of FOXJ1 in the node, and cooperatively induce the expression of ciliated genes (16). The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification (17). Mice carrying the equivalent of the human FOXJ1-c.1129delC mutation were generated and found to have disrupted motile cilia structure in trachea epithelial cells and randomized left-right patterning and hydrocephalus. Meanwhile, transcriptome results of this knock-in mouse heart tissues point to some genes involved in cardiac pathology, which may provide clues for the role of FOXJ1 in heart disease. We present this article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-27/rc).
Methods
Family and patient
A non-consanguineous Chinese Han family from Anhui Province was recruited for our study. The proband was a 51-day-old boy with dextrocardia who was found to have complex congenital heart disease. His diagnoses were confirmed by echocardiography, cardiac catheterization examinations, computed tomography, and other operation recordings. His affected mother has total situs inversus visceral. Clinical features were checked at Shanghai Children’s Medical Center (SCMC). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Shanghai Children’s Medical Center (SCMC) (No. SCMC-201015) and written informed consent was obtained from the parents of the child.
Whole-exome sequencing for candidate gene identification
Peripheral blood samples of all cases were collected, and genomic DNA was extracted using QIAamp® DNA Blood Mini Kit (QIAGEN, Hilden, Germany) by standard protocols. Exome sequencing was performed on the proband (III:1) and his parents (II:2, II:3) using the Agilent SureSelect capture kits (Agilent, Santa Clara, CA, USA). The captured libraries were subsequently sequenced using the Illumina HiSeq 2500 system. Sequencing data alignment and variant calling were performed as previously described (18). Given the nature of primary ciliary dyskinesia (PCD) genetics, we began to search for variants in known PCD-causing genes, considering rare truncating and missense variants. The pathogenicity of variants was evaluated using American College of Medical Genetics and Genomics (ACMG) standards and guidelines (19). FOXJ1-c.1129delC (p.L377Wfs*76) was classified as pathogenic based on the evidence of 1 very strong (PVS1) and 1 strong (PS2) and 1 moderate (PM2). Co-segregation with the phenotype were confirmed by Sanger sequencing. PCR primers are listed in Table S1.
Plasmid construction
The coding region of the wild-type (WT) and mutant (p.Leu377Trpfs*76) FOXJ1 containing a partial 3' untranslated region were cloned into pcDNA3.1 vector with a flag-tagged sequence in the 3' end. The human RFX3 promoter sequence (chr9:3,525,790-3,527,302, hg38) was cloned into the pGL4.10 vector (Promega, Madison, USA) to produce RFX3-promoter-Luc. All constructs were validated by Sanger sequencing.
Cell culture and transfection
HEK 293T cell line was obtained from the cell bank of the Committee on Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in high-glucose Dulbecco Modified Eagle Medium (#11965092, GibcoTM) supplemented with 10% fetal bovine serum (#10099141, GibcoTM) at 37 ℃ with 5% CO2. Transient transfection was performed with Lipofectamine 3000 Transfection Reagent (#L3000008, Invitrogen™) according to the manufacturer’s protocol.
Western blot and immunofluorescence
HEK293T cells were seeded in 12-well plates, transiently transfected with WT or mutant FLGA-FOXJ1 vectors, and lysed after 48 hours using sodium dodecyl sulfate (SDS) buffer for Western blot analyses. Polyvinylidene difluoride (PVDF) membranes were incubated with primary anti-FLAG antibody (#14793, CST) and loading control antibody GAPDH (#5174, CST), followed by anti-rabbit IgG secondary antibodies conjugated to horseradish peroxidase, then detected with the chemiluminescence system. Protein band intensities were quantified by ImageJ software (version 1.53t, National Institutes of Health, USA). For immunofluorescence staining, transfected HEK293T cells were fixed using 4% paraformaldehyde/phosphate-buffered saline (PFA/PBS) for 20 min, rinsed with PBS, permeabilized with 1% TritonX-100/PBS for 10 min, and blocked with 1% bovine serum albumin/PBS solution for 30 min at room temperature. The treated cells were incubated with anti-FLAG antibody at a dilution of 1:1,000 and then washed three times with PBS, followed by incubation with fluorescein isothiocyanate conjugated secondary antibody for 1 h. Finally, nuclei were stained with 4,6‐diamidino‐2‐phenylindole (DAPI), and images were captured using a Laser Scanning Confocal Microscope.
Dual-luciferase reporter assay
RFX3-Luc Photinus pyralis luciferase reporter plasmid, WT/mutant FOXJ1 constructs or empty vector, and internal control Renilla luciferase reporter plasmid pRL-TK were co-transfected in HEK293T cells. Briefly, HEK293T cells were transiently transfected for 24 hours using Lipofectamine 3000 with 200 ng of the RFX3-Luc, 20 ng of Renilla pRL-TK, and co-transfected with either: 150 ng pcDNA3.1 (empty vector); 75 ng pcDNA3.1 and 75 ng of WT FOXJ1; 75 ng pcDNA3.1 and 75 ng mutant FOXJ1; and 75 ng WT FOXJ1 plus 75 ng mutant FOXJ1. Both luciferase activities were determined using the Dual-Glo® Luciferase Assay System (#E2920, Promega) according to the supplier’s recommendations, and then data were expressed as the ratio of firefly/Renilla luciferase activity. Experiments were performed three times independently with three technical triplicates each.
Generation and histological analysis of Foxj1-c.1129delT mice
Briefly, the Foxj1-c.1129delT targeting construct was linearized by restriction digestion with NotI followed by phenol/chloroform extraction and ethanol precipitation. The linearized vector was transfected into C57BL/6N embryonic stem cells (ESC) according to Cyagen’s (Suzhou, China) standard electroporation procedures. Positive ESC clone was injected into C57BL/6 albino embryos, then re-implanted into CD-1 pseudo-pregnant females. Founder animals were identified by their coat color, and their germline transmission was confirmed by breeding with C57BL/6 females and subsequent genotyping of the offspring. The Neo cassette, flanked by SDA (self-deletion anchor) sites, is self-deleted in germ cells, thus, so the offspring is Neo cassette-free. Genotyping was confirmed on mouse tail snip DNA by Sanger sequencing. All experiments were performed under a project license (No. SCMC-LAWEC-2023-005) granted by institutional Animal Care and Use Committee of SCMC, in compliance with SCMC institutional guidelines for the care and use of animals. The sample size, genotype and age of the experimental models are indicated in the legends. At same age mice of each genotype were divided into groups for comparative analysis. No other treatment was given to the mice and no inclusion and exclusion were set. Mouse brains were collected and fixed in 4% PFA/PBS overnight, then were processed for paraffin embedding, sectioned at a thickness of 5 µm, and stained with hematoxylin-eosin using our reported procedures (18). Stained sections were imaged with a Leica DM6000 microscope.
Electron microscopy (EM)
Mouse trachea was isolated and prefixed with 2.5% (vol/vol) glutaraldehyde in 0.2 M phosphate buffer overnight at 4 ℃, washed and then post-fixed in 1% (wt/vol) osmium tetroxide for 1.5 h. Samples were prepared following standard transmission electron microscope (TEM) and scanning electron microscopy (SEM) protocols. We randomly watched three hundred cilia for each sample using twenty different microscope fields, but only those that could clearly distinguished between the normal and abnormal ciliary ultrastructure were counted and used for statistical analysis from about one hundred cilia. Ultrastructural abnormalities of the microtubule arrangement were counted by an expert.
RNA-Seq analysis
Total RNA isolated from the postnatal day 1 (P1) heart of three WT and three Foxj1c.1129delT/c.1129delT mice was for RNA sequencing, respectively. Library preparation and RNA-seq were performed on the NovaSeq 6000 System to generate 150 bp paired-end reads by Berry Genomics Corporation Ltd (Beijing, China). Clean reads were aligned to the reference genome (mm10) by HISAT2. DESeq2 package was used for differential expression genes (DEGs) analysis as we did before (20). Gene Ontology (GO) and KEGG pathway analysis of the DEGs were performed using the The Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources and its functional annotation tools (https://david.ncifcrf.gov/home.jsp).
Statistical analysis
GraphPad Prism (version 9.4.1, USA) was used for statistical analysis. The data for the comparison of two groups were analyzed using the independent samples t-test, and one-way analysis of variance (ANOVA) was used to compare data for three or more groups. P<0.05 was considered statistically significant.
Results
Clinical features and mutation analysis
In this study, a Chinese family with heterotaxy was identified (Figure 1A,1B). The proband (III:1) was a 51-day-old boy who had dextrocardia and was born with complex congenital heart disease including total anomalous pulmonary venous connection (TAPVC), atrial septal defect, pulmonary valve stenosis, and atrioventricular septal defect. He underwent a primary TAPVC repair with concomitant PA banding at 2-month-old, and subsequently a bidirectional Glenn procedure was performed at age of 1 year and postoperative monitoring showed an enhanced and blurred, and exudation in both lungs. This patient had progressive pulmonary hypertension and died of heart failure at 2.5 years of age. His mother (II:2) exhibited total situs inversus, and she was infertile that get pregnant by assisted reproductive technologies. These disease phenotypes are not seen in other family members. The proband reported a history of thick breathing sounds in both lungs after birth. To identify the causal genetic responsible for the disorders in this family, whole exome sequencing of the proband (III:1) and his parents (II:2 and II:3) were conducted. Based on the sequencing data, a heterozygous variant c.1129delC in FOXJ1 (RefSeq accession number NM_001454.4) was found in the proband and his affected mother. Further, we performed Sanger sequencing for two affected members (II:2 and III:1) and four unaffected members (I:1, I:2, II:1 and II:3) and confirmed c.1129delC variant co-segregation with the misplaced organ phenotype (Figure 1C). This deletion variant is predicted to lead to a shift in the reading frame at codon 377 (Leu377Trp) and altered the last 45 amino acids (aa), abolished the original stop codon (422Ter), and added 30aa (p.Leu377Trpfs*76) to the carboxyl-terminal transactivation domain (TAD) of FOXJ1 (Figure 1D). The protein sequence alignment showed that Leu377 through the remaining amino acids (377-421aa) of human FOXJ1 is highly conserved among mammals (Figure 1E). Moreover, the FOXJ1-c.1129delC variant has not previously been reported in ClinVar, ExAC, genomAD, and 1,000 Genomes databases and was classified as a pathogenic variant according to the ACMG guidelines.
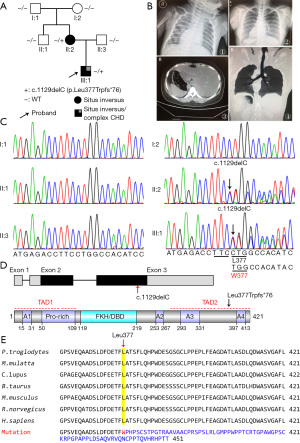
The p.Leu377Trpfs*76 mutation did not affect FOXJ1 expression levels and subcellular localization
To evaluate the functional consequence of the FOXJ1-c.1129delC mutation, we first detected the protein expression level and cellular localization of FLAG-FOXJ1-WT and -Leu377Trpfs*76 (p.L377Wfs*76) in HEK293T cells. Consistent with the predicted result that 30 more amino acids of FOXJ1 (mutant: 451aa) were produced than wild-type (WT) due to the c.1129delC mutation. We observed a higher molecular weight band of mutants and showed no significant difference in protein expression levels when compared to WT FOXJ1 according to the western blot analysis (Figure 2A,2B). Moreover, we found that both WT and mutant FOXJ1 proteins were explicitly localized in the nucleus (Figure 2C). These results show that FOXJ1-c.1129delC mutation produced longer non-canonical stable proteins with normal subcellular distribution as WT proteins are.
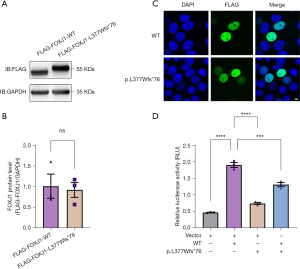
The FOXJ1 mutant has a dominant-negative effect on the RFX3 transcriptional activity
RFX3 is a key downstream of FOXJ1, interacts and functions as a co-activator with FOXJ1 to regulate the expression of cilia-related genes. Thus, we focused on studying the effect of FOXJ1-Leu377Trpfs*76 mutant on the transcriptional activity of RFX3. HEK293T cells transfected with plasmids encoding WT FOXJ1, empty vector, and reporter plasmid showed an approximately 4.1-fold higher luciferase activity than cells transfected with empty vector alone (Vector: 0.49±0.01; WT: 1.91±0.11). Transfection of HEK293T cells with FOXJ1 mutant led to a luciferase activity that was about 1.5-fold higher than the empty vector and was significantly reduced to 39%±11% compared with WT under the same experimental conditions (p.Leu377Trpfs*76: 0.74±0.04). However, when the plasmids encoding WT and mutant FOXJ1 were co-transfected (WT/p.Leu377Trpfs*76: 1.31±0.09), the reporter activity was reduced to 69%±9% of WT alone. Additionally, a significant luciferase activity after transfection of a mock vector could be due to endogenous FOXJ1 expression in HEK293T cells (Figure 2D). Thus, our dual-luciferase reporter assay results indicate that the FOXJ1 mutant exerts a dominant-negative effect on the RFX3 transcriptional activity.
Generation and phenotypic characterization of Foxj1-c.1129delT knock-in mice
To model the human phenotype in vivo and further elucidate the potential underlying mechanisms, we generated a knock-in mouse carrying a mutation c.1129delT in Foxj1 (orthologous mutation c.1129delC in human) by homologous recombination using a targeting vector containing exons 2 to 3 (Figure 3A). This alteration was also predicted to lead to a longer non-functional protein production (p.Leu377Trpfs*84, Figure S1). Heterozygous pups (Foxj1+/c.1129delT) were born at the expected Mendelian frequency, yet exhibited dramatic growth retardation, and all died within 2 months after birth with a mean survival time of 28 days (Figure 3B). In addition, Foxj1+/c.1129delT mice developed hydrocephalus at about 2 weeks of age and displayed abnormal head morphology with complete penetrance by 2 months (Figure 3C,3D). Homozygous (Foxj1c.1129delT/c.1129delT) mutation results in neonatal lethality in mice accompanied by severe hydrocephalus and few could survive 1 week (Figure 3E). Moreover, WT mice show completely normal organ placement and cardiac apical position. However, the Foxj1-c.1129delT mutants with dextrocardia or mirror image reversal of internal organ situs (Figure 3F). 11% (2 of 18 mutants) of the heterozygous mice showed laterality defects, such as situs inversus totalis or heterotaxis, and homozygous mutants presented with situs inversus at a rate of 71% (5 of 7 mutants) in live births based on the position of the heart and stomach (Figure 3G). Our results demonstrated that compared with WT mice, Foxj1-c.1129delT knock-in mice died early and presented with hydrocephalus and randomization of left/right body asymmetry.
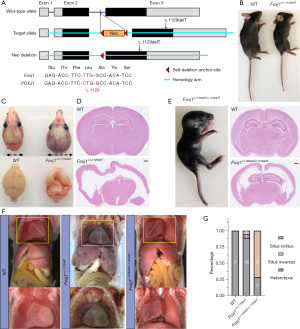
Foxj1-c.1129delT mice show reduced numbers of cilia and defective ciliary ultrastructure
Cilia have been implicated in the development of hydrocephalus and the determination of asymmetry of the heart and visceral organs. Hence, we characterized the ciliary morphology and ultrastructure in Foxj1-c.1129delT mutant mouse trachea epithelial cells. Immunofluorescence (IF) microscopy analyses with antibodies targeting acetylated-tubulin (a marker of the ciliary axonemes) and γ-tubulin (a marker of the centrosome) showed reduced numbers and densities of trachea cilia and disorganized along the surface of trachea epithelial cells in Foxj1+/c.1129delT mice as the images displayed with scanning electron microscopy (SEM), while the WT mouse cilia signal was continuously observed and well-organized along the surface of trachea epithelial cells (Figure 4A,4B). The homozygous Foxj1c.1129delT/c.1129delT mutant mouse exhibited a significant reduction in tracheal cilia and far fewer and shorter cilia formed in contrast to the abundant, long broom-like cilia of WT cells by SEM (Figure 4C). We then analyzed the ultrastructure of the mouse trachea cilia by transmission electron microscope (TEM). In Foxj1c.1129delT/c.1129delT mice, classical motile type cilia with a clearly defined 9+2 microtubule doublets ultrastructure were entirely destroyed in tracheal cells, but only about 23% (14 of 54 cilia) of tracheal cilia from Foxj1+/c.1129delT mouse showed a disorganized arrangement of the microtubule doublets by counting 20 TEM images from two mice (Figure 4D). Collectively, those results show that Foxj1-c.1129delT knock-in mutation affects microtube orientation and ciliogenesis.

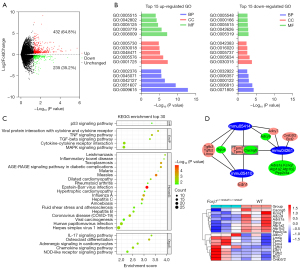
Transcriptomic analyses of Foxj1 mutant mouse heart tissues
To investigate the clues associated with abnormal heart phenotype in the proband, we performed RNA-Seq with P1 WT and Foxj1c.1129delT/c.1129delT mouse heart tissue. A total of 667 genes showed changes (differentially expressed genes, DEGs) in expression in the Foxj1c.1129delT/c.1129delT compared with their expression in the WT, including 432 up-regulated genes (64.8%) and 235 down-regulated genes (35.2%) (Figure 5A and available online https://cdn.amegroups.cn/static/public/tp-23-27-1.xls). The Gene Ontology (GO) functional analysis of the DEGs was divided into the biological process (BP), molecular function (MF) and cell component (CC). The top 15 results obtained from the GO enrichment analysis of the up-regulated and down-regulated DEGs are shown in Figure 5B, website: https://cdn.amegroups.cn/static/public/tp-23-27-2.xls and website: https://cdn.amegroups.cn/static/public/tp-23-27-3.xls. The up-regulated genes were mainly enriched in response to virus/immune process (Ontology: BP), the stress fiber/Z disc (Ontology: CC), and protein binding/cytokine activity (Ontology: MF) and the down-regulated genes were mainly enriched in potassium ion transport/transcription regulation, T-tubule/sarcolemma and (phospho-) lipid transporter activity/nucleotide (DNA) binding, respectively. The top 30 KEGG pathways for DEGs are shown in Figure 5C and website: https://cdn.amegroups.cn/static/public/tp-23-27-4.xls, which contain human diseases such as hypertrophic cardiomyopathy and dilated cardiomyopathy. In addition, pathway “adrenergic signaling in cardiomyocytes” was enriched among genes commonly down-regulated in Foxj1 mutant heart tissue (Figure 5D). These preliminary results may provide clues for the associations between Foxj1 and heart disease.
Discussion
In this study, we identified a novel heterozygous deletion mutation (c.1129delC/p.Leu377Trpfs*76) of FOXJ1 in one Chinese family with situs inversus, and the proband is complicated with complex congenital heart disease (CHD). This mutant p.Leu377Trpfs*76 protein, expressed with an almost equal level of the WT and localized normally in the nucleus, shows a dominant-negative effect on the expression of RFX3 in vitro. Moreover, we generated the first Foxj1 knock-in (c.1129delT, ortholog of the c.1129delC in human FOXJ1) mice that recapitulated the situs anomalies of our patients. Together, we report maternally inherited and a heterozygous dominant de-novo mutation in FOXJ1 with type 43 primary ciliary dyskinesia (CILD43, OMIM#618699). Additionally, transcriptome data of Foxj1 knock-in mouse hearts suggest that we should be aware of cardiac anomalies in individuals with FOXJ1 variants.
Even though Foxj1 null mice are well known for their abnormalities in left-right axis determination over two decades (11,13), the FOXJ1 mutations concerning human disorders are just identified over the past several years (3). To date, only eight FOXJ1 mutations have been reported, of which one is an amino acid substitution and seven are premature termination products on protein sequence, in 12 individuals (Table S2). Collectively, exon 3 of FOXJ1 seems to be a mutational hotspot and the majority of pathogenic variants were nonsense or frameshift type. The C-terminal region of FOXJ1 contains a transactivation region and removing the fourth acidic domain causes a significant decrease in its transcriptional activity (10). Consistent with this study, we found that c.1129delC alteration, predicted to disrupt the A4 region of FOXJ1, reduced its transcriptional activity on RFX3 via a dominant negative mechanism. Similar to our findings, action models like FOXJ1-c.1129delC mutation have been reported in other forkhead transcription factors. For example, Foxo1 lacking the C-terminal transactivation domain could act in a dominant-negative manner (21) and dominant-negative mutant of FOXE3 with an autosomal-dominant trait contributes to human ocular disease (22). However, the only functional study carried out so far indicates haploinsufficiency being the disease cause by 3'mRNA-seq of air-liquid interface-cultured respiratory epithelial cells from two controls and two FOXJ mutant individuals (3). Thus, it would be helpful to further test this dominant negative mutant in vivo by using Foxj1 knock-out and Foxj1-c.1129delT knock-in mouse models, and more research is needed to understand the action mechanism of FOXJ1 variants.
FOXJ1 is the master regulator of the transcriptional program that drives the assembly of motile cilia. Abnormal ciliary structure and function in Foxj1 deficient model organisms are considered as the causative of Foxj1-related disorders (11,23). In our study, heterotaxy and the rough breathing sounds of both lungs presented in the patients implied an impairment of the node cilia cells and ciliated pulmonary epithelial cells. Similarly, our Foxj1-c.1129delT knock-in mice presented with randomization of left/right body asymmetry. As expected, the number and density of tracheal cilia from Foxj1-c.1129delT mutant mice were reduced and cilia were disorganized along the surface of tracheal epithelial cells. The typical 9+2 cilia axoneme ultrastructure was entirely and partially destroyed in homozygous and heterozygous Foxj1 knock-in mice, respectively. Ciliary cross sections of respiratory epithelial cells from FOXJ1 affected individuals exhibited variable cilia structural defects, and some did not show any abnormalities of the 9+2 axonemal architecture. However, the number of cells with normal amounts of cilia in all sufferers was reduced. These affected individuals have an associated mucociliary clearance disorder, but only half have situs inversus (3). Thus, we noted that foxj1 dysfunction is not causing a uniform ciliary ultrastructure abnormality, but rather a decreased number and random changes in organization. Meanwhile, the proband’s mother was unable to conceive naturally. These reduced fertility conditions have been earlier reported in three patients with FOXJ1 mutation. One was unable to become pregnant, one had hydrosalpinx, and one male had severe oligoasthenoteratospermia (Table S2). In a mouse model, attempts on mating male or female Foxj1−/− mice with productive WT counterparts were unsuccessful (13). These data suggest that FOXJ1 mutation might influence ciliogenesis in the female reproductive tract and sperm flagella movement thereby affecting human fertility. Regretfully, Foxj1-c.1129delT knock-in mice died soon after birth, and the offspring number at birth was not evaluated. In addition, as shown in Table S2, hydrocephalus seems to be a fully penetrant condition from previously reported FOXJ1 mutant patients. Even though this phenotype is not informed in our patient, dilated ventricles were observed in almost all Foxj1-c.1129delT knock-in mice. This prominent phenotype could be explained by specific or preferential FOXJ1 expression in the brain ependymal cells, and defects in the ependymal cilia affect the cerebrospinal fluid passage during brain formation that led to hydrocephalus (24). Meanwhile, further detailed inspection is required to investigate the other common PCD-related symptoms and ciliary ultrastructure from the proband’s mother. Collectively, these data demonstrated that heterozygous Foxj1-c.1129delT mutation could results in mouse ciliary ultrastructural defects and this model presents primary ciliary dyskinesia related phenotypes and mimics the clinical symptoms of our patient.
In addition, another remarkable aspect, severe and complex CHD, is presented in the proband. Although assisted reproduction technologies have also been associated with higher risk of birth defects, such as congenital heart defects (25). Interestingly, CHD phenotypes were mentioned in other two cases with FOXJ1 variants, one presented with a ventricular septal cardiac defect and another with a small atrial septal defect (TableS 2). Besides, we screened two rare and predicted damaging FOXJ1 variants (c.266G>A/p.Gly89Asp, Exon2; c.637C>T/p.Arg213Trp, Exon3) in our cohort of patients with Tetralogy of Fallot (Table S3 and Figure S2). RNA-seq on cardiac cell line AC16 overexpressing wild-type or p.Arg213Trp mutant of FOXJ1 were performed and found that enriched KEGG pathways of down-regulated DEGs included the calcium signaling pathway and adrenergic signaling in cardiomyocytes, which is important for early gene expression of myocardial cells, and cardiomyocyte maturation and apoptosis (26,27) (Table S4). Similarly, cardiomyopathy-associated genes are differentially expressed [e.g., Tpm2/3 (28); Tgfb2/3 (29)] and adrenergic signaling in cardiomyocytes pathway are enriched from the transcriptome results of Foxj1 knock-in mouse heart tissues. While this does not exclude the possibility that these genetic changes result from structural cardiac anomalies rather than a direct Foxj1 effect. To date, the relationship between FOXJ1 variants and CHD is barely known, our preliminary findings may provide new information on the association between CHD and FOXJ1. Although hydrocephalus is common in animals with primary ciliary dyskinesia (PCD), these models distinctly have an increased incidence of CHD (30,31). Hence, CILD43 patients should be aware of the cardiac abnormality and CHD individuals could test for FOXJ1 variants.
In our report, both Foxj1c.1129delT/c.1129delT and Foxj1+/c.1129delT mice developed situs inversus and hydrocephalus, and showed a disruption of trachea cilia structure, whereas these abnormalities are only observed in previous reported Foxj1−/− mice and no aberration existed in single Foxj1 allele deletion (Foxj1+/−) mice. Foxj1−/− mice all died within postnatal days 40 (P40) and approximately half of the born had situs inversus and hydrocephalus (11,13). However, our Foxj1c.1129delT/c.1129delT mice seem to have a higher proportion reversal of internal organs, die earlier, and almost all mice developed hydrocephalus, though the numbers of mice examined was insufficient to reflect the proper incidence of situs inversus for Foxj1-c.1129delT mice early death. The proband died from severe CHD. Collectively, mice with c.1129delT mutation in Foxj1 showed a severer phenotype than Foxj1 allele deletion, which reflects a potential toxicity effect of the existence of aberrant Foxj1. In addition, hydrocephalus impacts the fundamental aspects of brain development, associated phenotypes such as intellectual disability and neurodevelopmental delay are not infrequent findings among patients (4), but the developmental delay simply told in three patients with congenital hydrocephalus who carry a FOXJ1 mutation (Table S2). And growth failure was observed in Foxj1−/− (13) and our Foxj1+/c.1129delT mice. In summary, Foxj1-c.1129delT knock-in mice generally showed the same traits as Foxj1−/− mice, but produced more severe effects, implying an adverse consequence of Foxj1-c.1129delT mutants.
Conclusions
Our findings expand the clinical and mutational spectrum of type 43 primary ciliary dyskinesia caused by mutations in FOXJ1. Meanwhile, much work remains to be done in unraveling the pathogenesis of FOXJ1 mutations. Identifying more patients with FOXJ1 variant will be toward a comprehensive understanding of the genotype-phenotype relationships and provide valuable diagnosis data. More distinct forms of Foxj1 knock-in mice can help to elucidate the mechanism underlying various clinical traits.
Acknowledgments
We would like to thank the patients and their family members for participating in this study.
Funding: The work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-27/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-27/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Shanghai Children’s Medical Center (SCMC) (No. SCMC-201015) and written informed consent was obtained from the parents of the child. Experiments were performed under a project license (No. SCMC-LAWEC-2023-005) granted by institutional Animal Care and Use Committee of SCMC, in compliance with SCMC institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wallmeier J, Nielsen KG, Kuehni CE, et al. Motile ciliopathies. Nat Rev Dis Primers 2020;6:77. [Crossref] [PubMed]
- Lucas JS, Davis SD, Omran H, et al. Primary ciliary dyskinesia in the genomics age. Lancet Respir Med 2020;8:202-16. [Crossref] [PubMed]
- Wallmeier J, Frank D, Shoemark A, et al. De Novo Mutations in FOXJ1 Result in a Motile Ciliopathy with Hydrocephalus and Randomization of Left/Right Body Asymmetry. Am J Hum Genet 2019;105:1030-9. [Crossref] [PubMed]
- Jin SC, Dong W, Kundishora AJ, et al. Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat Med 2020;26:1754-65. [Crossref] [PubMed]
- Shapiro AJ, Kaspy K, Daniels MLA, et al. Autosomal dominant variants in FOXJ1 causing primary ciliary dyskinesia in two patients with obstructive hydrocephalus. Mol Genet Genomic Med 2021;9:e1726. [Crossref] [PubMed]
- Kundishora AJ, Singh AK, Allington G, et al. Genomics of human congenital hydrocephalus. Childs Nerv Syst 2021;37:3325-40. [Crossref] [PubMed]
- Yu X, Ng CP, Habacher H, et al. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet 2008;40:1445-53. [Crossref] [PubMed]
- Blatt EN, Yan XH, Wuerffel MK, et al. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol 1999;21:168-76. [Crossref] [PubMed]
- Hackett BP, Brody SL, Liang M, et al. Primary structure of hepatocyte nuclear factor/forkhead homologue 4 and characterization of gene expression in the developing respiratory and reproductive epithelium. Proc Natl Acad Sci U S A 1995;92:4249-53. [Crossref] [PubMed]
- Lim L, Zhou H, Costa RH. The winged helix transcription factor HFH-4 is expressed during choroid plexus epithelial development in the mouse embryo. Proc Natl Acad Sci U S A 1997;94:3094-9. [Crossref] [PubMed]
- Brody SL, Yan XH, Wuerffel MK, et al. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 2000;23:45-51. [Crossref] [PubMed]
- Stauber M, Weidemann M, Dittrich-Breiholz O, et al. Identification of FOXJ1 effectors during ciliogenesis in the foetal respiratory epithelium and embryonic left-right organiser of the mouse. Dev Biol 2017;423:170-88. [Crossref] [PubMed]
- Chen J, Knowles HJ, Hebert JL, et al. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 1998;102:1077-82. [Crossref] [PubMed]
- Hagenlocher C, Walentek P, M, Ller C, et al. Ciliogenesis and cerebrospinal fluid flow in the developing Xenopus brain are regulated by foxj1. Cilia 2013;2:12. [Crossref] [PubMed]
- Beckers A, Adis C, Schuster-Gossler K, et al. The FOXJ1 target Cfap206 is required for sperm motility, mucociliary clearance of the airways and brain development. Development 2020;147:dev188052. [Crossref] [PubMed]
- Didon L, Zwick RK, Chao IW, et al. RFX3 modulation of FOXJ1 regulation of cilia genes in the human airway epithelium. Respir Res 2013;14:70. [Crossref] [PubMed]
- Bonnafe E, Touka M. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 2004;24:4417-27. [Crossref] [PubMed]
- Liang F, Wang B, Geng J, et al. SORBS2 is a genetic factor contributing to cardiac malformation of 4q deletion syndrome patients. Elife 2021;10:e67481. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Zhang X, Gao Y, Zhang X, et al. FGD5-AS1 Is a Hub lncRNA ceRNA in Hearts With Tetralogy of Fallot Which Regulates Congenital Heart Disease Genes Transcriptionally and Epigenetically. Front Cell Dev Biol 2021;9:630634. [Crossref] [PubMed]
- Nakae J, Kitamura T, Silver DL, et al. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 2001;108:1359-67. [Crossref] [PubMed]
- Reis LM, Sorokina EA, Dudakova L, et al. Comprehensive phenotypic and functional analysis of dominant and recessive FOXE3 alleles in ocular developmental disorders. Hum Mol Genet 2021;30:1591-606. [Crossref] [PubMed]
- Zhang M, Bolfing MF, Knowles HJ, et al. Foxj1 regulates asymmetric gene expression during left-right axis patterning in mice. Biochem Biophys Res Commun 2004;324:1413-20. [Crossref] [PubMed]
- Olstad EW, Ringers C, Hansen JN, et al. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr Biol 2019;29:229-241.e6. [Crossref] [PubMed]
- Wang C, Lv H, Ling X, et al. Association of assisted reproductive technology, germline de novo mutations and congenital heart defects in a prospective birth cohort study. Cell Res 2021;31:919-28. [Crossref] [PubMed]
- Giacomelli E, Meraviglia V, Campostrini G, et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 2020;26:862-879.e11. [Crossref] [PubMed]
- Papa A, Kushner J, Marx SO. Adrenergic Regulation of Calcium Channels in the Heart. Annu Rev Physiol 2022;84:285-306. [Crossref] [PubMed]
- Matyushenko AM, Levitsky DI. Molecular Mechanisms of Pathologies of Skeletal and Cardiac Muscles Caused by Point Mutations in the Tropomyosin Genes. Biochemistry (Mosc) 2020;85:S20-33. [Crossref] [PubMed]
- Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 2012;44:916-21. [Crossref] [PubMed]
- Brueckner M. Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation 2007;115:2793-5. [Crossref] [PubMed]
- Sakamoto K, Nakajima M, Kawamura K, et al. Ependymal ciliary motion and their role in congenital hydrocephalus. Childs Nerv Syst 2021;37:3355-64. [Crossref] [PubMed]


