
Newborn ornithine carbamyltransferase deficiency caused by new OTC gene mutations: a report of two cases and review of the literature on phenotype and genotype
Highlight box
Key findings
• The study identified two new mutations related to ornithine carbamyltransferase deficiency (OTCD), alongside exploring further the clinical and biochemical characteristics of neonatal OTCD patients.
What is known and what is new?
• OTCD is a common urea cycle disorder, with increased ammonia levels, decreased citrulline concentration, and poor prognosis being known characteristics.
• This manuscript discovers two new mutation sites associated with OTCD, expanding the genetic landscape of the disease.
What is the implication, and what should change now?
• These findings urge enhanced genetic testing protocols to include the newly found mutations and further research to understand their impact on OTCD’s progression and severity.
Introduction
Ornithine carbamyltransferase deficiency (OTCD; MIM # 311250) is the most common urea cycle disorder disease. It is an X-linked disease caused by the lack of ornithine carbamyltransferase (OTC), with an incidence rate of 1/14,000 live births (1) OTC catalyzes the conversion of ornithine and carbamoyl phosphate into citrulline in mitochondria. The lack of OTC results in the accumulation of ammonia, and excess stored ammonia leads to nervous system defects (2). The human OTC gene (NM_000531.5) is located in Xp21.1 and contains 10 exons and nine introns, with a total length of 73 Kb. More than 400 pathogenic mutations have been reported, most of which are missense mutations (3). The present study reports two cases of neonatal OTCD caused by new OTC gene mutations and reviewed the literature on the phenotype-genotype correlation between clinical and biochemical characteristics and OTC gene mutation sites of reported neonatal OTCD cases at home and abroad. Among the neonatal OTCD children retrieved in this study, the mortality rate was as high as 67%, and more than 50% of the survivors had neurological sequelae. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-437/rc).
Case presentation
Research object
The subjects of the study were two neonates recently diagnosed with OTCD and treated at Shenzhen Children’s Hospital. Data on the clinical manifestations, auxiliary examination, and treatment of these two patients were collected.
Research methods
Blood tandem mass spectrometry (MS/MS) and urine gas chromatography-mass spectrometry (GC/MS)
Blood samples were collected by dry filter paper; blood amino acids and acylcarnitine were analyzed by blood tandem MS/MS; and urine was collected and urine organic acids were analyzed by urine GC/MS. The blood tandem MS/MS and urine gas phase mass spectrometry analyses were completed by a third-party testing company.
Gene sequencing and data analysis
Four mL of peripheral blood was taken from the children and their parents and placed in an ethylene diamine tetraacetic acid (EDTA) anticoagulant tube, which was gently turned upside down and mixed 6–10 times. The sample was then sent to a third-party testing company. The whole exon group gene sequencing was performed on the case 1 sample, and 5,177 related genes were detected on the case 2 sample by high-precision clinical exon sequencing. The bioinformatics analysis of offline raw data included data quality control, filtering, and annotation. Finally, the detected mutation sites were screened to determine the gene sites related to the clinical phenotype of the children and classify the mutations into five categories (pathogenic, possibly pathogenic, clinically ambiguous, possibly benign, and benign) according to the international standards issued by the American Society of Medical Genetics and Genomics (ACMG), ClinGen, etc. (4).
Verification of gene mutation pedigree
DNA extraction
Human genomic DNA was extracted using QIAGEN’s whole blood DNA extraction kit (Germany). Please refer to the kit instructions for the specific procedures.
Primer design, polymerase chain reaction (PCR), and first-generation verification experiment
According to the sequencing results of the whole exon group of children, the OTC gene sequence in the GeneBank database and Primer Premier 5.0 software (Premier Biosoft, United States) were used to design primers for the OTC gene mutation sites, and National Center of Biotechnology Information (NCBI) Primer Blast was used to verify the primer specificity. The primer sequence is shown in Table 1. PCR was performed using the TaKaRa Ex Taq Hot Start kit of Dalian Bao Biological Co., Ltd. (Dalian, China), with a 50 µL mixed reaction system: including Ex Taq HS 1.25U, 10× Ex Taq Buffer 5 µL, deoxynucleotide triphosphate (dNTP) Mixture (2.5 mM each) 4 µL, OTC primer F 0.4 µM, OTC primer R 0.4 µM, and DNA template (50–200 ng), supplemented with sterile water to 50 µL. The PCR amplification conditions were as follows: 98 ℃ 10 s, (98 ℃ 10 s, 58 ℃ 30 s, and 72 ℃ 30 s) for 39 cycles, 72 ℃ 2 min, 4 ℃ at all times. After 2% agarose gel electrophoresis, the PCR amplification product was clearly the size of the target amplicon, and the band was bright, without obvious non-specific amplification. The PCR amplification product was then sent to Sangon Biotech (Shanghai, China) for the first-generation sequencing experiment and was finally identified using the ABI3730/3730XL gene analyzer (Applied Biosystems, Foster City, California, United States).
Table 1
| Primer name | Primer sequence |
|---|---|
| OTC primer F | 5’-GCTCCTTTGTCTCTCCCTTTGC-3’ |
| OTC primer R | 5’-TCCTCCATTCCTTGTTTCTTGC-3’ |
PCR, polymerase chain reaction; OTC, ornithine carbamyltransferase.
Results
Medical history collection results
Case 1: male, 3 days old, Gravida 1 Para 1, 39+6 weeks of pregnancy, delivered spontaneously, amniotic fluid was I degree polluted, birth weight 3,550 g, and the Apgar scores at 1, 5, and 10 min after birth were 8, 9, and 9, respectively. This patient was admitted to the hospital because of “decreased muscle tone for 3 days, dyspnea for 10 hours, and convulsions twice”.
Physical examination on admission: body temperature 37.7 ℃, heart rate 157 times/min, moderate yellowing of skin on the whole body, no rash, swelling of the limbs, visible mottling, slow light reflex, machine-controlled breathing, rough breathing sounds in both lungs; rough moist rales could be heard, and muscle tension in the limbs was low.
Biochemistry: blood ammonia 2,518.4 µmol/L, blood potassium 6.37 mmol/L, lactic acid 10.18 mmol/L, aspartate aminotransferase 122 IU/L, alanine aminotransferase 32 IU/L, creatine kinase isoenzyme 5.2 ng/mL, and troponin I 1.27 ng/mL.
Color ultrasound of the head: abnormal acoustic image of brain parenchyma, indicating brain injury, and reduced cerebral blood flow perfusion.
Color Doppler echocardiography: myocardial thickening, increased right atrial pressure index, and normal left ventricular overall systolic function index.
Electroencephalogram (EEG): severely abnormal newborn, flat wave.
Treatment: after admission, the patient received machine-assisted-assisted ventilation, continuous renal replacement therapy (CRRT), meropenem combined with penicillin to fight infection, fasting, blue light irradiation to reduce yellowing, fluid replacement to maintain stable blood glucose, arginine to reduce blood ammonia, and L-carnitine to supplement carnitine. During CRRT, the patient’s blood pressure was unstable, and a continuous intravenous infusion of dopamine, dobutamine, epinephrine, and norepinephrine was administered to maintain circulatory blood pressure. Also, plasma and cryoprecipitate were given as needed, and red blood cell transfusion was given to supplement blood volume. The blood ammonia and potassium of the patient gradually decreased, and his consciousness was still in a deep coma. Dynamic monitoring of the EEG showed flat waves. The parents gave up treatment 3 days later, and the child died 3 hours after.
Case 2: male, 3 days old, G7P5, 38+2 weeks of pregnancy delivered spontaneously, premature rupture of membranes for 2 hours, umbilical cord around the neck for 3 weeks, birth weight 3,300 g, and the Apgar scores at 1, 5, and 10 min after birth were 10 points, respectively. On the first day after birth, the patient had shortness of breath, foam vomited at the mouth, accompanied by groans, apnea, and cyanosis. The local hospital could not relieve the symptoms, and he was admitted to our hospital with “heart failure”.
Physical examination on admission: body temperature 37.1 ℃, heart rate 147 times/min, coma, no response to stimulation, mild yellowing of the skin, equal roundness of bilateral pupils, slightly slow reflection to light, rough breathing sound in both lungs, dry rales could be heard, and muscle tension was low.
EEG: neonate with a severe abnormality.
Chest and abdomen radiographs: pneumonia, slightly enlarged heart shadow, increased lung markings, increased abdominal intestinal inflation, and slightly expanded local intestines.
Head CT: small pieces of slightly high-density shadow in the bilateral occipital lobe, bleeding was not excluded. Combined with the patient’s medical history, OTCD was considered but ischemic hypoxic encephalopathy (HIE) was not excluded.
Head magnetic resonance imaging (MRI) + diffusion-weighted imaging (DWI) + magnetic resonance spectroscopy (MRS): abnormal signals in the brain with some cystic malacia, which, combined with MRS, could conform to the imaging manifestations of OTCD. A small amount of subdural hematoma in the left occipital region (subacute phase) was also observed.
Cardiac color ultrasound: newborn patent ductus arteriosus (restricted type considered), and lower limit of normal left ventricular systolic function index (EF56%).
Brain color ultrasound: brain edema-like sound image, right choroid plexus cyst.
Color Doppler ultrasound of liver, gallbladder, pancreas, and spleen: abnormal sound image in the gallbladder, indicating bile mud, and the liver was diffusely enlarged.
Color ultrasound of urinary system: mild dilatation of the bilateral renal pelvis.
Treatment: after admission, the patient received respirator-assisted ventilation, oxygen inhalation in the box, and other respiratory support. In addition, he also received meropenem, vancomycin, and ceftriaxone for anti-infection treatment. After checking blood ammonia, which was 1,550.1 µmol/L, he was immediately given CRRT treatment. The patient also received arginine, L-carnitine, citrulline, and other anti-hyperammonemia treatments; jejunitis, nasal feeding with sugar water, intravenous nutrition maintenance (high sugar, high calorie, low amino acid) supplementation. During this period, red blood cell suspension, plasma, cryoprecipitate, platelet, and albumin were transfused repeatedly to correct coagulation function, anemia, hypoproteinemia, etc. Additionally, the patient also received fentanyl, midazolam, and other sedatives; vascular active drugs such as dopamine, dobutamine, and adrenal gland maintain blood pressure; and blue light irradiation to reduce bilirubin, positively correct the electrolyte disorder, maintain the internal environment stability, and other symptomatic treatments.
After 12 days of CRRT treatment, the blood ammonia level of the child dropped to a minimum of 122 µmol/L. After CRRT was stopped, when the blood ammonia fluctuated below 500 µmol/L, the child could open his eyes and was conscious, but vomiting frequently occurred. See Figure 1 for the changes in blood ammonia during the treatment. On the 21st day after admission, the child developed a fever, and re-examination of infection indicators and blood ammonia increased significantly. Subsequently, the child developed coma, limb shaking, sobbing breathing, mottled skin, and other symptoms and died 6 days later.
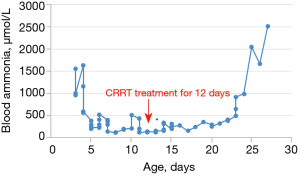
Family history: all three generations of the mother’s family had a history of premature death among boys after birth. The child had three healthy sisters and one brother that had died 3 days after birth.
Blood tandem MS/MS and urine gas mass spectrometry analyses
The results of case 1 blood tandem MS/MS showed decreased citrulline concentration (4.31 µM, reference value: 5.00–30.00 µM), while the concentrations of alanine (1,639.67 µM, reference value: 100.00–450.00 µM), glutamate (505.45 µM, reference value: 75.00–300.00 µM), methionine (212.46 µm; reference value: 9.50–45.00 µM), tyrosine (384.77 µm; reference value: 25.00–250.00 µM), leucine (317.89 µm; 60.00–250.00 µM), glutamine (137.59 µm, reference value: 1.50–85.00 µM), and other amino acids increased. The results of urine GC/MS showed that orotic acid was significantly increased (203.5 µM, reference value: 0.0–2.0 µM), and uracil was normal (7.1 µM, reference value: 0.0–8.0 µM). The concentration of citrulline in MS/MS test results was reduced, and the combination of GC/MS results showed that orotic acid was significantly increased, suggesting that ornithine aminotransferase deficiency was likely.
The MS/MS results in case 2 showed that the citrulline concentration (1.96 µM) decreased, while the concentrations of alanine (1.63.72 µM), glutamate (386.25 µM), methionine (170.68 µm), and tyrosine (371.92 µM) increased. The GC/MS results showed that orotic acid increased by 5.2 µM, while MS/MS test results showed that the citrulline concentration decreased, combined blood ammonia increased, and urea circulation disorders should be considered.
Gene sequencing and family verification results
Case 1: The statistical results of the clinical full exon detection data showed that the proportion of the Q30 base was 90.56%, the coverage of the target region was 99.84%, the average sequencing depth of the target region was 104X, the proportion of the average depth of the target region >20X site was 98.41%, and the sequencing data quality control was qualified. The missense mutation of c.961T>C (p. Ser321Pro) in exon 9 of the OTC gene was detected, and the sequencing depth of the half-zygote mutation site was 60X. The parental Sanger verification results showed that the mutation was inherited from the mother (Figure 2), who was a heterozygote and conformed to the X-linked genetic model.

The (NM_000531) c.961T>C mutation of the OTC gene has not been reported in the Human Gene Mutation Database (HGMD) or PubMed databases, which is a newly discovered mutation site. So far, the median gene frequency of the mutation in the normal population gene database (gnomAD database, 1,000 genome database, ExAC database, etc.) is 0. The mutation is located in the aspartate/ornithine binding domain, and the amino acid sequence of different species is highly conservative. SIFT, Polyphen2, MutationTaster, PROVEAN, and other software programs are more likely to predict that the mutation affects the protein structure/function. Combined with the clinical manifestations and test results of the submitter and according to the American ACMG variation classification guidelines, this variation is likely to cause disease.
Case 2: The statistical results of clinical full exon detection data showed that the average sequencing depth of the target area was 301±87X, the coverage area greater than 10X accounted for 99.9%, the coverage area greater than 20X accounted for 99.8%, and the sequencing data quality control was qualified. We detected that a short interspersed nuclear element (SINE) with a length of about 500 bp was inserted into exon 5 of the OTC gene (NM_000531) in the child, which was inherited from the mother. Fluorescent quantitative PCR (qPCR) was used to verify the mutation (Figure 3). The results showed that the relative expression amount (RQ value) of exon 5 of the OTC gene between the child and his mother was reduced, while the father of the child was normal. This mutation has not been reported in the HMGD, PubMed, or reference population databases; it is a newly discovered mutation site. Combined with the clinical manifestations and detection results of the examinee and according to the American ACMG variation classification guidelines, this mutation was rated as possibly pathogenic.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients’ parents or legal guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
OTCD is an X-linked recessive genetic disease. Male OTC hemizygous mutations are OTCD patients; due to the random inactivation of the X chromosome, a small number of female OTC heterozygous mutations can manifest as an OTCD phenotype. OTCD patients are divided into two types according to the age of onset: neonatal type and late-onset type (age of onset >28 days). Neonatal-type cases usually involve a serious condition, including the complete loss of OTC enzyme activity, and are mostly male hemizygote mutations (rarely female) (5). Neonatal children are asymptomatic at birth but have symptoms of hyperammonemia for hours or days, and their condition often worsens when they go to the hospital.
The clinical manifestations of the two cases of neonatal OTCD children reported in this study were quite different. Case 1 developed hypotonia within 1 day after birth, followed by dyspnea and convulsions, while case 2 began to develop dyspnea, heart failure, hypotonia, coma, etc. 1 day after birth. A total of 10 articles related to neonatal OTCD were retrieved from domestic journals, involving a total of 13 cases of neonatal OTCD (all males). Of these, six cases had a poor response, six cases had convulsions, four cases had shortness of breath/dyspnea, two cases had feeding difficulties, one case had vomiting, one case had coma, and one case had hypotonia. Lu et al. (6) performed a large sample single-center follow-up study of Chinese OTCD patients. Among 63 OTCD patients in their study, 15 were neonatal, including 13 males and two females. The median age of onset was 3 days (range, 1–27 days), and the common clinical manifestations were coma (10 cases), somnolence (10 cases), vomiting (7 cases), epilepsy (5 cases), etc. Therefore, it can be seen that the clinical manifestations of neonatal OTCD lack specificity. Gastrointestinal tract and nervous system symptoms are more common, and this condition is easily misdiagnosed as other diseases with nervous system symptoms, such as hypoxic-ischemic encephalopathy, intracranial infection, epilepsy, and other causes of hyperammonemia (7,8).
The diagnosis of OTCD is mainly based on clinical manifestations, blood ammonia, blood amino acid, urine organic acid detection, imaging examination, gene detection, etc. (9). Elevated blood ammonia is a common abnormal indication of OTCD; however, this is not a specific indication. Blood ammonia may be normal during the interval between onset and cannot be distinguished from other genetic metabolic diseases that cause hyperammonemia. MRI and other imaging findings also lack specificity. Diffuse brain edema as well as multiple and asymmetric abnormal signals are common in the acute phase. The MS/MS test results of typical OTCD patients mostly indicate that citrulline is reduced, while GC/MS test results show that orotic acid is significantly increased, which may be accompanied by an increase in uracil (9). However, a study has shown that lower citrulline levels are not a reliable marker for neonatal OTCD screening (10). Peng et al. (11) reported that the false positive rate of screening OTCD using citrulline level as a marker was high when using MS/MS for neonatal genetic metabolic disease screening. Staretz-Chacham et al. (12) showed that the combined screening of neonatal blood citrulline and urinary orotic acid levels can increase the sensitivity of OTCD screening. According to the latest diagnostic and management guidelines for urea circulation disorders published abroad, gene testing is strongly recommended for the diagnosis of OTCD. When gene testing is negative, it is recommended to measure the activity of OTC enzymes in the plasma, liver, or intestinal mucosa. However, due to the random inactivation of the X chromosome, the detection results of OTC enzyme activity cannot fully reflect the true enzyme activity of female heterozygotes (8).
The statistical results of this study on neonatal OTCD cases at home and abroad also showed that gene detection plays an important role in the diagnosis of OTCD. A total of 62 neonatal OTCD cases were retrieved. About 81% (39/48) of the patients had decreased serum citrulline concentration, about 91% (42/46) of the patients exhibited significantly increased urinary orotic acid, about 39% (18/46) of the patients could be accompanied by increased uracil, and 100% (51/51) of the patients had OTC gene mutations, which were mainly missense mutations, followed by nonsense mutations, frameshift mutations, splice site mutations, and exon deletion/duplication mutations, These mutations were mainly concentrated in exons 5, 6, 8, and 9.
The clinical manifestations of neonatal OTCD are not specific. For suspected patients, blood amino acid detection and urine organic acid detection should be performed as soon as possible, and combined with gene detection to make a clear diagnosis (9). Reviewing the diagnostic processes applied in the two cases in this study showed that they were consistent with the current mainstream diagnostic processes of OTCD at home and abroad. The two cases of neonatal OTCD had neurological symptoms and elevated blood ammonia in the acute stage of onset, suggesting metabolic disease. MS/MS indicated that citrulline concentration was reduced and GC/MS showed that orotic acid was significantly increased, and OTCD was highly suspected. Therefore, we performed gene testing on the children and Sanger verification on their parents. We found that the newly discovered c.961T>C missense mutation and SINE element insertion mutation were both inherited from their mothers, and finally diagnosed them with OTCD.
OTC gene mutation is an important basis for the diagnosis of OTCD. More than 500 types of OTC gene pathogenic mutations have been reported, of which 64.0% are missense mutations or nonsense mutations, 10.0% are splice mutations, 13.2% are small deletion or insertion mutations, 9.8% are large deletion or duplication mutations, and 1.3% are complex mutations, with high genetic heterogeneity (9). The two newly discovered OTC gene mutation sites reported in this study not only enrich our understanding of the mutation sites and types of the OTC gene but also play an important role in the clear diagnosis of OTCD combined with its clinical phenotypic characteristics.
The prognosis of OTCD patients is related to many factors, including the age of onset, early diagnosis, duration and peak value of hyperammonemia, and treatment measures. The prognosis of neonatal OTCD children is poor, and the mortality rate is as high as 43.4–74% (13). The 1-year survival rate of newborn male patients is about 40%, most of whom are stunted, and only 15% of them are normal after 1 year. Furthermore, the 1-year survival rate of female patients is also relatively high (about 57%) (5). Among the 62 cases of neonatal OTCD children retrieved in this study, the mortality rate of the 57 cases with known prognosis was as high as 67%, and more than 50% of the survivors had neurological sequelae such as mental retardation. Although the prognosis of neonatal OTCD children is poor, research shows that early diagnosis and treatment are expected to improve the survival rate of neonatal-type patients (14).
Treatment in the acute phase of OTCD is mainly aimed at limiting protein intake, intravenous arginine or sodium benzoate, hemodialysis, etc., to reduce blood ammonia and provide nutritional support (such as glucose solution and fat emulsion). Although the blood ammonia of our two patients decreased after active CRRT and other comprehensive treatments, they died after giving up due to severe hyperammonemia and serious nervous system damage in the early stage of the disease.
Conclusions
In summary, the clinical manifestations of neonatal OTCD patients lack specificity, and the early diagnosis of OTCD is of great significance for treatment and prognosis. Clinically, in addition to improving routine biochemical tests, blood MS/MS and urine GC/MS analysis should also be carried out as soon as possible for patients with high blood ammonia of unknown causes. If the blood citrulline level is reduced or normal and the urine orotic acid level is significantly increased, or the blood citrulline and urine orotic acid are normal but OTCD is highly suspected, the diagnosis can be made by combining gene testing. To increase the mutation detection ratio in the OTC gene, we can provide more accurate clinical and biochemical data of patients as soon as possible and supplement them in a timely manner in the future. Considering the poor prognosis of OTCD, the authors believe that in addition to improving gene detection as soon as possible, we can also improve the sensitivity and specificity of blood MS/MS and urine GC/MS analysis techniques to screen neonatal OTCD and intervene before serious symptoms appear. For families of probands with clear OTCD diagnosis, Sanger sequencing technology can be used to detect the carrying of pathogenic sites of family members as well as diagnose and intervene in patients with pre-symptoms in the family. At the same time, family members who are at risk of having children with OTCD will be given birth guidance, and prenatal diagnosis or preimplantation genetic diagnosis will be used to avoid the birth of such fetuses.
Acknowledgments
Funding: This study was supported by the Basic Research Program from Shenzhen Science and Techno1ogy Innovation Commission (No. JCYJ20180228175137465).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-437/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-437/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-437/coif). All authors report funding support from the Basic Research Program from Shenzhen Science and Techno1ogy Innovation Commission (No. JCYJ20180228175137465), and royalties or licenses for the TaKaRa Ex Taq Hot Start kit of Dalian Bao Biological Co., Ltd. (Dalian, China), and the first-generation sequencing experiment in Sangon Biotech (Shanghai, China). Fuman Jiang is from Shenzhen Jingke Genetic Science and Technology Co. Ltd, Shenzhen, China. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lichter-Konecki U, Caldovic L, Morizono H, et al. Ornithine Transcarbamylase Deficiency. 2013 Aug 29 [updated 2022 May 26]. In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023.
- Morris SM Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr 2002;22:87-105. [Crossref] [PubMed]
- Caldovic L, Abdikarim I, Narain S, et al. Genotype-Phenotype Correlations in Ornithine Transcarbamylase Deficiency: A Mutation Update. J Genet Genomics 2015;42:181-94. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Burgard P, Kölker S, Haege G, et al. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders--review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis 2016;39:219-29. [Crossref] [PubMed]
- Lu D, Han F, Qiu W, et al. Clinical and molecular characteristics of 69 Chinese patients with ornithine transcarbamylase deficiency. Orphanet J Rare Dis 2020;15:340. [Crossref] [PubMed]
- Yang Y, Sun F, Qian N, et al. Clinical and laboratory screening study of urea circulation disorders. Chinese Journal of Pediatrics 2005;5:331-4.
- Häberle J, Burlina A, Chakrapani A, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis 2019;42:1192-230. [Crossref] [PubMed]
- Kong Y, Han L, Yang Y, et al. Expert consensus on diagnosis and treatment of ornithine carbamyltransferase deficiency. J Zhejiang Da Xue Xue Bao Yi Xue Ban (Medical Edition) 2020;5:539-47. [Crossref] [PubMed]
- Cavicchi C, Malvagia S, la Marca G, et al. Hypocitrullinemia in expanded newborn screening by LC-MS/MS is not a reliable marker for ornithine transcarbamylase deficiency. J Pharm Biomed Anal 2009;49:1292-5. [Crossref] [PubMed]
- Peng G, Tang Y, Gandotra N, et al. Ethnic variability in newborn metabolic screening markers associated with false-positive outcomes. J Inherit Metab Dis 2020;43:934-43. [Crossref] [PubMed]
- Staretz-Chacham O, Daas S, Ulanovsky I, et al. The role of orotic acid measurement in routine newborn screening for urea cycle disorders. J Inherit Metab Dis 2021;44:606-17. [Crossref] [PubMed]
- Unsinn C, Das A, Valayannopoulos V, et al. Clinical course of 63 patients with neonatal onset urea cycle disorders in the years 2001-2013. Orphanet J Rare Dis 2016;11:116. [Crossref] [PubMed]
- Kido J, Nakamura K, Mitsubuchi H, et al. Long-term outcome and intervention of urea cycle disorders in Japan. J Inherit Metab Dis 2012;35:777-85. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


