
Neutrophil-to-lymphocyte ratio as a predictor of primary vesicoureteral reflux evolution in children with associated acute pyelonephritis
Highlight box
Key findings
• Neutrophil-to-lymphocyte ratio (NLR) may be considered as a simple and cost-effective predictor of clinical outcome of vesicoureteral reflux (VUR).
What is known and what is new?
• NLR has been recently postulated as an inflammatory biomarker for VUR diagnosis.
• NLR correlates with the increased risk of developing complications of primary VUR after an episode of APN during follow-up.
What is the implication, and what should change now?
• NLR should be included in the management algorithm for these patients.
Introduction
Primary vesicoureteral reflux (VUR) is a congenital disorder, typically resulting from a short submucosal tract at the junction between ureter and bladder, not associated with other obstructive, neurological or vascular abnormalities (1). It is one of the most common urological disease in childhood, with an estimated prevalence of 0.4–1.8% in the general pediatric population and up to 30% in children with a history of urinary tract infection (UTI) (2,3). In these patients, VUR plays an important role in the pathogenesis of UTIs as the relationship between acute pyelonephritis (APN), VUR and renal damage is well established (4). VUR increases the risk of APN when lower UTIs are present, with a higher rate of febrile infection in the affected population and an increased risk of upper urinary tract damage and renal scarring (5). This potential morbidity makes early diagnosis essential, as well as the determination of the clinical course of VUR, due to the high percentage of spontaneous resolution (SR) observed during its evolution (6).
Spontaneous VUR resolution is related to age at diagnosis, laterality, severity (grades of VUR), clinical presentation and ureteral anatomy. Faster SR is more common in children younger than one year of age at diagnosis, with low-grade VUR (grades I–III), and with an asymptomatic presentation (7). The presence of renal cortical defects, bladder dysfunction, and febrile UTIs have been reported as negative predictors for SR of VUR (8). Febrile UTI development has also shown a decreased likelihood of SR of primary VUR in children, although the role of APN and the systemic inflammatory response on the clinical course of VUR is still poorly understood. Furthermore, research on this topic is scarce, as most published studies only describe risk factors for the development of APN in patients with VUR (4).
Recently, neutrophil-to-lymphocyte ratio (NLR) has been postulated as an inflammatory biomarker in several childhood diseases with significant renal inflammation, such as lupus nephritis (9), acute glomerulonephritis (10) or Henoch-Schönlein purpura (11). In addition, some studies have reported its role as a diagnostic marker of APN with renal cortical defects and VUR (12,13). The aim of this study is to analyze the role of NLR as a predictor of SR of primary VUR in patients with APN. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-229/rc).
Methods
Study design and patient selection
A retrospective observational cohort study was conducted in patients with episodes of APN diagnosed at La Paz Children’s University Hospital between January 2013 and December 2019. APN was defined according to the following American Academy of Paediatrics criteria, including fever of ≥38.0 ℃, pyuria of >5 white blood count (WBC)/field, positive urine culture of ≥50,000 colony-forming units/mL on catheterisation and renal impairment demonstrated by imaging during the acute episode (14).
Only patients with associated primary VUR were included in the study, while those without VUR or with VUR secondary to other anatomical or functional alterations of the urinary tract or bladder were excluded. Urine cultures were collected using sterile urethral catheters prior to antibiotic treatment. Imaging studies performed included urinary ultrasound, Tc-99m-dimercaptosuccinic acid (DMSA) renal scan scintigraphy, voiding cystourethrography (VCUG). After APN diagnosis, urinary ultrasound and DMSA were performed during the first 5 days of admission. Subsequently, VCUG was performed 4 weeks after APN resolution, in order to evaluate the presence of VUR as well as the degree and laterality. Follow-up was performed by reviews in the outpatient clinic every 3 months, with repeat VCUG to monitor the VUR clinical course at 6-monthly intervals.
Patients were divided into two groups according to primary VUR evolution after the APN episode: SR group and complications development (CD) group (VUR CD during follow-up). Complications of VUR included the development of a new episode of APN during follow-up or worsening renal function of the kidney affected by VUR, with altered DMSA uptake on scintigraphy after APN (renal differential function higher than 20% between both kidneys). Antibiotic prophylaxis was initiated in all patients diagnosed with VUR after APN for at least 6 months, with subsequent withdrawal in those cases where no further episodes of APN occurred and VUR resolved spontaneously on control isotopic cystography. Surgical treatment was indicated in patients who developed the aforementioned VUR-related complications (new APN episodes or impaired renal function). Endoscopic injection of hyaluronic acid (Deflux®, Palette Life Sciences, Sweden) was chosen initially in these cases. In patients who did not respond to endoscopic treatment, surgical correction (ureteral reimplantation) was performed.
Study variables
Demographics (gender, age, weight and height at diagnosis of APN episode), prenatal data (antenatal care, gestational age and type of delivery), laboratory, microbiological and scintigraphic features (DMSA findings) of the APN episode, as well as variables related to primary VUR (diagnosis prior to APN episode, antibiotic prophylaxis and type of prophylaxis, laterality and grade of VUR, presence of post-mictional residue and SR or not) were analysed. Laboratory data were obtained from blood tests performed in the emergency department on patient arrival, including haemogram and biochemical parameters. VUR diagnosis was made in all cases by VCUG. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of La Paz Children’s University Hospital (No. PI-50147). Individual consent was waived due to the retrospective nature of this study, the absence of human or animal samples and the anonymous collection of analytical data.
Statistical analysis
Data were collected in Microsoft Excel software 2010 (Redmond, WA, USA), and analysed with SPSS Statistic 22 (Chicago, IL, USA). Continuous variables were expressed as mean and standard deviation, if normally distributed, or as median and interquartile range (IQR) if not normally distributed. To test whether the variables were normally distributed, Kolmogorov-Smirnoff and Shapiro-Wilk tests were used. For normally distributed continuous variables, Student’s t-test was used for independent samples, and Mann-Whitney test was used when applicable. Qualitative variables were expressed as frequency and percentage, and were analysed using Chi-square test, or Fisher’s test when the former could not be applied. Odds ratios (OR) were calculated with 95% confidence intervals. Statistical significance was established at a value of P<0.05. Sensitivity and specificity for SR of the different parameters analysed were determined using receiver operating characteristic (ROC) curves. Subsequently, the cut-off point of maximum diagnostic accuracy for each parameter was calculated using the Youden index (15).
Results
A total of 1,146 APN episodes diagnosed in the Emergency Department of La Paz Children’s University Hospital were analysed, of which 403 (35.2%) had associated VUR. Of these, 273 patients had associated primary VUR and were finally included in the study, with a median age at APN diagnosis of 11 months (IQR: 3–22 months). Spontaneous VUR resolution occurred in 169 patients (SR group), while CD were observed in the remaining 104 patients (CD group). Figure 1 shows the flow chart of patient selection. Female gender was frequent in both groups. No differences in demographic or perinatal features were observed when comparing both groups, which are shown in Table 1.
Table 1
| Demographic and perinatal features | Spontaneous resolution group (n=169) | Complications development group (n=104) | P value |
|---|---|---|---|
| Gender, n (%) | 0.867 | ||
| Males | 73 (43.2) | 46 (44.2) | |
| Females | 96 (56.8) | 58 (55.8) | |
| Age at diagnosis (months), median [IQR] | 11 [4–22] | 10 [3–23] | 0.241 |
| Weight (kg), median [IQR] | 7.9 [5.1–11.3] | 13 [9.5–17.5] | 0.324 |
| Height (cm), median [IQR] | 65 [57–75] | 71 [63.7–89] | 0.621 |
| Antenatal care, n (%) | 165 (97.6) | 101 (97.1) | 0.793 |
| Gestational age, n (%) | 0.672 | ||
| Full-term newborn | 150 (88.8) | 94 (90.4) | |
| Preterm newborn | 19 (11.2) | 10 (9.6) | |
| Type of delivery, n (%) | 0.879 | ||
| Vaginal | 127 (75.1) | 79 (76.0) | |
| Cesarean-section | 42 (24.9) | 25 (24.0) |
IQR, interquartile range.
E. coli was the most frequently isolated microorganism in urine cultures (74.6% in SR group vs. 80.8% in CD group), followed by E. faecalis growth, with no differences between groups. CD group patients had an abnormal uptake of DMSA on renal scintigraphy in 66.3% of them, compared to 46.7% in SR patients (P=0.009). CD patients presented a higher differential renal function between both kidneys in the scintigraphic study when compared to SR group (21.4% vs. 14.3%; P=0.021). Table 2 shows microbiological and scintigraphic features of APN episodes in both groups.
Table 2
| Microbiological and DMSA scintigraphic features | Spontaneous resolution group (n=169) | Complications development group (n=104) | P value |
|---|---|---|---|
| Microorganism in urine culture, n (%) | 0.214 | ||
| E. coli | 126 (74.6) | 84 (80.8) | |
| E. coli + E. faecalis | 2 (1.2) | 3 (2.9) | |
| E. faecalis | 12 (7.1) | 5 (4.8) | |
| E. cloacae | 6 (3.6) | 2 (1.9) | |
| P. aeruginosa | 6 (3.6) | 4 (3.8) | |
| P. mirabilis | 2 (1.2) | 3 (2.9) | |
| K. pneumoniae | 0 | 1 (1.0) | |
| K. oxytoca | 6 (3.6) | 0 | |
| K. aerogenes | 5 (3.0) | 0 | |
| H. haemolyticus | 1 (0.6) | 1 (1.0) | |
| S. aureus | 0 | 1 (1.0) | |
| S. epidermidis | 2 (1.2) | 0 | |
| S. mitis | 1 (0.6) | 0 | |
| Altered renal uptake in DMSA scintigraphic, n (%) | 79 (46.7) | 69 (66.3) | 0.009 |
| Differential renal function (difference in percentage between both kidneys), mean (SD) | 14.3 (4.5) | 21.4 (7.8) | 0.021 |
DMSA, dimercaptosuccinic acid; SD, standard deviation.
More than 60% of patients in each group were diagnosed prior to the episode of APN, mainly in the study of prenatal ureterohydronephrosis, followed by previous UTI, with no differences between the two groups. All patients with a previous diagnosis of primary VUR were on antimicrobial prophylaxis at the time of the APN episode, with trimethoprim being the most commonly used antibiotic regimen in both groups, with no significant differences between them. Most patients presented bilateral VUR (54.4% in SR group and 65.4% in CD group), followed by left-sided involvement. When comparing the grade of VUR determined by VCUG, more than 40% of patients in both groups had grade III VUR, followed by grade IV (26.6% in SR group vs. 29.8% in CD group) and grade II (17.8% in SR group vs. 16.3% in CD group) with no statistically significant differences between them. There was neither difference in post-mictional residue, which was less than 4% in both groups. Diagnostic and radiological features of VUR are shown in Table 3.
Table 3
| Diagnostic and radiological features | Spontaneous resolution group (n=169) | Complications development group (n=104) | P value |
|---|---|---|---|
| Previous VUR diagnosis, n (%) | 102 (60.4) | 66 (63.5) | 0.608 |
| Prenatal | 52 (30.8) | 35 (33.7) | |
| Previous UTI | 45 (26.6) | 29 (27.9) | |
| Incidental ectasia | 5 (3.0) | 2 (1.9) | |
| Prophylactic antibiotic, n (%) | 102 (60.4) | 66 (63.5) | 0.581 |
| Trimethoprim | 86 (84.3) | 56 (84.8) | |
| Fosfomycin | 3 (1.8) | 3 (2.9) | |
| Amoxicillin | 13 (12.7) | 7 (10.6) | |
| Laterality, n (%) | 0.314 | ||
| Bilateral | 92 (54.4) | 68 (65.4) | |
| Right | 24 (14.2) | 12 (11.5) | |
| Left | 53 (31.4) | 24 (23.1) | |
| Grade of VUR (VCUG), n (%) | 0.222 | ||
| I | 1 (0.6) | 0 | |
| II | 30 (17.8) | 17 (16.3) | |
| III | 68 (40.2) | 43 (41.3) | |
| IV | 45 (26.6) | 31 (29.8) | |
| V | 25 (14.8) | 13 (12.5) | |
| Post-mictional residue, n (%) | 4 (2.4) | 4 (3.8) | 0.256 |
VUR, vesicoureteral reflux; UTI, urinary tract infection; VCUG, voiding cystourethrography.
Regarding laboratory data, CD group patients had significantly higher leucocytes, neutrophils, CRP and plasma creatinine values when compared to group A. NLR was also significantly higher in CD group (5.9 vs. 1.4; P<0.001). In contrast, patients in SR group had statistically significantly higher absolute values of lymphocytes and eosinophils. No differences were found in the remaining haemogram parameters, plasma glucose and ionogram, shown in Table 4.
Table 4
| Laboratory data | Spontaneous resolution group (n=169) | Complications development group (n=104) | P value |
|---|---|---|---|
| Leukocytes (103/µL) | 13,780 [8,750–18,950] | 18,155 [11,732–23,125] | 0.004 |
| Neutrophils (103/µL) | 6,185.5 [2,926.5–10,820.5] | 13,711 [8,778–18,927] | <0.001 |
| Lymphocytes (103/µL) | 4,652 [3,190–6,349] | 1,741 [1,058–3,128] | <0.001 |
| Monocytes (103/µL) | 1,018.5 [580.9–1,684.3] | 1,001 [382–1,597] | 0.588 |
| Eosinophils (103/µL) | 160 [39.8–339.5] | 42.6 [0–108.6] | <0.001 |
| Basophils (103/µL) | 43.4 [13.6–86] | 34.1 [0–73.8] | 0.857 |
| NLR | 1.4 [0.76–2.14] | 5.9 [3.41–8.70] | <0.001 |
| Platelets (103/µL) | 380 [397–483] | 397 [185.75–372] | 0.253 |
| CRP (mg/L) | 39.9 [14.2–81.3] | 93.7 [30.1–103.7] | <0.001 |
| Glucose (mg/dL) | 91 [82–106] | 82.5 [71–103.7] | 0.051 |
| Creatinine (mg/dL) | 0.36 [0.27–0.48] | 0.43 [0.36–0.59] | <0.001 |
| Ionogram (mEq/L) | |||
| Na+ | 136.9 [135–139] | 135 [131.4–137] | 0.153 |
| K+ | 4.6 [4.3–4.9] | 4.2 [3.8–4.6] | 0.320 |
| Cl− | 105 [103–107] | 104 [101–106] | 0.201 |
Medians and interquartile ranges [Q1–Q3] for each value are presented. APN, acute pyelonephritis; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein.
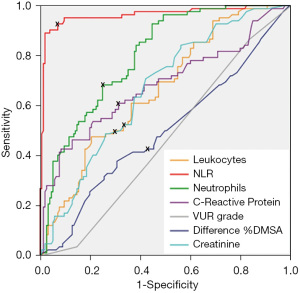
When comparing the sensitivity and specificity of the different laboratory and radiological parameters for predicting the absence of SR of primary VUR, NLR presented the highest area under the curve (AUC =0.962), and a cut-off point of 3.42, with a maximum sensitivity and specificity of 92.7% and 91.1% for the cut-off point of 3.42, respectively. Table 5 shows AUC, sensitivity and specificity of the all the parameters analysed. ROC curve for the absence of SR of primary VUR after the APN episode is plotted in Figure 2.
Table 5
| Parameters | AUC | Cut-off point | Sensitivity (%) | Specificity (%) | P value |
|---|---|---|---|---|---|
| NLR | 0.962 | 3.42 | 92.7 | 91.1 | <0.001 |
| Neutrophils (103/µL) | 0.800 | 9,900 | 78.1 | 77.5 | <0.001 |
| CRP (mg/L) | 0.682 | 55.0 | 64.6 | 61.5 | <0.001 |
| Leukocytes (103/µL) | 0.663 | 12,995 | 70.7 | 67.5 | <0.001 |
| VUR grade | 0.465 | 3 | 56.2 | 53.1 | 0.369 |
| Diff% DMSA | 0.498 | 11.7 | 51.2 | 50.6 | 0.961 |
| Creatinine (mg/dL) | 0.666 | 0.39 | 63.4 | 62.6 | <0.001 |
AUC, area under the curve; VUR, vesicoureteral reflux; APN, acute pyelonephritis; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; Diff% DMSA, differential renal function difference between both kidneys in DMSA (dimercaptosuccinic acid).
Discussion
This study examines for the first time the role of NLR as a predictor of SR of primary VUR in patients with APN. Since the increased risk of APN is what may determine the potential morbidity of VUR, it is crucial to identify children with a low probability of SR of VUR, to avoid delays in appropriate treatment and to prevent further episodes of APN. This is particularly important due to the high percentage of children with episodes of APN that are associated with primary VUR. Skoog et al. found that the prevalence of VUR in children with a history of APN is close to 30% (2), similar to the results obtained in our study, where 1,146 episodes of APN were analysed, of which 403 (35.2%) had associated VUR. Although it is now accepted that sterile reflux does not cause renal injury, the development of APN may increase the risk of renal scarring and subsequent sequelae such as hypertension, proteinuria and chronic kidney disease (16). The presence of VUR increases the risk of APN and, in turn, the development of APN is a negative predictor of SR of VUR (8).
Computational models have been designed to attempt to predict SR of VUR based on radiological data such as the degree of VUR and renal cortical defects observed on DMSA scintigraphy. However, these models do not include laboratory data assessing the inflammatory response associated with the injuries observed in patients with VUR and APN. On this aspect, NLR is a specific marker of acute inflammation involved in different pathologies where the systemic inflammatory response is the common denominator (17). It has recently been described as an independent positive predictive factor for the presence of DMSA uptake defects in patients with APN (AUC =0.713), as well as for the presence of associated VUR (AUC =0.638) (12,13). NLR has numerous advantages over imaging indicators due to its simple calculation and high immediate availability on a haemogram analysis. For these reasons, the aim of this study was to determine the usefulness of NLR as a biomarker predicting the clinical course of primary VUR in patients with associated APN. However, the absence of previous studies on this aspect precluded a comparative analysis of the results obtained.
In this context, NLR showed significant superiority over the other laboratory parameters analysed, such as total leukocyte count, absolute neutrophil count, C-reactive protein and plasma creatinine, and also over radiological parameters such as the degree of VUR determined by VCUG and differential renal function alteration on DMSA. A hypothetical explanation for this finding may lie in the pathophysiology of the systemic inflammatory response triggered by the bacterial infection in the episode of APN, which leads to an increase in neutrophil proliferation mediated by growth factors on haematopoietic stem cells, and an increase in lymphocyte apoptosis mediated by tumour necrosis factor-alpha (TNF-alpha) (17). This increase of neutrophils in the bloodstream leads to an increased influx of these cells into the urothelium of the urinary tract affected by APN, with an increased local inflammatory response at that level. The release of inflammatory mediators and cytokines could favour the development of residual fibrosis in the urothelium of the affected urinary tract, the ultimate consequence of which would be the persistence of VUR. This hypothesis may explain that elevated NLR values of these children during the episode of APN correlate with a higher percentage of VUR CD and conservative treatment failure. Although our study does not include histological data to support this hypothesis, these outcomes are consistent with the findings described by Oswald et al. who analysed samples of the ureterovesical junction obtained from children with VUR undergoing surgical treatment, in which they observed a high degree of muscle atrophy and degeneration, an altered fibre arrangement with increased accumulation of extracellular matrix collagen and a significant increase in CD68-positive macrophages, indicating inflammatory processes at this point (18).
In our study, both groups of patients can be considered comparable, as there were no differences in the demographic, prenatal or microbiological features. In patients without SR of VUR, surgical correction was indicated on a case-by-case basis, considering the patient’s age, degree of VUR, presence of renal scarring and clinical course. This explains why CD group patients presented greater alteration in DMSA uptake and greater alteration of the differential renal function than patients in SR group. The most frequent finding in both groups was the presence of bilateral VUR. These data are similar to those reported in other studies (6,9,10).
The relevance of this work lies in analysing for the first time the usefulness of the NLR as a predictor of the clinical course of VUR. The identification of patients with a low probability of SR of primary VUR after an episode of APN is helpful in clinical decision making regarding the surgical treatment option. NLR is a simple and inexpensive parameter to calculate as it can be determined in all laboratories. Our results suggest that NLR correlates negatively with SR of primary VUR after an episode of APN, such that patients with low NLR will have a high probability of SR of primary VUR. Avoiding disproportionate surgical treatment in patients with primary VUR and associated APN is mandatory, therefore a balance must be found between cases of APN with low systemic inflammatory response and patients with high NLR who will most likely require surgical correction of VUR due to persistence of the VUR.
However, this study has several limitations, mainly due to its retrospective and single-centre design, which justify its acknowledgement. Although we have a cohort of patients from a single institution, the sample size obtained has allowed us to detect significant differences. In addition, the absence of previous studies makes it impossible to compare and extrapolate the results obtained. There is also the possibility of false negative in VCUG, both in VUR diagnosis and detection of resolution. However, these false negatives are usually low grades of VUR (mainly grade I and II), which have a high probability of SR and low risk of complications. Furthermore, the timing of blood sampling after fever was probably different for each individual patient, which might be another limitation of the study (19). For all these reasons, prospective studies with data from multiple institutions are needed, in order to include a larger number of patients to achieve higher statistical power. Despite these limitations, the cut-off point of an NLR >3.41 in the laboratory study of an episode of APN predicted the likelihood of needing surgical treatment for VUR resolution with an AUC of 0.966.
Conclusions
NLR may be considered as a simple and cost-effective predictor of clinical outcome of VUR, which correlates with the increased risk of developing complications of primary VUR after an episode of APN during follow-up. Therefore, it should be included in the management algorithm for these patients, although future prospective studies are still required to confirm these results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-229/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-229/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-229/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-229/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board of La Paz Children’s University Hospital (No. PI-50147). Written informed consent was not required due to the retrospective nature of this study and the anonymous collection of analytical data, in line with institutional guidelines.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coleman R. Early management and long-term outcomes in primary vesico-ureteric reflux. BJU Int 2011;108:3-8. [Crossref] [PubMed]
- Skoog SJ, Peters CA, Arant BS Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel Summary Report: Clinical Practice Guidelines for Screening Siblings of Children With Vesicoureteral Reflux and Neonates/Infants With Prenatal Hydronephrosis. J Urol 2010;184:1145-51. Erratum in: J Urol 2011;185:365. [Crossref] [PubMed]
- Sargent MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol 2000;30:587-93. [Crossref] [PubMed]
- Jang HC, Park YJ, Park JS. Predicting factors of breakthrough infection in children with primary vesicoureteral reflux. Yonsei Med J 2012;53:748-52. [Crossref] [PubMed]
- Lopez PJ, Celis S, Reed F, et al. Vesicoureteral reflux: current management in children. Curr Urol Rep 2014;15:447. [Crossref] [PubMed]
- Peters C, Rushton HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol 2010;184:265-73. [Crossref] [PubMed]
- Estrada CR Jr, Passerotti CC, Graham DA, et al. Nomograms for predicting annual resolution rate of primary vesicoureteral reflux: results from 2,462 children. J Urol 2009;182:1535-41. [Crossref] [PubMed]
- Sjöström S, Sillén U, Jodal U, et al. Predictive factors for resolution of congenital high grade vesicoureteral reflux in infants: results of univariate and multivariate analyses. J Urol 2010;183:1177-84. [Crossref] [PubMed]
- Rianthavorn P, Prurapark P. Risk factors of infection-associated mortality in children with lupus nephritis in under-resourced areas. Lupus 2019;28:1727-34. [Crossref] [PubMed]
- Demircioglu Kılıc B, Akbalık Kara M, Buyukcelik M, et al. Pediatric post-streptococcal glomerulonephritis: Clinical and laboratory data. Pediatr Int 2018;60:645-50. [Crossref] [PubMed]
- Yakut HI, Kurt T, Uncu N, et al. Predictive role of neutrophil to lymphocyte ratio and mean platelet volume in Henoch-Schönlein purpura related gastrointestinal and renal involvement. Arch Argent Pediatr 2020;118:139-42. [Crossref] [PubMed]
- Lee JW, Park JS, Park KB, et al. Prediction of renal cortical defect and scar using neutrophil-to-lymphocyte ratio in children with febrile urinary tract infection. Nuklearmedizin 2017;56:109-14. [Crossref] [PubMed]
- Han SY, Lee IR, Park SJ, et al. Usefulness of neutrophil-lymphocyte ratio in young children with febrile urinary tract infection. Korean J Pediatr 2016;59:139-44. [Crossref] [PubMed]
- Subcommittee on Urinary Tract Infection. Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011;128:595-610. [Crossref] [PubMed]
- Hu X, Li C, Chen J, et al. Confidence intervals for the Youden index and its optimal cut-off point in the presence of covariates. J Biopharm Stat 2021;31:251-72. [Crossref] [PubMed]
- Lin KY, Chiu NT, Chen MJ, et al. Acute pyelonephritis and sequelae of renal scar in pediatric first febrile urinary tract infection. Pediatr Nephrol 2003;18:362-5. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14.
- Oswald J, Brenner E, Schwentner C, et al. The intravesical ureter in children with vesicoureteral reflux: a morphological and immunohistochemical characterization. J Urol 2003;170:2423-7. [Crossref] [PubMed]
- Karavanaki KA, Soldatou A, Koufadaki AM, et al. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr 2017;106:149-54. [Crossref] [PubMed]



