
Effects of urinary tract infection during the first years of life in subsequent growth: a nationwide comparative matched cohort study
Highlight box
Key findings
• History of urinary tract infection (UTI) during infancy is associated with high body mass index measured at 30–36 months.
What is known and what is new?
• While several childhood illnesses have been known to affect growth status, the impact of UTI on subsequent childhood growth has not been well elucidated.
• In this nationwide population-based matched cohort study involving 84,519 children with a history of UTI during infancy and an equal number of controls, the height differences were statistically insignificant, but the body mass index standard deviation score was significantly higher among children who had UTI.
What is the implication, and what should change now?
• The findings suggest that UTI during infancy might be associated with a higher body mass index in early childhood, potentially indicating a long-term impact on children’s growth, particularly their weight.
Introduction
Urinary tract infection (UTI) is among the most common bacterial infections in children (1,2). The prevalence of UTIs is approximately 7.0% in febrile infants aged <24 months and 8% in older children (3). In general, UTIs are considered to follow a benign course, but complications may develop, such as sepsis, kidney abscess, acute kidney injury, and kidney scar. Kidney scar is a potential complication in around 15% of children with the first UTI and can result in long-term consequences such as hypertension and chronic kidney disease (CKD) (4,5). Calderon-Margalit et al. found that adolescents with a history of childhood pyelonephritis had a higher prevalence of end stage kidney disease (ESKD) than those with no history of childhood kidney disease (6).
Childhood infectious diseases modulate weight gain and linear growth by influencing metabolism, nutrition, and the microbiome (7,8). Notably, several childhood diseases, such as allergic disorders, inflammatory bowel disease, and dental caries, have been associated with impaired future growth (9-11). Furthermore, CKD and kidney scar resulting from UTIs are known to have a detrimental impact on growth in childhood through various mechanisms, including anorexia, malnutrition, and decreased insulin-like growth factor-1 expression (12). In addition, recent studies have indicated that early antibiotic exposure in infancy is associated with childhood growth and obesity with gut microbiota alteration (13,14). Considering the common practice of administering oral or intravenous antimicrobial medication for 7–14 days to treat UTI in children (15), broad-spectrum antibiotics may potentially affect their growth parameters in terms of height and weight. However, to the best of our knowledge, despite the significant burden of UTIs during childhood, the impact of pediatric UTIs on growth outcomes remains unexplored.
In this nationwide population-based matched cohort study, we aimed to identify the impact of UTI during the first year of life on future growth, using data from the Korean National Health Information Database (NHID) and the Korean National Health Screening Program for Infants and Children (NHSPIC) data. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-361/rc).
Methods
Data source
We conducted a nationwide population-based matched cohort study of Korean children born between January 1, 2007, and December 31, 2019, using NHID and NHSPIC data. Briefly, NHID is provided by the Korean National Health Insurance System (NHIS), a Korean Ministry of Health and Welfare-affiliated mandatory healthcare system covering 99.4% of the 51 million people in South Korea. NHID contains the patient’s demographics and insurance claims, including all information regarding utilization of medical facilities, such as visiting medical institutions and International Classification of Diseases, 10th revision (ICD-10) codes. The NHIS database’s validity, variables’ definitions, and utilization of NHIS in epidemiological research have been validated externally and internally (16,17). Korean NHSPIC is a health screening program for infants and children during well-baby check-up visits (18,19). All children are eligible to participate in the NHSPIC seven times: 4–6 months (1st), 9–12 months (2nd), 18–24 months (3rd), 30–36 months (4th), 42–48 months (5th), 54–60 months (6th), and 66–71 months (7th). Each program contains history taking, physical examination, anthropometric measurements including height and weight, questionnaires with developmental screening, and anticipatory guidance for their age. NHSPIC participation rates among all Korean children were 74.5–83.0% in 2018–2020 (20), which implies that the results of NHSPIC can represent the health status of Korean children.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Public Institutional Review Board Designated by the Korean Ministry of Health and Welfare (IRB No. P01-202207-01-029). The requirement for written informed consent forms was waived due to the anonymous and retrospective nature of the study.
Inclusion and exclusion criteria
Children who had claims data for UTI before 12 months old and participated in the 4th NHSPIC examination between January 2018 and December 2020 were eligible for the study group. UTIs were identified according to ICD-10 codes, which include acute pyelonephritis (N10), acute cystitis (N30.0, N30.8, N30.9, and B37.4), and unspecified UTI (N39.0 and P39.3). Controls were selected as children who had visited the well-baby clinic and undergone the 4th NHSPIC examination without a history of UTI diagnosis. They were matched one-to-one with the study group based on birth year, birth month, and sex using a multi-way stratification method. Children with complex chronic conditions were excluded, derived from the Pediatric complex chronic conditions classification system version 2 by Feudtner et al. (CCC V2) (21). In addition, children who did not participate in the 1st NHSPIC examination at 4–6 months of age were excluded since this examination contains important covariates such as birth weight, prematurity, and breastfeeding status during infancy.
Outcomes
The primary outcomes were height and body mass index (BMI) measured on the 4th NHSPIC examination (30–36 months of age). The standard deviation scores (SDSs) of height and BMI were determined and utilized as outcome variables for accurate comparison of children across various age groups or genders. We calculated SDS using reference height-for-age and BMI-for-age growth chart, which was released in 2017 by the Korean Disease Control and Prevention Agency (22), using reported L, M, and S parameters according to the child’s age and sex with the following equation:
where X is the measurement (height and BMI) and L, M, and S are the values from the growth chart corresponding to the age in months of the child (23). We conducted three subgroup analyses by dividing UTIs into various groups. Firstly, we categorized UTIs into acute pyelonephritis (APN) group and unspecified UTI group (including acute cystitis), based on their diagnosis code and analyzed. Secondly, to assess a dose-response relationship, we divided and analyzed children into two groups based on their history of UTI: those having a single episode and those with multiple episodes. UTI diagnosis codes with more than 1-month intervals were considered separate events. Lastly, we investigated whether the timing of the first UTI episode was associated with future growth, examining the occurrence of UTI in infants younger than 3 months versus those aged 4 to 12 months.
Covariates
We considered the following variables as potential confounders or their surrogates: breastfeeding status, prematurity, low birth weight, parents’ socioeconomic status, and place of residence (categorized into capital, metropolitan, and rural). Breastfeeding status was determined using the results of the 1st NHSPIC examination at 4–6 months of age, which were categorized as exclusive breastfeeding, formula feeding, mixed feeding, and unknown status (non-responder).
Statistical analysis
To conduct a balanced analysis, we used inverse probability of treatment weighting (IPTW), and all measured covariates were included in the IPTW calculation. Standardized differences were calculated to evaluate the quality of balancing after IPTW. We then conducted a weighted linear regression analysis to produce a doubly robust estimated β coefficient with 95% confidence intervals (CIs) for each study outcome.
Furthermore, several sensitivity analyses were conducted. Firstly, IPTW unadjusted regression analysis using was performed. Secondly, we excluded children who received a diagnosis code of vesicoureteral reflux (VUR) within the first 12 months of age, as they are at a particular risk of prolonged prophylactic antibiotic exposure and developing reflux nephropathy, and reanalyzed the results. However, the data regarding the voiding cystourethrography were not obtained. Thirdly, growth status measured on the 5th NHSPIC examination, conducted at 42–48 months, was used. Finally, logistic regression analysis was performed to estimate the odds ratio with 95% CI for the dichotomous outcome of short stature and obesity. Short stature was defined as height percentile of 3rd or less, while obesity was defined as BMI percentile of 95th or more. All statistical analyses were performed using R-project version 4.2.6 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at a P value <0.05.
Results
Baseline characteristics
A total of 116,017 children who experienced UTI before the first year of age, received the 4th NHSPIC between January 1, 2018, and December 31, 2020, and conducted the 4th NHSPIC exam were included. Children who did not conduct 1st NHSPIC exam (N=20,545) and children with complex chronic conditions were excluded (N=10,953). Finally, 84,519 children and 84,519 birth year- and sex-matched control were eligible for the main analysis (Figure 1). After IPTW, the two groups were balanced for all covariates with an absolute standardized mean difference (SMD) <0.05 (Table 1, Table S1 and Figure S1).
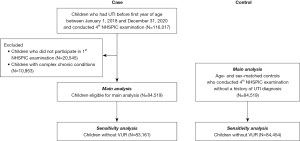
Table 1
| Characteristics | Before IPTW | After IPTW† | |||||
|---|---|---|---|---|---|---|---|
| Control group (n=84,519) | UTI group (n=84,519) | SMD | Control group (n=84,322) | UTI group (n=84,519) | SMD | ||
| Age at 4th NHSPIC examination, months (SD) | 34.96 (2.27) | 34.97 (2.29) | 34.96 (2.27) | 34.98 (2.29) | |||
| Male, No. (%) | 41,036 (48.6) | 45,051 (53.3) | 0.048 | 40,947 (48.6) | 43,426 (51.4) | 0.028 | |
| Income status, No. (%) | |||||||
| <20 percentiles | 8,159 (9.7) | 8,445 (10.0) | 0.003 | 8,138 (9.7) | 8,458 (10.0) | 0.003 | |
| 20–<40 percentiles | 6,691 (7.9) | 6,929 (8.2) | 0.003 | 6,677 (7.9) | 6,934 (8.2) | 0.003 | |
| 40–<60 percentiles | 13,682 (16.2) | 13,952 (16.5) | 0.004 | 13,657 (16.2) | 13,954 (16.5) | 0.003 | |
| 60–80 percentiles | 27,842 (32.9) | 27,913 (33.0) | 0.001 | 27,762 (32.9) | 27,903 (33.0) | 0.001 | |
| >80 percentiles | 24,713 (29.2) | 23,841 (28.2) | −0.010 | 24,658 (29.2) | 23,829 (28.2) | −0.010 | |
| Others‡ | 3,432 (4.1) | 3,439 (4.1) | <0.001 | 3,430 (4.1) | 3,441 (4.1) | <0.001 | |
| Place of residence, No. (%) | |||||||
| Capital | 12,956 (15.3) | 11,569 (13.7) | −0.017 | 12,919 (15.3) | 11,541 (13.7) | −0.017 | |
| Metropolitan | 22,560 (26.7) | 22,562 (26.7) | <0.001 | 22,532 (26.7) | 22,565 (26.7) | <0.001 | |
| Rural | 49,003 (58.0) | 50,388 (59.6) | 0.016 | 48,871 (58.0) | 50,413 (59.6) | 0.016 | |
| Breastfeeding status at infant, No. (%) | |||||||
| Exclusive breastfeeding | 10,778 (12.8) | 9,565 (11.3) | −0.014 | 10,744 (12.7) | 9,562 (11.3) | −0.014 | |
| Mixed feeding | 6,436 (7.6) | 5,550 (6.6) | −0.011 | 6,420 (7.6) | 5,525 (6.5) | −0.013 | |
| Formula feeding | 15,409 (18.2) | 14,378 (17.0) | −0.010 | 15,368 (18.2) | 14,339 (17.0) | −0.010 | |
| Missing | 51,896 (61.4) | 55,026 (65.1) | 0.037 | 51,790 (61.4) | 55,093 (65.1) | 0.037 | |
| Low birth weight, No. (%) | 5,472 (6.5) | 4,717 (5.6) | 0.009 | 5,474 (6.5) | 4,740 (5.6) | 0.009 | |
| Preterm birth, No. (%) | 3,063 (3.6) | 2,910 (3.4) | 0.002 | 3,055 (3.6) | 2,909 (3.4) | 0.002 | |
†, propensity scores were computed by using the following variables: sex, income quartile, place of residence, breastfeeding status, low birth weight, and preterm birth; ‡, parents with a special occupation such as military personnel or shipping labor union. IPTW, inverse probability of treatment weighting; UTI, urinary tract infection; SMD, standardized mean difference; NHSPIC, National Health Screening Program for Infants and Children; SD, standard deviation.
Association of growth status with UTI experience
At 30 to 36 months of age, the mean height SDS was −0.20862; height SDS did not show a significant difference between the UTI and control groups after adjusting for covariates (β coefficient =−0.0034, 95% CI: −0.0121 to 0.0054). The mean BMI SDS was 0.280. BMI SDS at 30–36 months of age showed a positive correlation with UTI history in infancy (β coefficient =0.0426, 95% CI: 0.0304 to 0.0547) (Table 2).
Table 2
| Outcomes | β coefficient† | 95% CI |
|---|---|---|
| Main analysis | ||
| Height SDS‡ | −0.0034 | −0.0121 to 0.0054 |
| BMI SDS‡ | 0.0426 | 0.0304 to 0.0547 |
| Subgroup analysis—main diagnosis§ | ||
| Height SDS—unspecified UTI | −0.0073 | −0.0164 to 0.0018 |
| Height SDS—APN | 0.0171 | 0.0014 to 0.0327 |
| BMI SDS—unspecified UTI | 0.0404 | 0.0277 to 0.0530 |
| BMI SDS—APN | 0.0540 | 0.0302 to 0.0778 |
| Subgroup analysis—number of UTI episodes§ | ||
| Height SDS—single episode of UTI | −0.0034 | −0.0125 to 0.0057 |
| Height SDS—multiple episodes of UTI | −0.0033 | −0.0187 to 0.0122 |
| BMI SDS—single episode of UTI | 0.0420 | 0.0292 to 0.0548 |
| BMI SDS—multiple episodes of UTI | 0.0454 | 0.0235 to 0.0672 |
| Subgroup analysis—timing of first UTI§ | ||
| Height SDS—first UTI before 3 months old | −0.0061 | −0.0156 to 0.0035 |
| Height SDS—first UTI after 3 months old | 0.0028 | −0.0096 to 0.0152 |
| BMI SDS—first UTI before 3 months old | 0.0422 | 0.0286 to 0.0558 |
| BMI SDS—first UTI after 3 months old | 0.0435 | 0.0264 to 0.0606 |
†, the β coefficient of height or BMI SDS, along with 95% CI, was calculated using weighted multiple logistic regression analysis. The analysis was adjusted for sex, income quintile, residual area, breastfeeding status, prematurity, and low birth weight; ‡, the β coefficient of height or BMI SDS for UTI experience during infancy; §, the exposure variables—UTI experience during infancy—were subgrouped into various group, and the respective β coefficient was calculated for each subgroup. UTI, urinary tract infection; CI, confidence interval; SDS, standard deviation score; BMI, body mass index; APN, acute pyelonephritis.
We conducted several subgroup analyses. Compared with the control group, the height SDS of the APN group was higher (β coefficient =0.0171, 95% CI: 0.0014 to 0.0327). However, no significant difference was observed in the height SDS of the unspecified UTI group. In addition, there was no significant difference in height SDS between the control group and children with multiple episodes of UTI. No significant difference was found in the height SDS between those who experienced UTI at 3 months of age or younger and the control group. However, the BMI SDS was significantly different between the control group and APN (β coefficient =0.0540, 95% CI: 0.0302 to 0.0778) or unspecified UTI group (β coefficient =0.0404, 95% CI: 0.0277 to 0.0530). In addition, significant differences were found in the BMI SDS between the control group and children who had multiple UTIs or who had UTIs under 3 months of age (Table 2). The numbers of cases and controls for subgroup analysis and sensitivity analysis are presented in Table S1.
Sensitivity analysis
Various sensitivity analyses showed consistent results with the main analysis, as shown in Table 3. The analysis included IPTW-unadjusted analysis, analysis excluding children with VUR, growth indices at 5th NHSPIC (conducted at 42–48 months of age), and outcome as dichotomous variables (short status and obesity). The sensitivity analyses’ results indicated no association between UTI experience and height SDS in childhood. However, every sensitivity analysis demonstrated a positive relationship between UTI history and BMI SDS.
Table 3
| Outcomes | β coefficient/odds ratio | 95% CI |
|---|---|---|
| Model 1: IPTW unadjusted analysis | ||
| Height SDS | −0.0040a | −0.0126 to 0.0045 |
| BMI SDS | 0.0417a | 0.0296 to 0.0538 |
| Model 2: excluding children with VUR | ||
| Height SDS | −0.0042a | −0.0129 to 0.0045 |
| BMI SDS | 0.0420a | 0.0299 to 0.0540 |
| Model 3: 5th NHSPIC results (conducted on 42–48 months of age) | ||
| Height SDS | −0.0095a | −0.0223 to 0.0002 |
| BMI SDS | 0.0405a | 0.0260 to 0.0550 |
| Model 4: dichotomous outcome | ||
| Short stature† | 1.001b | 0.999 to 1.002 |
| Obesity‡ | 1.002b | 1.001 to 1.005 |
†, defined as height percentile of 3rd or less; ‡, defined as BMI percentile of 95th or more. a, β coefficient; b, odds ratio. CI, confidence interval; IPTW, inverse probability of treatment weighting; SDS, standard deviation score; BMI, body mass index; VUR, vesicoureteral reflux; NHSPIC, National Health Screening Program for Infant and Children.
Discussion
Main findings
In this nationwide population-based cohort study, we found that UTI in the first 12 months was not associated with height status but with high BMI at 30 to 36 months. Our study provides robust evidence that UTI can be a risk factor for childhood obesity through the diverse subgroup and sensitivity analyses. These findings indicate that monitoring growth indices, particularly BMI status, may be necessary for over two years in children who have experienced UTI during infancy.
Comparison with previous studies
UTI is one of the most common bacterial infections among children, resulting in frequent hospitalization and a high economic burden. However, there has been limited research directly investigating the impact of UTI on childhood growth. A single-center study conducted in Iran concluded that weight-height index and height SDS were not associated with a history of UTI (24). Various cross-sectional studies found that obesity is one of the risk factors for UTI in young children (25,26). To our knowledge, this is the first longitudinal study demonstrating the possibility that UTI precedes obesity and suggesting that high BMI status can persist more than 2 years after UTI diagnosis. The elevation of SDS BMI in children might serve as an initial indicator for several health hazards, including metabolic syndrome and cardiovascular diseases (27,28). These underscore the importance of early intervention strategies for these at-risk children to alleviate the potential negative consequences.
Biological plausibility
What could be the underlying reason for the positive relationship between a history of UTI and obesity? One potential explanation is the possibility of direct kidney damage resulting from UTI. Kidney scar acquired after UTI can result in hypertension, proteinuria, and CKD through an inflammatory process, interstitial fibrosis, and loss of functioning nephrons (29). Kidney scars, observed as defects on dimercapto succinic acid (DMSA) scan, can develop in approximately 15% of cases within 12 months after UTI, especially among children with recurrent APN or VUR (30,31). The subgroup analysis results of this study suggest the possibility of a positive relationship between kidney scar and obesity, as children with a history of APN and recurrent UTIs tended to be more obese than unspecified UTIs and single UTIs. The relationship between VUR and growth index has been investigated in several studies. However, the findings have been inconsistent (24,32,33). In addition, it is well known that overt CKD causes detrimental effects on growth. One population-wide study in Israel in 2018 reported a three-fold increase in subsequent ESKD in children with a history of pyelonephritis (6). However, the likelihood of infantile UTI history causing kidney dysfunction or CKD in childhood is very low. This can partly explain the result that there is no association between UTI history and height growth in this study. Given that childhood UTIs can lead to kidney scar and raise the risk of developing CKD in later stages of the disease (34), it is plausible that UTIs may still impact final growth and weight during adolescence and adulthood, despite the absence of impact on height growth during early childhood in this study.
UTI is also one of the important diseases that cause antibiotic administration in infants and young children, along with otitis media and pneumonia (35). The resultant changes in the intestinal microbiota due to antibiotic administration could affect the occurrence of obesity (36). Park et al. reported that childhood obesity occurs more frequently in children who experienced UTI before 2 years of age in a dose-dependent manner (37). One study found adverse effects of antibiotic administration on BMI in mouse experiment (14). Based on these different results regarding the association of antibiotic administration with obesity, broad-spectrum antibiotics might have been affecting the BMI of children diagnosed with UTIs.
Strengths and limitations
A great strength of our study was the population-based inclusion of over 80,000 UTI children. Despite the limitation of our study being confined to individuals with the 4th NHSPIC results, potentially leading to selection bias, we observed the NHSPIC participation rate of up to 83%. Considering this rate is higher than the 70% participation rate of adult health screening in Korea (20), the present study can provide greater reliability than a single-center study. However, our study had several limitations. Firstly, since the patient group was defined only by the diagnostic codes of APN, cystitis, and unspecified UTI, there may be a detection bias of UTI cases. However, Korean NHIS code data was reported to be reliable in more than 75% of cases in thorough validation (38). Another important limitation is the absence of growth status data at the time of UTI diagnosis in children, which makes it uncertain whether initial obesity raises the chances of UTI development and its persistence until age three, or if obesity develops after contracting a UTI. In addition, the lack of long-term observational data makes it challenging to draw conclusions about the impact of UTI on final height and weight. Large-scale, long-term cohort studies are necessary to track individuals with a history of UTI from childhood through adolescence and into adulthood. Lastly, due to the nature of our data source, we could not assess the precise clinical presentation, clinical severity, and complications such as kidney abscess and kidney scarring, imaging test results such as DMSA scan and kidney ultrasonography, and the data related to antibiotic management. Nonetheless, we analyzed using the data of VUR in the sensitivity analysis, which is closely associated with kidney scar in childhood. This limitation warranted a prospective cohort study for children who developed kidney scar after UTI to demonstrate a more accurate prognosis on the growth of children with UTI history. Lastly, there was insufficient adjustment for recent nutritional status. However, we did adjust for breastfeeding status, which may partially account for nutritional confounders.
Conclusions
In conclusion, UTI during the first 12 months was associated with high BMI between 30–36 months but not related to height status. These findings suggest the following directions for further research expanding the follow-up period to establish the impact of childhood UTI experience on future growth.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-361/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-361/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-361/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-361/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Public Institutional Review Board Designated by the Korean Ministry of Health and Welfare (IRB No. P01-202207-01-029). The requirement for written informed consent forms was waived due to the anonymous and retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaufman J, Temple-Smith M, Sanci L. Urinary tract infections in children: an overview of diagnosis and management. BMJ Paediatr Open 2019;3:e000487. [Crossref] [PubMed]
- Yoo YM, Park BS, Lee SY, et al. An Epidemiologic Study on Hosts and Pathogens of Urinary Tract Infection in Urban Children of Korea (2012–2017). Child Kidney Dis 2019;23:29-35.
- Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 2008;27:302-8. [Crossref] [PubMed]
- Wennerström M, Hansson S, Jodal U, et al. Renal function 16 to 26 years after the first urinary tract infection in childhood. Arch Pediatr Adolesc Med 2000;154:339-45. [Crossref] [PubMed]
- Shaikh N, Craig JC, Rovers MM, et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr 2014;168:893-900. [Crossref] [PubMed]
- Calderon-Margalit R, Golan E, Twig G, et al. History of Childhood Kidney Disease and Risk of Adult End-Stage Renal Disease. N Engl J Med 2018;378:428-38. [Crossref] [PubMed]
- Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis 2008;46:1582-8. [Crossref] [PubMed]
- Stephensen CB. Burden of infection on growth failure. J Nutr 1999;129:534S-8S. [Crossref] [PubMed]
- Jin HY, Lim JS, Lee Y, et al. Growth, puberty, and bone health in children and adolescents with inflammatory bowel disease. BMC Pediatr 2021;21:35. [Crossref] [PubMed]
- Alkarimi HA, Watt RG, Pikhart H, et al. Dental caries and growth in school-age children. Pediatrics 2014;133:e616-23. [Crossref] [PubMed]
- Chen W, Yang H, Hou C, et al. The influence of childhood asthma on adult height: evidence from the UK Biobank. BMC Med 2022;20:94. [Crossref] [PubMed]
- Rodig NM, McDermott KC, Schneider MF, et al. Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol 2014;29:1987-95. [Crossref] [PubMed]
- Li P, Chang X, Chen X, et al. Early-life antibiotic exposure increases the risk of childhood overweight and obesity in relation to dysbiosis of gut microbiota: a birth cohort study. Ann Clin Microbiol Antimicrob 2022;21:46. [Crossref] [PubMed]
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014;158:705-21. [Crossref] [PubMed]
- Strohmeier Y, Hodson EM, Willis NS, et al. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev 2014;2014:CD003772. [Crossref] [PubMed]
- Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev 2012;13:6163-8. [Crossref] [PubMed]
- Lee CK, Ha HJ, Oh SJ, et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance Service database. J Gastroenterol Hepatol 2020;35:760-8. [Crossref] [PubMed]
- Moon JS. Review of National Health Screening Program for Infant and Children in Korea. Journal of the Korean Medical Association 2010;53:377-85.
- Eun BL, Moon JS, Eun SH, et al. The current child and adolescent health screening system: an assessment and proposal for an early and periodic check-up program. Korean J Pediatr 2010;53:300-6.
- NHIS. 2021 National Health Insurance statistical yearbook. Available online: https://www.nhis.or.kr/nhis/together/wbhaec06300m01.do?mode=view&articleNo=10812384&article.offset=0&articleLimit=10. Accessed June 6 2023.
- Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. [Crossref] [PubMed]
- Kim JH, Yun S, Hwang SS, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr 2018;61:135-49. [Crossref] [PubMed]
- Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305-19. [Crossref] [PubMed]
- Malaki M, Sayedzadeh SA, Shoaran M. Growth indices in urinary tract infection children with or without vesicoureteral reflux. Saudi J Kidney Dis Transpl 2011;22:723-6.
- Renko M, Salo J, Ekstrand M, et al. Meta-analysis of the Risk Factors for Urinary Tract Infection in Children. Pediatr Infect Dis J 2022;41:787-92. [Crossref] [PubMed]
- Hsu PC, Chen SJ. Obesity and risk of urinary tract infection in young children presenting with fever. Medicine (Baltimore) 2018;97:e13006. [Crossref] [PubMed]
- Gregory JW. Prevention of Obesity and Metabolic Syndrome in Children. Front Endocrinol (Lausanne) 2019;10:669. [Crossref] [PubMed]
- Friedemann C, Heneghan C, Mahtani K, et al. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ 2012;345:e4759. [Crossref] [PubMed]
- Li B, Haridas B, Jackson AR, et al. Inflammation drives renal scarring in experimental pyelonephritis. Am J Physiol Renal Physiol 2017;312:F43-53. [Crossref] [PubMed]
- Han JH, Rhie S, Lee JH. Predictors of renal scars in infants with recurrent febrile urinary tract infection: a retrospective, single-center study. Child Kidney Dis 2022;26:52-7.
- Kosmeri C, Kalaitzidis R, Siomou E. An update on renal scarring after urinary tract infection in children: what are the risk factors? J Pediatr Urol 2019;15:598-603. [Crossref] [PubMed]
- Fu LS, Hong YT, Shu SG. Height and weight growth in children with vesicoureteral reflux diagnosed before one year old. Urology 2009;74:1314-7. [Crossref] [PubMed]
- Polito C, La Manna A, Capacchione A, et al. Height and weight in children with vesicoureteric reflux and renal scarring. Pediatr Nephrol 1996;10:564-7. [Crossref] [PubMed]
- Park E, Lee HJ, Choi HJ, et al. Incidence of and risk factors for short stature in children with chronic kidney disease: results from the KNOW-Ped CKD. Pediatr Nephrol 2021;36:2857-64. [Crossref] [PubMed]
- Desai S, Aronson PL, Shabanova V, et al. Parenteral Antibiotic Therapy Duration in Young Infants With Bacteremic Urinary Tract Infections. Pediatrics 2019;144:e20183844. [Crossref] [PubMed]
- Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol 2015;31:69-75. [Crossref] [PubMed]
- Park YJ, Chang J, Lee G, et al. Association of class number, cumulative exposure, and earlier initiation of antibiotics during the first two-years of life with subsequent childhood obesity. Metabolism 2020;112:154348. [Crossref] [PubMed]
- Kimm H, Yun JE, Lee SH, et al. Validity of the diagnosis of acute myocardial infarction in korean national medical health insurance claims data: the korean heart study (1). Korean Circ J 2012;42:10-5. [Crossref] [PubMed]


