
Experience of video-assisted thoracic surgery treatment of congenital pulmonary airway malformation in infants less than 3 months of age
Highlight box
Key findings
• Video-assisted thoracic surgery (VATS) is relatively safe and effective in treating infants with congenital pulmonary airway malformation (CPAM) accompanied by severe clinical symptoms less than 3 months of age.
What is known and what is new?
• At present, VATS to treat CPAM has gradually become mainstream which has more advantages compared with thoracotomy.
• There are few studies on the use of VATS to treat CPAM in infants of less than 3 months of age. We shared our treatment experience in this article.
What is the implication, and what should change now?
• With the improvement of minimally invasive technology and anesthesia management, VATS is a safe and effective surgical approach. But the learning curve may be longer than that of thoracotomy.
Introduction
Congenital pulmonary airway malformation (CPAM) is the most common type of congenital lung malformation (CLM), a congenital pulmonary hamartoma-like lesion characterized by excessive proliferation and dilatation of terminal bronchioles (1). Additionally, it often manifests as a single-chamber or multi-chamber cyst or honeycomb structure in lung parenchyma (1). The prevalence of CPAM accounts for about 1/35,000–1/7,200 of the live fetuses, and this rate is gradually increasing (2). For asymptomatic children with CPAM after birth, the appropriate age for operation is above 3 months old (3,4). Notably, a few children were born with serious respiratory and circulatory disorders due to the compression of the heart and lungs and mediastinal deviation. Generally, these children tend to have larger cystic lesions, and emergency treatment should be taken after birth. At present, video-assisted thoracic surgery (VATS) to treat CPAM has gradually become mainstream. However, there are few studies on the use of VATS to treat CPAM in infants of less than 3 months old. Therefore, we collected the case data of CPAM in infants under 3 months of age admitted to Shanghai Children’s Hospital and reported on our treatment experience. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-475/rc).
Methods
Clinical data
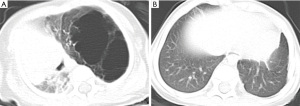
The relevant data of 12 children with CPAM admitted to our hospital from January 2019 to December 2022 were collected (Table 1). There were 10 males and 2 females. The age ranged from 13 to 89 days, averaging 60.09±30.13 days. Additionally, the body weight ranged from 2.8 to 7.5 kg, averaging 5.12±1.56 kg. All 12 cases were found with lung lesions by ultrasonic examination from 18 to 24 weeks of gestation, and all were born at term. Moreover, the CPAM volume-ratio (CVR) values ranged from 0.68 to 4.12, averaging 1.95±1.03. As the huge CPAM was discovered in utero, all these children were admitted to the neonatal intensive care unit directly after they were born in the local hospitals. Most of them were born with symptoms such as tachypnea. Due to lack of medical conditions for surgery in those local hospitals, the parents of these children chose our hospital for surgery. Six children were admitted because of tachypnea, accompanied by dyspnea or cyanosis of the mouth and lips. Four cases had a history of repeated cough and fever, chest X-ray suggesting pulmonary inflammation, and the conservative treatment had poor results. One of them suffered from lung consolidation owing to repeated infections in the lower lobe of the right lung. Furthermore, two cases were treated in the intensive care unit of a local hospital due to respiratory failure combined with pulmonary infection, requiring mechanical ventilation. They were subsequently transferred by ambulance to our hospital for treatment. All children were examined by computerized tomography (CT) before operation (please see Figures 1-4 for CT of some cases).
Table 1
| Pt No. | Age (d) | Weight (kg) | CVR | Operative method | OPT (min) | MVT (h) | ITOTDT (d) | HST (d) | Stocker type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | 7.5 | 1.79 | Lobectomy | 135 | 4 | 3 | 9 | 1 |
| 2 | 89 | 6.4 | 0.69 | Lobectomy | 100 | 5 | 3 | 6 | 3 |
| 3 | 31 | 4.6 | 1.62 | Lobectomy | 190 | 8 | 5 | 8 | 1 |
| 4 | 13 | 2.8 | 2.20 | Lobectomy | 180 | 49 | 4 | 11 | 1 |
| 5 | 41 | 3.9 | 2.27 | Lobectomy | 130 | 26 | 9 | 10 | 1 |
| 6 | 47 | 4.8 | 1.73 | Lobectomy | 150 | 18 | 3 | 9 | 1 |
| 7 | 88 | 5.6 | 1.27 | Lobectomy | 90 | 3 | 4 | 7 | 1 |
| 8 | 80 | 7.5 | 0.68 | Lobectomy | 135 | 3 | 4 | 6 | 2 |
| 9 | 44 | 5.4 | 2.40 | Lobectomy | 40 | 5 | 2 | 5 | 2 |
| 10 | 89 | 3.5 | 4.12 | Atypical resection | 145 | 4 | 4 | 12 | 1 |
| 11 | 80 | 6 | 1.18 | Segmentectomy | 120 | 4 | 3 | 8 | 1 |
| 12 | 21 | 3.4 | 3.40 | Lobectomy | 75 | 24 | 3 | 10 | 1 |
Pt, patient; d, days; kg, kilogram; CVR, CPAM volume-ratio; CPAM, congenital pulmonary airway malformation; OPT, operation time; MVT, mechanical ventilation time; ITOTDT, indwelling time of thoracic drainage tube; HST, hospital stay time.



Operative method
All children underwent VATS at Shanghai Children’s Hospital. Operative methods were divided into lobectomy, segmentectomy, and lung-sparing resection. The three-dimensional CT imaging was carefully analyzed before the operation to determine the resection range, which may be adjusted according to intraoperative findings during the operation. One-lung ventilation was selected for anesthesia (bronchial occluder was used to selectively occlude the main bronchus of the affected side), a closed thoracic cavity was established, and artificial pneumothorax was maintained (pressure was 3–4 mmHg, flow rate was about 1 L/min) (1 mmHg =0.133 kPa). For children with giant vesicles, we tried to gain more operating space by cauterizing the giant vesicles. In the lateral position of the child, three-hole method was adopted. The 5th intercostal chest in the anterior axillary line was selected as the observation well. The 8th intercostal chest in the midline axillary line and the 5th intercostal chest in the posterior axillary line were usually selected as the operation holes. The operation holes were appropriately adjusted according to the lesion position.
We chose two 5-mm Trocars and one 10-mm Trocar. In children with lobectomy or segmentectomy, LigaSure (Ethicon Endo-surgery, LLC, Somerville, NJ, USA) was used to free the pulmonary arteries, veins, and bronchi, clamped with absorbable clips, and cut off with an ultrasonic scalpel (Figures 5-7). Notably, the proximal bronchial margin was ligated with non-absorbable silk thread. The boundary between normal and diseased lung tissue in children with atypical resection was clear. Firstly, the edge of diseased lung tissue was marked with an electric hook, and the diseased tissue was removed along the boundary using an ultrasonic scalpel or Endo-GIA (Covidien LLC, Mansfield, MA, USA). Children with repeated pneumonia had severe adhesions in the chest cavity. Thus, it was necessary to use an electric hook to free the adhesion between lung tissue and chest wall and expose the affected lung before lobectomy, segmentectomy, or lung-sparing resection.



Statistical analysis
The SPSS22.0 software was used to organize and analyze the data. Results are presented as mean and standard deviation (SD).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of the Shanghai Children’s Hospital (No. 2023R064-E01). The informed consent was waived by the Medical Ethics Committee of the Shanghai Children’s Hospital due to the retrospective design of the study.
Results
All the children underwent VATS, and no cases were converted to thoracotomy. All cases were completed by the same surgeon and the same chief anesthesiologist. The operation time ranged from 40 to 190 minutes, with an average of 124.17±42.74 minutes. In this study, ten children underwent lobectomy, one underwent segmentectomy whose lesion was located in the posterior segment of the upper lobe of the left lung and the scope of the lesion was limited, and one underwent lung-sparing resection whose lesion was located both in the left upper lung and in the left lower lung. Among them, the scope of resection included two cases of left upper lobe, three cases of left lower lobe, two cases of right upper lobe, two cases of right lower lobe, one case of right upper lobe and middle lobe, one case of posterior segment of left upper lobe, and one case of irregular resection of left upper lobe and left lower lobe.
Postoperative mechanical ventilation time ranged from 3 to 49 hours, with an average of 12.75±14.16 hours. Ventilatory weaning was performed when the children were awake from anesthesia, spontaneous breathing resumed, and arterial blood gas analysis showed that there was no carbon dioxide retention (arterial carbon dioxide partial pressure lower than 50 mmHg). The indwelling time of the thoracic drainage tube ranged from 2 to 9 days, averaging 3.92±1.78 days. The postoperative hospital length of stay ranged from 5 to 12 days, averaging 8.42±2.15 days. All these children were hospitalized for a few more days because of their young age, and they were discharged after ensuring that there were no signs of discomfort. Furthermore, postoperative pathological examination showed that nine cases were type 1, two were type 2, and one was type 3.
There were no postoperative complications such as bleeding, bronchopleural fistula, or atelectasis. Postoperative pulmonary air leakage occurred in one case. The child’s lesion involved the right upper lobe, and the boundary between the right and middle lobe was unclear. The right upper lobe was resected during the operation, and the wound was large. Moreover, the lung air leakage disappeared after adjusting the drainage tube’s position and prolonging the thoracic drainage tube’s indwelling time (the total time of indwelling the tube was 9 days).
The follow-up time ranged from 3 months to 4 years. All the children recovered well, with no serious respiratory infection or pneumonia except occasional slight upper respiratory infection during the follow-up. And all of them received chest CTs for about 1 year after operation, results showing that no residual lesions were found in the reexamined chest CT; the exudation of patchy inflammation in lung disappeared; neither lung consolidation nor lung atelectasis was found; there was no apparent residual cavity in the affected chest. In addition, electrocardiogram (ECG), echocardiography and pulmonary ventilation function test or other examinations were performed if necessary.
Discussion
In 1949, CPAM was called congenital cystic adenomatoid malformation (CCAM), the lung’s most common congenital cystic malformation. In 2002, Stocker (1) renamed the disease as CPAM according to its origin, pathological features, and clinical features and divided it into five types according to histological manifestations: 0, 1, 2, 3 and 4.
Children with asymptomatic CPAM after birth do not need immediate surgery. Notably, Kulaylat reports that children with CPAM operated before 3 months of age have a higher incidence of complications than those operated after 3 months of age (3). An imaging examination should be performed during the follow-up after birth for those with prenatally identified lesions by ultrasound, and it is necessary to comprehensively consider the radiation dose for the child and the family’s assessment of the risk and choose the operation according to the change of the condition. Verhalleman indicated that in his university hospital, if possible, the first 3 months of life are preserved for lung growth and general development (4). Elective surgery in patients with a high risk for clinical symptoms is recommended before 12 months of age, although no specific guidelines exist (5-8). Referring to the opinions of many scholars, it is suggested that the operation should be performed between 3 months and 1 year old (9-13). So when the children have persistent tachypnea or recurrent pneumonia, early surgery can be considered. Additionally, a few children were born with serious respiratory and circulatory disorders due to the compression of the heart and lungs and mediastinal deviation. Most of these children have huge cystic lesions and emergency surgery should be carried out in time after birth. In our study, all the children had severe respiratory symptoms. Some of them had persistent tachypnea owing to the compression of the heart and lungs; some had recurrent pneumonia; some even required mechanical ventilation because of respiratory failure. So all the surgeries were emergent.
For children under 3 months old with CPAM, thoracotomy is often selected, and good short-term results have been observed. However, it also greatly increases the risk of thoracic and/or spinal deformity (14,15). Because the skeletal muscle of early infants is in the development stage, muscle atrophy may occur after cutting the latissimus dorsi and/or serratus anterior muscle in posterolateral thoracotomy, leading to deformity (16). With the improvement of minimally invasive technology and anesthesia management, it is possible to treat CPAM in infants under 3 months old by thoracoscopy. Compared with thoracotomy, VATS has the advantages of smaller incisions, less drainage time, and shorter hospitalization time (17-19). However, there are few related studies at home and abroad because thoracoscopic surgery for infants under 3 months old has narrow chest space. Excessive stretching and squeezing of lung tissue will cause respiratory and circulatory instability. Therefore, how to improve the narrow operating space as much as possible to ensure the stability of the vital signs of the children is the key to the operation’s success.
In this study, all the children did not switch to thoracotomy, and the surgical effect was satisfactory. We have some experience in improving the surgical operation space. First and foremost, one-lung ventilation is necessary to obtain sufficient visualization and anatomical space. At present, the bronchial occluder is mainly used to selectively occlude the main bronchus of the affected side to achieve one-lung ventilation. Anesthesiology collaboration is required because the patient’s trachea is small under 3 months of age, and it is difficult to place the occluder. Moreover, the left and right main bronchi are short, and the placed occlude easily migrates into different aspects of the airway. If the occlude is dragged into the main bronchi to cause blockage, it will cause acute hypoxia, which will be life-threatening in severe cases. Due to the accumulated clinical experience of bronchial occlusion in children with CPAM, all 12 children in this group were successfully occluded. Usually, the anesthesiologist will first keep the children in the supine position and place the bronchial occluder under endoscope, then make them in the lateral position. It is important to observe the bronchial occluder under endoscope immediately after changing the body position to ensure that there is no migrations. Even if it is displaced, it is relatively easy to adjust. It is difficult to place bronchial occluder in the lateral position. Additionally, the anesthesiologist closely monitored the respiratory condition during the operation, and there was no case that the occluder was removed due to respiratory instability. Second, artificial pneumothorax can gain more operating space, but too high pressure will affect the vital signs of children. For children less than 3 months of age, we suggest setting the pressure to below 5 mmHg. Third, for artificial pneumothorax and suspension of lung tissue, in the view of the thoracoscope, select a suitable position in the intercostal space on the skin, pull out the needle after the needle silk thread is punctured into the chest cavity, and suspend the diseased lung tissue on the chest wall with the silk thread to expose the hilum, which is convenient for operation (Figure 8). Fourth, if possible, use the electric hook to cauterize the huge vesicles which look like balloons under the enlarged visual field of thoracoscope, so as to reduce the volume of the lesion and increase the operating space (Figure 9). However, attention should be taken not to touch the blood vessels to avoid bleeding affecting the surgical visual field.

Fetal edema is the leading risk factor for poor prognosis of children with CPAM, and the occurrence of fetal edema is directly related to the size of the CPAM. Thus, the risk of fetal edema is often predicted by evaluating the size of the focus in clinical practice. CVR is the specific measurement index, and the calculation formula is (length × height × width × 0.523 of the lesion)/head circumference in centimeter. The larger the CVR value, the larger the relative volume of the lesion and the more pronounced the thoracic space-occupying effect. Notably, when CVR >1.6, the risk of edema in the fetus of children with CPAM increases, Timothy reports those with CVRs greater than 1.6 have the greatest risk of edema, death, and possible fetal intervention (20). Ocal and his colleagues’ research also predicts early surgery with high sensitivity and specificity when getting a threshold value of 0.76 for CVR (21). In this study, the children with CVR >1.6 did not have obvious edema during pregnancy, so no cases were undergoing intrauterine intervention, such as thoraco-amniotic shunt placement. Furthermore, this study shows that although the prognosis of fetal edema is not good, the incidence of fetal edema is low. However, there are few statistical studies on the incidence of fetal edema in China, and a large sample and multi-center study are needed. In addition, we hold that CVR value is not the primary factor to determine the timing of operation, but it is an important factor.
The limitation of this study was that the number of cases in this study was small, and it could only provide limited treatment experience. It is necessary to accumulate a large number of cases to improve the treatment technology further. Additionally, the follow-up time of this study was short. Although the effect is good during the follow-up period, the long-term effect outside the follow-up period is unknown. In the future follow-up process, we will pay close attention to the children’s lung development and lung function.
Conclusions
In short, infants with CPAM accompanied by severe clinical symptoms less than 3 months of age as discussed in the preceding content should be operated as soon as possible. VATS is an alternative surgical approach. With the improvement of minimally invasive technology and anesthesia management, VATS is relatively safe and effective in treating children with such conditions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-475/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-475/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-475/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-475/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethical Committee of the Shanghai Children’s Hospital (No. 2023R064-E01). The informed consent was waived by the Medical Ethics Committee of the Shanghai Children’s Hospital due to the retrospective design of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stocker JT. Congenital pulmonary airway malformation: a new name for and an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathology 2002;41:424-30.
- Stocker LJ, Wellesley DG, Stanton MP, et al. The increasing incidence of foetal echogenic congenital lung malformations: an observational study. Prenat Diagn 2015;35:148-53. [Crossref] [PubMed]
- Kulaylat AN, Engbrecht BW, Hollenbeak CS, et al. Comparing 30-day outcomes between thoracoscopic and open approaches for resection of pediatric congenital lung malformations: Evidence from NSQIP. J Pediatr Surg 2015;50:1716-21. [Crossref] [PubMed]
- Verhalleman Q, Richter J, Proesmans M, et al. Congenital cystic adenomatoid malformations of the lung: a retrospective study of diagnosis, treatment strategy and postoperative morbidity in surgically treated patients. Eur J Cardiothorac Surg 2022;62:ezac464. [Crossref] [PubMed]
- Makhijani AV, Wong FY. Conservative post-natal management of antenatally diagnosed congenital pulmonary airway malformations. J Paediatr Child Health 2018;54:267-71. [Crossref] [PubMed]
- Kane SC, Da Silva Costa F, Crameri JA, et al. Antenatal assessment and postnatal outcome of fetal echogenic lung lesions: a decade's experience at a tertiary referral hospital. J Matern Fetal Neonatal Med 2019;32:703-9. [Crossref] [PubMed]
- Berman L, Jackson J, Miller K, et al. Expert surgical consensus for prenatal counseling using the Delphi method. J Pediatr Surg 2018;53:1592-9. [Crossref] [PubMed]
- Kapralik J, Wayne C, Chan E, et al. Surgical versus conservative management of congenital pulmonary airway malformation in children: A systematic review and meta-analysis. J Pediatr Surg 2016;51:508-12. [Crossref] [PubMed]
- Stanton M, Njere I, Ade-Ajayi N, et al. Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J Pediatr Surg 2009;44:1027-33. [Crossref] [PubMed]
- Laberge JM, Puligandla P, Flageole H. Asymptomatic congenital lung malformations. Semin Pediatr Surg 2005;14:16-33. [Crossref] [PubMed]
- Conforti A, Aloi I, Trucchi A, et al. Asymptomatic congenital cystic adenomatoid malformation of the lung: is it time to operate? J Thorac Cardiovasc Surg 2009;138:826-30. [Crossref] [PubMed]
- Jelin EB, O'Hare EM, Jancelewicz T, et al. Optimal timing for elective resection of asymptomatic congenital pulmonary airway malformations. J Pediatr Surg 2018;53:1001-5. [Crossref] [PubMed]
- Kim YT, Kim JS, Park JD, et al. Treatment of congenital cystic adenomatoid malformation-does resection in the early postnatal period increase surgical risk? Eur J Cardiothorac Surg 2005;27:658-61. [Crossref] [PubMed]
- Wagenaar AE, Tashiro J, Satahoo SS, et al. Resection of pediatric lung malformations: National trends in resource utilization & outcomes. J Pediatr Surg 2016;51:1414-20. [Crossref] [PubMed]
- Dukleska K, Teeple EA, Cowan SW, et al. Outcomes in Children Undergoing Surgery for Congenital Pulmonary Airway Malformations in the First Year of Life. J Am Coll Surg 2018;226:287-93. [Crossref] [PubMed]
- Makita S, Kaneko K, Ono Y, et al. Risk factors for thoracic and spinal deformities following lung resection in neonates, infants, and children. Surg Today 2017;47:810-4. [Crossref] [PubMed]
- Zhang N, Zeng Q, Chen C, et al. Video-assisted thoracoscopic lobectomy in children: a retrospective single-center study with 175 consecutive cases. Chin J Pediatr Surg 2017;38:591-4.
- Esposito C, Bonnard A, Till H, et al. Thoracoscopic Management of Pediatric Patients with Congenital Lung Malformations: Results of a European Multicenter Survey. J Laparoendosc Adv Surg Tech A 2021;31:355-62. [Crossref] [PubMed]
- Raitio A, Vilkki V, Pakkasjärvi N. Introduction of pediatric thoracoscopic lung resections in a low-volume center - feasibility, outcome and cost analysis. Acta Chir Belg 2023;123:497-501. [Crossref] [PubMed]
- Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg 2002;37:331-8. [Crossref] [PubMed]
- Ocal A, Demirci O, Dizdaroğulları GE, et al. Can we predict the need for postnatal surgery in patients with prenatal fetal lung masses detected by CVR value? J Gynecol Obstet Hum Reprod 2023;52:102526. [Crossref] [PubMed]



