
This article has an erratum available at: http://dx.doi.org/10.21037/tp-2024-02 the article has been update on 2024-07-19 at here.
Maintaining excellent outcomes: the impact of age cutoff reclassification on reduced therapy for neuroblastoma patients
Neuroblastoma, accounting for nearly 12–15% of childhood cancers, is the most prevalent and fatal extracranial solid malignancy affecting children. Nevertheless, despite its low incidence, with approximately 10 cases per million children under 15 years of age (8–10% of the total), neuroblastoma remains a significant clinical concern (1).
Neuroblastoma primarily originates in the adrenal gland from neural crest precursor cells, that usually differentiate into adrenal chromaffin and sympathetic ganglion cells. However, it can emerge anywhere along the sympathetic nervous system chain. The exceptional feature of neuroblastoma lies in its diverse clinical behavior, as some tumors regress or mature, while others persist and progress despite intensive multimodal treatments. This variability in behavior closely correlates with a range of clinical and biological characteristics (2).
Over the past few decades, extensive efforts have been made to increase the accuracy of the neuroblastoma risk classification system by integrating a variety of clinical and biological parameters. These advancements have facilitated the categorization of patients into low-, intermediate-risk, and high-risk groups.
The Children’s Oncology Group (COG) applies a set of criteria to categorize patient risk, which includes the patient’s age at the time of diagnosis, the disease’s extent as per the International Neuroblastoma Staging System (INSS), tumor characteristics determined by the International Neuroblastoma Pathology Classification (INPC) criteria, the MYCN gene status, and the DNA index or tumor cell ploidy (3).
Older age has long been associated with poorer outcomes in neuroblastoma since the 1970’s. Previous studies indicated that children over 12 months of age at diagnosis had inferior outcomes (4). This evidence was also supported by evidence generated from neuroblastoma mass screening programs conducted in Japan, Quebec and North America, and UK (5). Later on, a retrospective analysis by London et al. from Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) studies revealed that 18 months was a better age cut-off for risk stratification (6).
Regarding to the disease stage, Evans et al. described the first staging system for neuroblastoma in 1970, based on both, the site of origin, metastatic spread and the clinical behavior of the tumor (7). Later, an international panel of experts came together to establish a surgical staging system with the aim of facilitating the comparison of outcomes and treatment approaches across different countries. In 1988, the INSS was initially introduced and later revised in 1993. It took into account factors such as the extent of tumor removal, involvement of nearby lymph nodes, tumor infiltration across the body’s midline. The difference between infants with a specific metastatic disease pattern (INSS stage 4S) that primarily affected the liver, skin, and bone marrow, and other children with metastatic disease (INSS stage 4) was also introduced (8). Shimada and colleagues created the initial histological grading framework for categorizing neuroblastic tumors, considering factors such as the presence of stroma, the level of differentiation, and the mitosis-karyorrhexis index. Subsequently, in 1999, the INPC was introduced, largely built upon Shimada’s original classification (9). Furthermore, genetic elements like MYCN status and DNA index were incorporated as well (10). MYCN amplification was linked to more advanced tumor stages (10) and reduced progression-free survival across all disease stages (11). Conversely, a higher DNA index was associated with improved treatment response in infants with inoperable tumors (10).
Regarding the clinical management of these patients. low-risk disease, typically seen in newborn infants or diagnosed prenatally, can exhibit spontaneous regression. Survival rates for patients with INSS stage 1 neuroblastoma are excellent with surgery alone (12) and a subset of infants (stage 4S neuroblastoma without MYCN amplification) often undergo spontaneous regression without any treatment (13). Chemotherapy can be used as a salvage therapy for relapsed cases. For patients with biologically favorable but completely resected localized tumors (INSS stage 2A and 2B), chemotherapy can be omitted in the majority of cases, resulting in a survival rate higher than 95%. Chemotherapy or low-dose radiotherapy is recommended for patients with large tumors or massive hepatomegaly causing mechanical obstruction, respiratory insufficiency, or liver dysfunction.
The intermediate-risk group encompasses a wide spectrum of diseases. Surgical removal of the tumor and a moderate-intensity combination of multiple chemotherapy agents (two to eight initial cycles) form the core of neuroblastoma treatment (13). The prognosis for patients with INSS stage 3 disease heavily relies on the histologic and biological features of the tumor. For children whose tumors show favorable characteristics, the combination of surgical resection and moderate-intensity chemotherapy, which includes cisplatin, doxorubicin, etoposide, and cyclophosphamide, leads to a survival rate exceeding 95%.
High-risk neuroblastoma is characterized by the presence of MYCN oncogene amplification or metastatic disease (stage M) diagnosed at 18 months of age or older (13), although specific definitions may exhibit slight variations within certain subgroups across various cooperative organizations. High-risk tumors display a high level of aggressiveness, and their long-term overall survival (OS) rate is only around 40% to 50%. The majority of patients have metastatic disease, most frequently affecting the bone, bone marrow, and liver (14). These cohorts of patients undergo a regimen of five to six rounds of initial chemotherapy followed by surgical intervention. The consolidation therapy involves the administration of high-dose myeloablative chemotherapy, followed by either a single or tandem autologous hematopoietic stem-cell transplantation, radiation therapy, and subsequent post-consolidation immunotherapy, which includes the use of an anti-GD2 antibody (15).
Despite the above-described aggressive multi-model treatment, nearly half of the patients will not be cured (16). Numerous investigations have provided valuable insights into the fact that individuals who have successfully overcome high-risk neuroblastoma often experience significant and diverse long-term consequences. The LEAHRN study conducted with a cohort of high-risk neuroblastoma survivors treated with contemporary therapy between 2000 and 2006, showed that there is a range of severe and multiple late effects, including endocrine complications such as hypothyroidism, growth failure, and hypogonadism (17). Poor linear growth is common, particularly among those exposed to total body irradiation or radiation-induced damage. Survivors with delayed growth should undergo growth hormone stimulation testing. Testicular or ovarian gonadal failure is prevalent, and associated with high-dose alkylators and/or radiation. Female survivors are at risk of absent or delayed puberty and must be closely monitored by endocrinologists. Both genders may experience infertility due to treatment. Profound hearing loss is highly prevalent and linked to learning problems. Pulmonary and cardiac diseases, including cardiomyopathy and stroke, are also frequent risks for high-risk neuroblastoma survivors and should take periodic pulmonary and cardiac screenings. Furthermore, high-risk neuroblastoma survivors have higher rates of subsequent solid malignant neoplasms (SMNs), necessitating early screening for breast, colorectal, and skin cancers (17).
In 2005, several studies pointed out that certain high-risk neuroblastoma patients aged 12–18 months had favorable outcomes (18). Then, in 2006, with the aim to optimize treatment outcomes while minimizing exposure to treatment-related toxicities, the COG changed the age cut-off, from 12 to 18 months, which reclassified some patients from high to intermediate risk (Figure 1) (19).
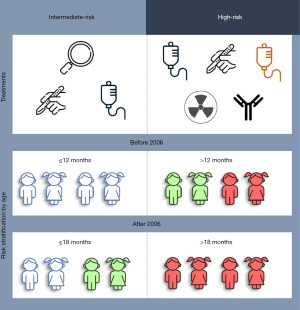
While this re-classification saved children to be exposed to unnecessary treatments, their outcome should remain unaltered. Thus, Bender et al. analyzed the outcome after the reclassification of the toddler’s groups diagnosed with neuroblastoma from the high-risk category to intermediate risk (19).
Being conscious that the change in the classification system was not homogeneously applied among all COG centers, the authors decided on December 31st 2006 as the date to discriminate between patients treated before 2006 (≤2006) or after (>2006). The criteria for eligibility were enrolment on a COG biology study between 1990 and 2018, age at diagnosis less than 3 years, and known survival data. From 8,523 patients with known risk group, 28.46 % (≤2006, n=1,581 and >2006, n=845) were initially classified as high-risk, 30.55% (≤2006, n=1,160 and >2006, n=1,444) were intermediate-risk and 40.98% (≤2006, n=2,124 and >2006 n=1,369) were low-risk. Two cohorts of patients were reassigned from high- to intermediate-risk. On the one hand, patients between 12 and 18 months of age, and with tumors stage 4 and favorable biology (12–18mo/Stage4/FavBiology; ≤2006, n=40 and >2006, n=55) and, on the other hand, 12–18 months old patients with tumors that had MYCN non-amplified and unfavorable histology (12–18mo/stage3/MYCN-NA/Unfav; ≤2006, n=6 and >2006, n=4).
Survival analyses revealed that there were no significant differences in event-free-survival after the reassignment being 89%±5.1% (≤2006) versus 94%±3.2% (>2006). The similar trend was observed for OS showing 91%±4.4% (≤2006) versus 88%±4.3% (>2006). The small increase in OS observed in the group of patients treated >2006 could be due to the better response of a small subset of patients that relapse.
Next, the authors compared the outcomes of the 12–18 months cohorts with biologically favorable MYCN non-amplified disease (classified as high-risk in ≤2006) versus the rest of high-risk patients. These analyses showed that the second group (i.e., the rest of high-risk patients) had a remarkably worse outcome compared to the first group, both in event-free and OS. However, when the same comparison was analyzed in the cohort of intermediate-risk patients diagnosed >2006, no significant differences were observed neither in event-free survival (EFS) nor OS. This stark contrast supported the decision to move the 12–18 months cohorts from the high-risk category to the intermediate-risk category. Nevertheless, the study had some limitations that could affect the conclusions of the study. For example, from the cohort of 105 patients, only 20 received treatment through the enrolment in clinical trials. It is also not clear whether those patients that were not in the trials, received high-risk therapy before 2006 and intermediate-risk therapy after 2006.
In conclusion, the authors have effectively shown that the adjustment in age cut-off was justified, and the decision made by COG to assign reduced therapy to these specific subgroups was indeed successful.
Even the number of patients that benefited from this change was relatively small (105 patients), their benefit for them and their families is high, since they will have lower risk of toxicities and late effects.
Conclusion and future perspectives
Over the past few years, it has become progressively evident that the neuroblastoma community responded to the requirement to establish a worldwide agreement regarding the classification of pre-treatment risk levels for pediatric patients with neuroblastoma. Groups such as the INRG, continue working towards greater precision in risk stratification and unifying the stratification criteria for neuroblastoma patients. Most likely, mining all the molecular data generated into the paediatric oncology precision medicine programs, will yield novel elements to further refine the risk groups and offer tailored treatments for these patients, and improve not only their outcome but also their quality of lives.
Acknowledgments
Funding: This work was funded by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-391/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-391/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Segura MF, Soriano A, Roma J, et al. Methodological advances in the discovery of novel neuroblastoma therapeutics. Expert Opin Drug Discov 2022;17:167-79. [Crossref] [PubMed]
- Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289-97. [Crossref] [PubMed]
- Sokol E, Desai AV. The Evolution of Risk Classification for Neuroblastoma. Children (Basel) 2019;6:27. [Crossref] [PubMed]
- Breslow N, McCann B. Statistical estimation of prognosis for children with neuroblastoma. Cancer Res 1971;31:2098-103.
- Murphy SB, Cohn SL, Craft AW, et al. Do children benefit from mass screening for neuroblastoma? Consensus Statement from the American Cancer Society Workshop on Neuroblastoma Screening. Lancet 1991;337:344-6. [Crossref] [PubMed]
- London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 2005;23:6459-65. [Crossref] [PubMed]
- Evans AE, D'Angio GJ, Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer 1971;27:374-8. [Crossref] [PubMed]
- Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol 1988;6:1874-81. [Crossref] [PubMed]
- Shimada H, Chatten J, Newton WA Jr, et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst 1984;73:405-16. [Crossref] [PubMed]
- Look AT, Hayes FA, Nitschke R, et al. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med 1984;311:231-5. [Crossref] [PubMed]
- Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985;313:1111-6. [Crossref] [PubMed]
- Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children's Oncology Group study P9641. J Clin Oncol 2012;30:1842-8. [Crossref] [PubMed]
- Nuchtern JG, London WB, Barnewolt CE, et al. A prospective study of expectant observation as primary therapy for neuroblastoma in young infants: a Children's Oncology Group study. Ann Surg 2012;256:573-80. [Crossref] [PubMed]
- Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am 2015;62:225-56. [Crossref] [PubMed]
- Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol 2015;33:3008-17. [Crossref] [PubMed]
- Morgenstern DA, Marzouki M, Bartels U, et al. Phase I study of vinblastine and sirolimus in pediatric patients with recurrent or refractory solid tumors. Pediatr Blood Cancer 2014;61:128-33. [Crossref] [PubMed]
- DuBois SG, Macy ME, Henderson TO. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am Soc Clin Oncol Educ Book 2022;42:1-13. [Crossref] [PubMed]
- Schmidt ML, Lal A, Seeger RC, et al. Favorable prognosis for patients 12 to 18 months of age with stage 4 nonamplified MYCN neuroblastoma: a Children's Cancer Group Study. J Clin Oncol 2005;23:6474-80. [Crossref] [PubMed]
- Bender HG, Irwin MS, Hogarty MD, et al. Survival of Patients With Neuroblastoma After Assignment to Reduced Therapy Because of the 12- to 18-Month Change in Age Cutoff in Children's Oncology Group Risk Stratification. J Clin Oncol 2023;41:3149-59. [Crossref] [PubMed]


