
Plasma proteoglycan 4: a novel biomarker for acute lung injury after pediatric cardiac surgery
Highlight box
Key findings
• Proteoglycan 4 (PRG4) can be used as a biomarker for acute lung injury after pediatric cardiac surgery.
What is known and what is new?
• Patients with lower serum levels of PRG4 after surgery have lower lung compliance, higher oxygen demand and longer mechanical ventilation duration.
What is the implication, and what should change now?
• There is some evidence of the utility of PRG4 as biomarker for acute lung injury after cardiopulmonary bypass surgery.
IntroductionOther Section
The use of cardiopulmonary bypass (CPB) during surgical repair for infants with heart disease is associated with systemic inflammatory response syndrome (SIRS) and variable end organ injury (1-3). The SIRS response can have significant impact on lung injury, but the extent of the injury varies amongst patients and its mechanism is not fully understood (4,5).
Over the past decade, several plasma and bronchoalveolar lavage biomarkers for lung injury have been investigated and have provided a better understanding of the pathophysiological mechanism of lung injury (6). Although many of these biomarkers are awaiting validation, they have the potential of playing a prognostic and therapeutic role in caring for patients with lung injury (7).
Proteoglycan 4 (PRG4) is a mucin-like glycoprotein originally discovered in synovial fluid, and classically studied as a boundary lubricant, that is present throughout the body at various biointerfaces, tissues, and fluid. Its biological properties in modulating the inflammatory and innate immunologic response have been explored in recent studies (8-11), as well as its ability to modulate smooth muscle cell phenotype during vascular remodeling and intimal calcification (12). In terms of a systemic biomarker, altered levels of serum PRG4 were found to offer prognostic value for adult patients with chronic obstructive pulmonary disease (13).
In a recent pilot study, our group identified PRG4 as potential biomarker for prolonged mechanical ventilation in infants with complex cardiac surgery using both an unbiased proteomic analysis and enzyme-linked immunosorbent assay (ELISA) testing (14). In order to further validate our findings and to better correlate the PRG4 levels with respiratory and clinical outcomes we analyzed serum PRG4 levels in a larger cohort of infant patients before and after CPB. We hypothesized that decreased PRG4 plasma levels pre and postoperative were associated with longer mechanical ventilation duration, worse lung compliance and increased oxygen requirement after surgery. We present this article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-194/rc).
MethodsOther Section
Patient selection
All patients younger than 6 years old at Children’s of Alabama who underwent cardiac surgery were approached for participation in the congenital heart center biorepository under a separate institutional review board (IRB) approval. Our current study was reviewed and approved by the University of Alabama at Birmingham’s IRB (approval No. IRB-300003366; approval date February 02, 2022; study title: “Validation of Specific Serum Biomarkers Role as Prognostic Surrogates for Acute Lung Injury after Cardiac Surgery Requiring CPB in Pediatric Patients”) and informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients who had preoperative and 48-hour postoperative samples in this repository and had surgical repair for tetralogy of Fallot (TOF), ventricular septal defect (VSD) or atrioventricular septal defect (AVSD) at 1 year or younger were included in our study. Patients who required mechanical ventilation before surgery were excluded. We had a total of 86 patients from 2012 to 2020 who met the inclusion criteria and had available samples in the biorepository. We divided our studied patients into quartiles based on duration of mechanical ventilation. We had 21 patients in the control group (<25th percentile of mechanical ventilation duration) and 21 patients in the study group (>75th percentile of mechanical ventilation duration). We randomly selected 20 patients in each group and tested their plasma level for PRG4 at two time points, preoperative and 48-hour postoperative. We did not test the remaining samples of the remaining patients (n=46) who met the inclusion criteria. ELISA testing was conducted, in a blinded matter at the University of Connecticut Health Center under a research collaboration and biological material transfer agreement which was established on Jun 01, 2022 with award number 2025674. The fund of our study allowed us to test only 40 patients for PRG4 (two sample for each patient).
Plasma samples
Blood samples pre- and post-operative were collected by the bedside nurses and stored in heparinized tubes. Samples were handled by centrifugation and plasma was isolated and stored in the heart center biorepository in −80 ℃ centigrade freezer. One sample (250 µL) at each time point (preoperation and 48-hour postoperation) for each included patient (n=40 patients) was transferred to the University of Connecticut Health Center.
PRG4 ELISA analysis
PRG4 concentration in plasma samples was quantified using a commercially available sandwich ELISA (PRG4 DuoSet ELISA, DY9829-05, R&D Systems, Minneapolis, MN, USA), essentially as per the manufacturers’ instruction. The one important deviation was using full-length recombinant human PRG4 (rhPRG4, Lubris LLC, Framingham, MA, USA) (15,16) to generate the standard curve (1–1,000 µg/mL) instead of the supplied PRG4 peptide. Samples were assayed at 1:500 dilution, in technical duplicate, and 96-well plates were read at 450 nm on a microplate reader (i3x, Molecular Devices, San Jose, CA, USA). The plate-based ELISA assay employed here requires an overnight incubation step and then approximately 7 hours of work, which is a typical duration for bench ELISA analysis. However, PRG4 has also been quantified using a homogenous bead-based assay employing the AlphaLISA platform (Molecular Devices, Shelton, CT, USA) (17), which has the potential for high-throughput analysis of clinical samples.
Statistical analysis
Descriptive analyses [means, standard deviations, medians, interquartile ranges, frequency distributions (%)] were used to describe patient demographics, clinical characteristics, clinical outcomes and respiratory variables. Student’s t-tests and paired t-tests were used to compare continuous measures between groups as appropriate. Mann-Whitney U test were used to compare non-normal continuous measure and Chi-square, Fisher’s exact tests were used to compare categorical variables. Multivariable linear regression was performed for the prolonged mechanical ventilation outcome with adjustment for the weight and age at time of surgery. All hypothesis tests were two-tailed. A P value of <0.05 indicated statistical significance. All analyses were performed in SAS (version 9.4, Cary, NC, USA).
Clinical outcomes
The secondary outcomes of our analysis included intensive care unit (ICU) and hospital length of stay (day), duration of inotropic support (hours) rather than vasopressor inotrope score, duration of inotropic support was defined as the total duration of inotropic infusion start (or admission time if they are admitted on inotropic support) to the time of weaning infusion off. Lactate level at admission and creatinine level at post-operative days 1 and 2.
ResultsOther Section
Patient characteristics
Patients in the study group were younger (median 91 days) and had lower weight (mean 4.28 kg) at time of surgery (Table 1). There were more males in the control group compared to the study group (55.0% vs. 40.0%). The majority (65.0%) of our cohort were White. Only one patient in our cohort had genetic abnormality (trisomy 21 syndrome). There was no statistical difference in regard to CPB and aortic cross clamp time among groups. There were total of 20 patients in our cohort with AVSD (9 in control vs. 11 in study), 8 with VSD (7 in control vs. 1 in study), and 12 with TOF (4 in control and 8 in study). All our patients are admitted to the cardiac ICU (CICU) intubated and on mechanical ventilation. Patients are extubated after admission whenever they are ready from the respiratory and hemodynamic standpoint.
Table 1
| Characteristics | Control (n=20) | Study (n=20) | P value |
|---|---|---|---|
| Age (days) | 146 [120, 197] | 91 [39, 129] | 0.0013 |
| Weight (kg) | 5.19±0.98 | 4.28±1.34 | 0.0197 |
| Cardiopulmonary bypass (min) | 69.5 [55.0, 97.0] | 88.5 [67.0, 123.0] | 0.1635 |
| Aortic cross clamp (min) | 48.5 [36.5, 59.5] | 51.5 [45.0, 67.5] | 0.6649 |
| Gender, male | 11 (55.0) | 8 (40.0) | 0.3422 |
| Race, White | 13 (65.0) | 13 (65.0) | 0.9900 |
Data are presented as mean ± standard deviation, median [interquartile range] or n (%).
PRG4 plasma levels
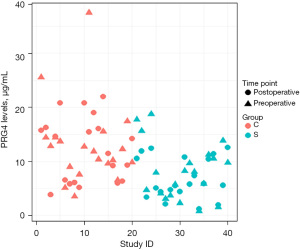
Preoperative and postoperative levels of PRG4 were significantly lower in the study group compared to control group (preoperative mean 8.15 vs. 13.01 µg/mL, P=0.0232; postoperative mean 6.88 vs. 12.25 µg/mL, P=0.0016). There was a trend toward lower levels of PRG4 in postoperative samples in the study group (6.88 vs. 8.15 µg/mL, P=0.0869); however, it was not statistically significant (Table 2, Figure 1).
Table 2
| Testing period | PRG4 plasma concentration (µg/mL) | P value (control vs. study) |
||
|---|---|---|---|---|
| Control (n=20) | Study (n=20) | Difference (control–study) | ||
| Pre-operative | 13.01±7.50 | 8.15±7.50 | 4.86±6.49 (0.70, 9.01) | 0.0232 |
| Post-operative | 12.25±5.97 | 6.88±3.76 | 5.37±4.99 (2.18, 8.57) | 0.0016 |
| Difference (pre-operative–post operative) |
0.76±7.40 (−2.72, 4.23) | 1.27±3.16 (−0.20, 2.75) | – | – |
| P value (pre- vs. post-operative) | 0.6500 | 0.0869 | – | – |
Data are presented as mean ± standard deviation (95% CI). P values are based on t-test or paired t-test as appropriate. PRG4, proteoglycan 4; CI, confidence interval.
Respiratory variables
The median duration of mechanical ventilation for subjects in the control group was 11.9 vs. 78.5 hours in the study group (P<0.0001). Patients in the study group had significantly lower lung compliance (median 2.1 vs. 3.1) and higher mean airway pressure (median of 10 vs. 9) at the admission time to the CICU following surgery (P=0.003 and 0.021, respectively). Patients in the control group required less oxygen support at admission time to the CICU and 4 hours after admission. The tidal volume was indifferent among groups and this can be related to the volume control mode of ventilation that is used widely in our center and is routinely set at 7 to 8 mL/kg (Table 3).
Table 3
| Variables | Control (n=20) | Study (n=20) | P value |
|---|---|---|---|
| Admission | |||
| FiO2 | 0.45 [0.40, 0.59] | 0.57 [0.46, 0.95] | 0.021 |
| PaO2 (mmHg) | 144 [106, 231] | 78 [65, 102] | <0.001 |
| PaO2/FiO2 ratio | 377.5 [237.8, 475.3] | 141.0 [70.7, 243.0] | <0.001 |
| MAP (cmH2O) | 9 [8, 9] | 10 [9, 11] | 0.021 |
| ETCO2 (mmHg) | 40.5 [36.3, 44.0] | 33.0 [30.5, 40.5] | 0.017 |
| Tidal volume (mL/kg) | 8.1 [7.6, 9.1] | 7.8 [7.3, 8.9] | 0.424 |
| Lung compliance (mL/mmHg) | 3.1 [2.7, 3.8] | 2.1 [1.4, 2.7] | 0.003 |
| 4 hours post-admission | |||
| FiO2 | 0.40 [0.30, 0.48] | 0.60 [0.40, 0.83] | 0.012 |
| PaO2 (mmHg) | 117.0 [84.8, 159.5] | 82.3 [55.2, 89.2] | <0.001 |
| PaO2/FiO2 ratio | 330.4 [180.4, 400.8] | 143.4 [64.9, 209.2] | <0.001 |
| Length of ventilation (hours) | 11.9 [10.1, 12.8] | 78.5 [55.6, 145.3] | <0.0001 |
Data are presented as median [interquartile range]. FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen in the arterial blood; MAP, mean airway pressure; ETCO2, end tidal carbon dioxide.
Clinical outcomes
In general, patients in the study group had worse clinical outcomes in comparison to patients in the control group. The CICU length of stay was significantly longer for patients in study group (median of 14.5 vs. 3.0 days, P<0.001). The hospital length of stay was longer for patients in the study group (median of 21.5 vs. 6.5 days, P<0.001). The duration of inotropic support was longer and serum lactate at admission were higher in the study group (8.5 vs. 1.0 hour, median of 2.5 vs. 1.4 mmol/L, P<0.001) (Table 4).
Table 4
| Outcome | Control (n=20) | Study (n=20) | P value |
|---|---|---|---|
| Lactate admission (mmol/L) | 1.4 [1.2, 1.6] | 2.5 [1.8, 3.4] | <0.001 |
| Creatinine POD1 (mg/dL) | 0.30 [0.29, 0.39] | 0.50 [0.39, 0.60] | <0.001 |
| Creatinine POD2 (mg/dL) | 0.26 [0.20, 0.30] | 0.40 [0.30, 0.56] | <0.001 |
| Duration of inotropes (hours) | 1.0 [1.0, 1.0] | 8.5 [4.3, 11.0] | <0.001 |
| CICU LOS (days) | 3.0 [2.0, 4.0] | 14.5 [8.3, 29.5] | <0.001 |
| HLOS (days) | 6.5 [5.0, 7.0] | 21.5 [11.0, 36.8] | <0.001 |
Data are presented as median [interquartile range]. POD, postoperative day; CICU, cardiovascular intensive care unit; LOS, length of stay; HLOS, hospital LOS.
PRG4 plasma levels as predictor of prolonged mechanical ventilation with adjustment for weight and age
Using logistic regression, we studied the ability of PRG4 plasma levels in predicting prolonged mechanical ventilation as shown in Table 5. For each 1 unit decrease in preoperative PRG4 level, there was 13.7% higher likelihood of prolonged mechanical ventilation [odds ratio: 0.863, 95% confidence interval (CI): 0.751–0.991, P=0.0374]. For each 1 unit decrease in postoperative PRG4 level, there was 20% higher likelihood of prolonged mechanical ventilation (odds ratio 0.804, 95% CI: 0.689–0.939, P=0.0060). After adjustment for weight and age, preoperative PRG4 levels were not able to predict prolonged mechanical ventilation (95% CI: 0.745–1.000, P=0.0580). However, each one unit decrease in the postoperative PRG4 level, there was 20% higher likelihood of prolonged mechanical ventilation (95% CI: 0.668–0.960, P=0.0167) as shown in Table 5. The receiver operative curve for postoperative PRG4 level in predicting prolonged mechanical ventilation after adjustment for weight and age is shown in Figure S1.
Table 5
| PRG4 levels | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Preoperative | 0.863 | 0.751–0.991 | 0.0374 |
| Adjusted preoperative | 0.865 | 0.745–1.000 | 0.0580 |
| Postoperative | 0.804 | 0.689–0.939 | 0.0060 |
| Adjusted postoperative | 0.801 | 0.668–0.960 | 0.0167 |
Logistic regression. For each 1 unit decrease in PRG4 level, there is 13.7% and 20% higher chance of having prolonged mechanical ventilation for preoperative and postoperative levels respectively. After adjustment for weight and age, for each 1 unit decrease in postoperative PRG4 levels, there is 20% higher chance of having prolonged mechanical ventilation. PRG4, proteoglycan 4; CI, confidence interval.
DiscussionOther Section
Recent evidence suggests a role of PRG4 in regulating the inflammatory response in variable organs including the lung. PRG4 can bind to toll like receptors and mediate the anti-inflammatory response to injury (8,10,18). Additionally, PRG4 may have a critical role in maintaining tissue hemostasis as well. Proteoglycan depositions were found to contribute to lung pathology and variable serum levels were correlated with the disease prognosis for patients with chronic obstructive pulmonary disease (13-19). Recently, PRG4 was found to play a protective role in prevention adhesions after cardiotomy and loss of PRG4 in the pericardial fluids after cardiotomy might had induced adhesion formation (20).
Our prior pilot study suggested a correlation between low PRG4 plasma levels with long mechanical ventilation in pediatric patients following CPB (21). This comparative study between patients with and without prolonged mechanical ventilation supports the findings of our pilot study as well as correlates lower serum levels of PRG4 with worse lung compliance, higher mean airway pressure and increased need for supplemental oxygen.
In addition, patients with lower PRG4 plasma levels had a longer hospital stay and longer duration of inotropic support. The length of CPB and aortic cross clamp was indifferent among the two groups and there was no difference statistically when we compared the postoperative PRG4 levels to the preoperative levels in both groups. This finding leads us to speculate about the possibility of a lower serum PRG4 level predicting prolonged mechanical ventilation postoperatively. Lower levels of PRG4 in the study group may suggest a native predisposition for adverse lung injury after cardiac surgery rather than the exposure to CPB.
At this stage and as evident in our study, lower levels of PRG4 are correlated with worse clinical and respiratory outcomes. We were limited by the number of patients that had matching cardiac defects and available samples in our study; thus, we were not able to discover the association of longer CPB time with the postoperative levels of PRG4. Our study did not investigate the association of different cardiac lesions with the length of mechanical ventilation. We divided our patients based on the length of mechanical ventilation rather than the cardiac lesion. Thus, we are unable to have any conclusion relating to the association of cardiac defect and PRG4 levels. In addition, we were unable to discover the impact of certain demographic characteristics on the PRG4 levels. Study group had lower postoperative levels of PRG4 compared to preoperative levels, but that was statistically insignificant. The duration of inotropic support was clinically and statistically longer in the study group, due to the retrospective nature of our study, we are unable to determine if this hemodynamic variation is related to acute lung injury or heart failure or both.
ConclusionsOther Section
Following cardiac surgery, infants with prolonged mechanical ventilation had lower levels of plasma PRG4. Lower levels of PRG4 were associated with worse ventilatory and clinical outcomes. PRG4 level may have prognostic value in regard to acute lung injury for infants with cardiac surgery. We plan to validate our finding and further explore the association of PRG4 levels with worse outcomes in a larger and more diverse group. Testing PRG4 levels in other bodily fluids might shine the light on the pathophysiological role of PRG4 in adverse lung injury.
AcknowledgmentsOther Section
Funding: Funding for this study was provided through
FootnoteOther Section
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-194/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-194/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-194/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-194/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the University of Alabama at Birmingham Institutional Review Board (approval No. IRB-300003366) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Vohra HA, Whistance R, Modi A, et al. The inflammatory response to miniaturised extracorporeal circulation: a review of the literature. Mediators Inflamm 2009;2009:707042. [Crossref] [PubMed]
- Giacinto O, Satriano U, Nenna A, et al. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat Inflamm Allergy Drug Discov 2019;13:158-73. [Crossref] [PubMed]
- Kirklin JK. Prospects for understanding and eliminating the deleterious effects of cardiopulmonary bypass. Ann Thorac Surg 1991;51:529-31. [Crossref] [PubMed]
- Monteverde E, Fernández A, Poterala R, et al. Characterization of pediatric patients receiving prolonged mechanical ventilation. Pediatr Crit Care Med 2011;12:e287-91. [Crossref] [PubMed]
- Gaies M, Tabbutt S, Schwartz SM, et al. Clinical Epidemiology of Extubation Failure in the Pediatric Cardiac ICU: A Report From the Pediatric Cardiac Critical Care Consortium. Pediatr Crit Care Med 2015;16:837-45. [Crossref] [PubMed]
- Bhargava M, Wendt CH. Biomarkers in acute lung injury. Transl Res 2012;159:205-17. [Crossref] [PubMed]
- Barnett N, Ware LB. Biomarkers in acute lung injury--marking forward progress. Crit Care Clin 2011;27:661-83. [Crossref] [PubMed]
- Das N, Schmidt TA, Krawetz RJ, et al. Proteoglycan 4: From Mere Lubricant to Regulator of Tissue Homeostasis and Inflammation: Does proteoglycan 4 have the ability to buffer the inflammatory response? Bioessays 2019;41:e1800166. [Crossref] [PubMed]
- Iqbal SM, Leonard C, Regmi SC, et al. Lubricin/Proteoglycan 4 binds to and regulates the activity of Toll-Like Receptors In Vitro. Sci Rep 2016;6:18910. [Crossref] [PubMed]
- Alquraini A, Garguilo S, D'Souza G, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther 2015;17:353. [Crossref] [PubMed]
- Menon NG, Suhail Y, Goyal R, et al. Recombinant Human Proteoglycan 4 (rhPRG4) Downregulates TNFα-Stimulated NFκB Activity and FAT10 Expression in Human Corneal Epithelial Cells. Int J Mol Sci 2022;23:12711. [Crossref] [PubMed]
- Seime T, Akbulut AC, Liljeqvist ML, et al. Proteoglycan 4 Modulates Osteogenic Smooth Muscle Cell Differentiation during Vascular Remodeling and Intimal Calcification. Cells 2021;10:1276. [Crossref] [PubMed]
- Lee KY, Chuang HC, Chen TT, et al. Proteoglycan 4 is a diagnostic biomarker for COPD. Int J Chron Obstruct Pulmon Dis 2015;10:1999-2007. [PubMed]
- Asfari A, Hock KM, Byrnes JW, et al. Biomarkers for Adverse Lung Injury Following Pediatric Cardiopulmonary Bypass. Crit Care Explor 2021;3:e0528. [Crossref] [PubMed]
- Samsom ML, Morrison S, Masala N, et al. Characterization of full-length recombinant human Proteoglycan 4 as an ocular surface boundary lubricant. Exp Eye Res 2014;127:14-9. [Crossref] [PubMed]
- Lambiase A, Sullivan BD, Schmidt TA, et al. A Two-Week, Randomized, Double-masked Study to Evaluate Safety and Efficacy of Lubricin (150 μg/mL) Eye Drops Versus Sodium Hyaluronate (HA) 0.18% Eye Drops (Vismed®) in Patients with Moderate Dry Eye Disease. Ocul Surf 2017;15:77-87. [Crossref] [PubMed]
- Das N, Menon NG, de Almeida LGN, et al. Proteomics Analysis of Tears and Saliva From Sjogren's Syndrome Patients. Front Pharmacol 2021;12:787193. [Crossref] [PubMed]
- Melrose J. A Perspective on the Potential Utility of a Viscosupplement Multifunctional Biotherapeutic: Proteoglycan-4: From mere lubricant to regulator of tissue homeostasis and inflammation. Bioessays 2019;41:e1800215. [Crossref] [PubMed]
- Bensadoun ES, Burke AK, Hogg JC, et al. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:1819-28. [Crossref] [PubMed]
- Park DSJ, Regmi SC, Svystonyuk DA, et al. Human pericardial proteoglycan 4 (lubricin): Implications for postcardiotomy intrathoracic adhesion formation. J Thorac Cardiovasc Surg 2018;156:1598-1608.e1. [Crossref] [PubMed]
- Carey RJ, Kenney S. Operant conditioning and haloperidol-induced hypokinetic effects. Neuropsychobiology 1987;18:199-204. [Crossref] [PubMed]


