
Short-term change of tibial torsion in children with spastic cerebral palsy after selective dorsal rhizotomy
Highlight box
Key findings
• Selective dorsal rhizotomy (SDR) exhibits short-term efficacy in improving internal tibial torsion among children with spastic cerebral palsy.
What is known and what is new?
• SDR effectively reduces lower limb muscle tone in children with spastic cerebral palsy.
• Children with internal tibial torsion were observed an increase in transmalleolar angles after SDR, particularly in those with younger age (≤4.8 years old) and without severe spasticity on their hamstring pre-op (modified Ashworth Scale ≤3).
What is the implication, and what should change now?
• SDR emerges as a promising surgical intervention for children with spastic cerebral palsy, addressing the improvement of tibial torsion. This approach not only provides a conceptual framework but also offers a viable and practical method.
Introduction
Spastic cerebral palsy (CP) is a prevalent cause of motor dysfunction in children, and its clinical presentation varies based on the degree of increased muscle tone (1). In addition to spasticity in the extremities, patients with spastic CP often experience secondary musculoskeletal deformities, including tibial torsion (2). Tibial torsion can be categorized into internal or external rotation and can have a detrimental impact on gait and overall motor function (3,4). In children with spastic CP, tibial torsion may result from abnormality of multiple lower limb muscles, such as the hip adductor, medial hamstring, and tibialis muscles (2). Timely diagnosis and treatment of tibial torsion are crucial, as persistent rotational misalignment of the lower extremity can lead to lever arm dysfunction. This dysfunction manifests as excessive rotation of the lower extremity and can cause the foot to shift outward or inward, thereby altering knee extension and ankle flexion (5).
Traditional treatments for tibial torsion primarily involve soft tissue surgeries, such as intramuscular decompression, tendon lengthening, or transfer (6). In severe cases, bone remodeling surgeries may be necessary (7-9). Selective dorsal rhizotomy (SDR) offers a promising approach by reducing spasticity in the lower extremity muscles, potentially leading to improvements in tibial torsion (10-14). However, very few studies have investigated the specific effects of SDR on tibial torsion. To address this gap, we conducted a retrospective study to investigate the impact of SDR on tibial torsion. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-339/rc).
Methods
Inclusion criteria
We conducted a retrospective review of consecutive cases diagnosed with spastic CP who underwent lumbosacral SDR at Shanghai Children’s Hospital from July 2019 to November 2022. The study cohort included patients who met the following inclusion criteria: confirmed diagnosis of CP by a multi-disciplinary team, age ranging from 3 to 18 years, no history of relevant orthopedic surgeries such as tendon lengthening or joint surgeries, good cognitive ability, and completion of both pre-operative and post-operative physical examinations performed by a single physiotherapist. Ethical approval for this study was obtained from the Ethics Committee of Shanghai Children’s Hospital (approval No. 2020R069-E02). This study was conducted in accordance with the relevant guidelines and the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all the patients or patients’ legal guardians.
SDR procedure
All SDR surgeries were performed by a consistent team of surgeons with expertise in the procedure (15). The surgical intervention was carried out under general anesthesia. Intraoperative electromyography recordings were obtained using needle electrodes placed in specific muscle groups, including the anal sphincter, bilateral hip adductors, quadriceps, hamstrings, tibialis anterior, medial and lateral gastrocnemius, and peroneus longus. During the procedure, intraoperative neurophysiological monitoring was employed to ensure the integrity and functionality of the nervous system. To maintain the stability of the monitoring system, the minimum alveolar concentration of sevoflurane was carefully controlled and kept below 0.5, while the patient’s core body temperature was maintained within the range of 36 to 37 ℃. The patient was positioned prone with the head lowered, and a single-level laminotomy was performed below the conus medullaris, typically at the L2–L3 level. This approach allowed for exposure of the cauda equina after opening the dura. The nerve roots/rootlets were electrically tested using a bipolar probe to identify the motor and sensory nerve fibers (16). Following the identification of specific dorsal roots/rootlets that met the rhizotomy protocol, partial transection was performed (17,18). Once all nerve roots/rootlets were tested and addressed, the surgical incision was meticulously closed layer by layer. This standardized surgical technique and monitoring protocol were consistently followed for all SDR procedures in the study (19).
Physical assessment before and after SDR
The motor function, muscle tone and joint range of motion of all children were assessed by one single physiotherapist before SDR. Motor function was measured by two methods, the gross motor function classification system (GMFCS) and gross motor function measure-66 (GMFM-66) (20,21). GMFCS is a five-grade classification system for determination of motor function of CP. Patients classified as the highest GMFCS level I were those who can walk without limitations, and those evaluated as the worst level V were those who needed to be dependent on humans and equipment to move. GMFM-66 is an observational clinical tool for evaluation of motor function in patients suffering from CP. The GMFM-66 scoring system is a four-point scale that consists of 66 items organized into five dimensions of gross motor function. In this system, a five-year-old child without motor disabilities achieves the maximum score of 100. To assess the muscle tone of bilateral lower extremities in all patients, the modified Ashworth Scale (mAS) was used (22,23). The mAS system includes six grades:
- Grade 0 (mAS score =0): no increase in muscle tone.
- Grade 1 (mAS score =1): slight increase in muscle tone, observed as a catch and release or minimum resistance at the end of the range of motion when the affected part is moved in flexion or extension.
- Grade 1+ (mAS score =2): slight increase in muscle tone, characterized by a catch followed by minimal resistance throughout the remaining range of movement.
- Grade 2 (mAS score =3): moderate increase in muscle tone.
- Grade 3 (mAS score =4): significant increase in muscle tone.
- Grade 4 (mAS score =5): affected part rigid in flexion or extension.
In this study, the muscles assessed included bilateral hip adductors, quadriceps femoris, hamstrings, gastrocnemius, and soleus. The target muscles, except for the quadriceps femoris, were considered muscles evaluated as mAS level 2 or higher before the SDR procedure. Two weeks after SDR, patients were discharged and received another physical examination.
Measurement and definition of tibial torsion
In this study, we employed the transmalleolar angle (TMA) as a measurement to evaluate the presence of internal/external tibial torsion in patients before undergoing SDR (22-24). To measure the TMA, the patient was positioned in a supine position, with both feet extended beyond the edge of the bed. The midpoint of the patella was marked at the center of the medial and lateral sides of the knee. Subsequently, the highest points of the inner and outer ankles were marked on both sides. With the midpoint of the patella directed upwards and the knee in a neutral position, a gravity goniometer was placed at the highest points of the inner and outer ankles on each side to accurately measure the TMA (Figure 1). The TMA was assessed both before and after the SDR procedure, with a larger degree indicating a greater external rotation of the lower tibial segment (foot end) in relation to the upper tibial segment (knee end). The classification of normal and internal/external tibial torsion was based on the age-matched normal range of TMA provided by the Shanghai Disabled Persons’ Federation (Table 1). All limbs included in the study were categorized into three groups: the internal tibial torsion group, consisting of limbs with measured values below the normal range; the normal group, comprising limbs within the normal range; and the external tibial torsion group, consisting of limbs with measured values above the normal range.

Table 1
| Age (years) | Normal range for TMA (°) |
|---|---|
| 0< age <2 | 0< TMA <2 |
| 2≤ age <4 | 2≤ TMA <4 |
| 4≤ age <5 | 4≤ TMA <8 |
| 5≤ age <6 | 8≤ TMA <13 |
| age ≥6 | 13≤ TMA ≤18 |
TMA, transmalleolar angle.
Statistical analysis
SPSS 26.0 (IBM, Illinois, USA) was used for statistical analysis. All continuous data were demonstrated by mean ± standard deviation. Preoperative and postoperative comparisons were analyzed by the Wilcoxon paired rank sum test, and comparisons between groups of data were made by the student t-test or Mann-Whitney rank sum test as appropriate. Fisher exact test or Pearson χ2 test was used for comparison of categorical variables. A P value less than 0.05 was considered as statistically different.
Results
A total of 148 children (113 males, 35 females) met the inclusion criteria for this study. The mean age was 6.0±2.2 years, ranging from 3 to 16 years. Among them, 18 (12.2%) had hemiplegia, 106 (71.6%) had diplegia, and 24 (16.2%) had quadriplegia as their topographical subtype. Prior to the surgery, 16 (10.8%) children were classified as GMFCS level I, 50 (33.8%) as level II, 55 (37.2%) as level III, 25 (16.9%) as level IV, and 2 (1.4%) as level V. The preoperative GMFM-66 score was 62.1±11.8.
During the SDR procedure, an average of 55.9±11.6 nerve roots/rootlets were electrically stimulated, and a total of 7.2±3.2 dorsal roots/rootlets (left side: 4.1±2.3, right side: 3.1±2.1) were partially transected according to the rhizotomy protocol. Following SDR, there was a significant decrease in muscle tone in the lower extremities (refer to Table 2). The mAS score of bilateral adductors decreased after SDR (left side: 2.9±1.3 vs. 1.7±0.9, P<0.0001; right side: 2.9±1.2 vs. 1.7±0.8, P<0.0001). Additionally, the value of mAS scores for quadriceps decreased (left side: 0.9±1.2 vs. 0.1±0.4, P<0.0001; right side: 0.8±1.2 vs. 0.1±0.4, P<0.0001), and the muscle tone of bilateral hamstrings also exhibited a significant reduction after the operation (left side: 3.2±1.1 vs. 2.7±0.9, P<0.0001; right side: 3.3±1.0 vs. 2.8±0.8, P<0.0001). Furthermore, the mAS score of bilateral anterior tibialis decreased (left side: 0.6±0.5 vs. 0.3±0.5, P<0.0001; right side: 0.6±0.6 vs. 0.3±0.4, P<0.0001), Similarly, the mAS in bilateral gastrocnemius displayed a significant decrease (left side: 4.6±0.7 vs. 3.9±0.8, P<0.0001; right side: 4.8±0.5 vs. 4.0±0.7, P<0.0001), and the muscle tone of bilateral soleus also decreased (left side: 4.1±1.0 vs. 3.0±0.9, P<0.0001; right side: 4.2±0.8 vs. 3.1±0.9, P<0.0001).
Table 2
| Characteristics | Pre-operational status | Post-operational status | P value |
|---|---|---|---|
| Spasticity (modified Ashworth Scale Score) | |||
| Hip adductors | |||
| Left | 2.9±1.3 | 1.7±0.9 | <0.0001 |
| Right | 2.9±1.2 | 1.7±0.8 | <0.0001 |
| Quadriceps | |||
| Left | 0.9±1.2 | 0.1±0.4 | <0.0001 |
| Right | 0.8±1.2 | 0.1±0.4 | <0.0001 |
| Hamstrings | |||
| Left | 3.2±1.1 | 2.7±0.9 | <0.0001 |
| Right | 3.3±1.0 | 2.8±0.8 | <0.0001 |
| Tibialis anterior | |||
| Left | 0.6±0.5 | 0.3±0.5 | <0.0001 |
| Right | 0.6±0.6 | 0.3±0.4 | <0.0001 |
| Gastrocnemius | |||
| Left | 4.6±0.7 | 3.9±0.8 | <0.0001 |
| Right | 4.8±0.5 | 4.0±0.7 | <0.0001 |
| Soleus | |||
| Left | 4.1±1.0 | 3.0±0.9 | <0.0001 |
| Right | 4.2±0.8 | 3.1±0.9 | <0.0001 |
| Range of motion (°) | |||
| Hip abduction | |||
| Left | 74.7±12.5 | 82.3±8.6 | <0.0001 |
| Right | 75.0±12.5 | 82.0±9.3 | <0.0001 |
| Knee flexion | |||
| Left | 150.1±4.3 | 151.4±3.7 | <0.0001 |
| Right | 150.1±4.6 | 151.3±3.8 | <0.05 |
| Ankle dorsiflexion (knee extended) | |||
| Left | −6.3±15.0 | 6.9±10.6 | <0.0001 |
| Right | −6.7±14.0 | 3.9±14.8 | <0.0001 |
| Ankle dorsiflexion (knee flexed) | |||
| Left | 6.2±12.8 | 15.8±7.6 | <0.0001 |
| Right | 5.5±11.8 | 14.5±7.8 | <0.0001 |
| Tibial torsion degree | |||
| Left | 3.1±2.9 | 6.0±2.1 | <0.0001 |
| Right | 4.2±3.5 | 6.7±2.6 | <0.0001 |
Data are presented as mean ± standard deviation.
The range of motion in three joints (hip, knee, and ankle) increased after SDR. The range of motion for bilateral hip abduction increased (left side: 74.7±12.5 vs. 82.3±8.6, P<0.0001; right side: 75.0±12.5 vs. 82.0±9.3, P<0.0001). Left ankle dorsiflexion with knee extended also showed an increase (−6.3±15.0 vs. 6.9±10.6, P<0.0001), and right ankle dorsiflexion with knee extended exhibited an increase as well (−6.7±14.0 vs. 3.9±14.8, P<0.0001). The range of motion for left ankle dorsiflexion with knee flexed increased (6.2±12.8 vs. 15.8±7.6, P<0.0001), and right ankle dorsiflexion with knee flexed also increased (5.5±11.8 vs. 14.5±7.8, P<0.0001). Additionally, bilateral TMA increased after SDR. The degree of TMA increased on the right side (3.1±2.9 vs. 6.0±2.1, P<0.0001) and on the left side (4.2±3.5 vs. 6.7±2.6, P<0.0001).
A total of 296 limbs from 148 cases were assessed in this study. Prior to the surgery, 257 (86.8%) limbs were classified as having internal tibial torsion, 35 (11.8%) were classified as normal, and 4 (1.4%) were classified as having external tibial torsion. It was observed that limbs with internal tibial torsion tended to belong to older patients compared to limbs with a normal angle (6.7±2.2 vs. 5.2±2.2 years old, P<0.001). The muscle tone of the tibialis anterior and soleus was higher in limbs with internal tibial torsion compared to those with a normal angle. Additionally, the limbs with a normal angle exhibited greater joint mobility, particularly in hip abduction and ankle dorsiflexion, when compared to limbs with internal rotation (Table 3).
Table 3
| Characteristics | Internal tibial torsion (n=257) | Normal (n=35) | External tibial torsion (n=4) |
|---|---|---|---|
| Age (years) | 6.7±2.2 | 5.2±2.2*** | 5.2±3.2 |
| Pre-op gross motor function classification system | 2.6±0.9 | 2.7±0.9 | 2.8±1.3 |
| Pre-op gross motor function measurement-66 | 62.2±11.7 | 61.4±11.8 | 62.0±18.5 |
| Spasticity (modified Ashworth Scale Score) | |||
| Hip adductors | 2.9±1.2 | 3.0±1.3 | 3.0±2.0 |
| Quadriceps | 0.9±1.2 | 0.9±1.2 | 0.3±0.5 |
| Hamstrings | 3.2±1.1 | 3.3±1.0 | 3.3±1.5 |
| Tibialis anterior | 0.6±0.5 | 0.4±0.5* | 0.3±0.5 |
| Gastrocnemius | 4.7±0.6 | 4.7±0.6 | 4.5±1.0 |
| Soleus | 4.2±0.9 | 3.9±0.8* | 4.0±1.4 |
| Range of motion | |||
| Hip abduction | 74.4±12.6 | 78.6±11.1* | 68.8±11.8 |
| Knee flexion | 150.1±4.6 | 150.3±3.4 | 150.0±0.0 |
| Ankle dorsiflexion (knee extended) | −7.2±14.6 | −0.6±11.7** | −11.3±18.0 |
| Ankle dorsiflexion (knee flexed) | 5.2±12.5 | 10.7±9.7** | 6.3±13.8 |
| Tibial torsion degree | 3.2±2.9 | 5.9±3.6*** | 9.8±7.5 |
Data are presented as mean ± standard deviation. *, P<0.05 compared with group classified as internal tibial torsion. **, P<0.01 compared with group classified as internal tibial torsion. ***, P<0.001 compared with group classified as internal tibial torsion.
In order to investigate the factors contributing to internal rotation of the tibia, we further examined the clinical data of 18 hemiplegic children, using the intact limbs of these patients as a control group in comparison to the affected limbs. No significant difference was found in the TMA between the intact and affected extremities, although the spasticity of muscles in the affected limbs was much higher than that of the intact limbs (Table 4).
Table 4
| Characteristics | Affected limbs (n=18) | Intact limbs (n=18) | P value |
|---|---|---|---|
| Age, years | 6.8±2.0 | – | |
| Gender | |||
| Boys | 26 (72.2) | – | |
| Girls | 10 (27.8) | – | |
| Gross motor function classification system | |||
| Level I | 24 (66.7) | – | |
| Level II | 12 (33.3) | – | |
| Level III | – | – | |
| Level IV | – | – | |
| Level V | – | – | |
| Gross motor function measurement-66 | 78.83±5.57 | – | |
| Spasticity (modified Ashworth Scale Score) | |||
| Hip adductors | 0.9±0.7 | 0.3±0.6 | <0.01 |
| Quadriceps | 0.0±0.0 | 0.0±0.0 | >0.99 |
| Hamstrings | 2.1±0.9 | 0.7±0.7 | <0.0001 |
| Tibialis anterior | 0.2±0.4 | 0.1±0.2 | 0.296 |
| Gastrocnemius | 4.9±0.2 | 3.2±0.9 | <0.0001 |
| Soleus | 4.8±0.4 | 1.9±1.0 | <0.0001 |
| Range of motion | |||
| Hip abduction | 86.1±3.7 | 87.2±3.9 | 0.299 |
| Knee flexion | 151.4±2.9 | 151.4±2.9 | >0.99 |
| Ankle dorsiflexion (knee extended) | −16.7±12.9 | 12.8±5.5 | <0.0001 |
| Ankle dorsiflexion (knee flexed) | −10.3±12.3 | 17.2±4.3 | <0.0001 |
| Tibial torsion degree | 4.4±3.3 | 6.2±5.5 | 0.301 |
Data are presented as mean ± standard deviation or n (%).
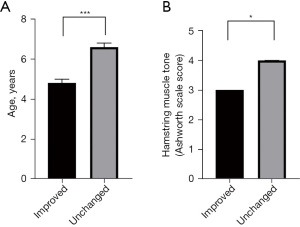
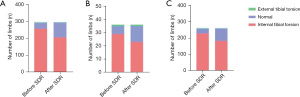
The preoperative and postoperative changes in the TMA for the 296 assessed limbs are presented in Figure 2. Among the limbs with internal tibial torsion, 21.0% (54/257) improved and changed to a normal angle after SDR, although the other 79% (203/257) were still grouped as internal tibial torsion, 65% (166/257) of them showed an outward change in the TMA angle after SDR, indicating that the improvement of internal tibial torsion could be seen in a majority of limbs. Additionally, one limb (25.0%) with external tibial torsion improved and changed to a normal angle. When comparing the differences between the 54 improved limbs and the 203 unchanged limbs with internal tibial torsion, several observations were made. Firstly, the limbs that showed improvement tended to belong to younger patients at the time of surgery, which reached statistical significance (Figure 3A, P<0.0001). Furthermore, limbs with a lower preoperative mAS score for the hamstrings were more likely to improve compared to those with a higher mAS score for the hamstrings (Figure 3B). The change in tibial torsion after SDR was significant when compared to the preoperative status, as confirmed by a chi-square test involving all 296 limbs, with a P value less than 0.0001 (Figure 4A). No significant difference was observed when comparing the preoperative and postoperative statuses of the limbs in the 36 limbs of hemiplegic children (Figure 4B). However, when excluding these 36 limbs and considering the remaining 260 limbs, a statistically significant difference was observed (Figure 4C), with a P value less than 0.0001.

Discussion
Previous study has reported an association between tibial torsion in children with CP and abnormal muscle tone in the hip adductors, hamstrings, and posterior tibial muscles, which aligns with our findings (2). Our study revealed that limbs classified as internal rotation had higher muscle tone in the soleus compared to normal limbs. Additionally, we observed an association between elevated muscle tone in the anterior tibial muscles and internal tibial rotation. In normally developing children, physiological internal tibial rotation is present at a young age and gradually resolves by 4–5 years old (24). However, children with CP appear to have difficulty in naturally correcting internal tibial rotation during development. In our cohort study, we found that limbs with internal tibial rotation tended to belong to older children. This may be attributed to the fact that younger patients were affected by spasticity for a shorter period, resulting in a relatively normal tibial angle. However, it remains unclear whether patients with a normal angle would deteriorate over time.
SDR is a safe and effective surgical approach for reducing muscle spasticity in the lower limbs of children with spastic CP (25). The patients included in this study demonstrated a significant improvement in TMA after the surgery, with a statistically significant increase in values compared to the preoperative period. It is important to note that these results reflect short-term changes after surgery, excluding the influence of postoperative rehabilitation and other interventions. Based on these findings, it is reasonable to speculate that the decrease in muscle tone in the lower limbs contributed to the improvement of tibial torsion. Furthermore, it can be presumed that elevated muscle tone in the lower limbs might be a contributing factor to tibial torsion in individuals with spastic CP. However, it is important to consider that tibial torsion in this population may not solely result from spasticity. Another potential cause of this phenomenon could be the development of secondary skeletomuscular deformities due to increased muscle tone. This speculation is supported by the observation that patients with younger age and lower spasticity in the hamstrings tended to experience greater improvement in tibial torsion following SDR.
In an attempt to identify potential causes of tibial torsion, we reviewed the data of hemiplegic children, as their unaffected limbs could serve as a control group. However, we did not find any significant differences in TMA among the 18 hemiplegic patients included in this study. It is possible that the lack of significant differences could be attributed to the limited sample size of hemiplegic patients and the compensatory mechanisms present in the intact limbs of these individuals. Further studies with larger sample sizes are warranted to better understand this issue and provide more conclusive findings.
However, we observed a single case where the tibial angle changed from normal to internal rotation after surgery. Upon further examination of this case, we found that the joint movement angles were significantly lower in comparison to the normal group, Additionally, the spasticity levels of the adductor and soleus muscles were higher than the normal level. Based on these findings, we speculate that the previously normal tibial angle may have been a result of compensatory mechanisms. Although there was improvement in muscle tone and joint range of motion following surgery, the TMA shifted into the abnormal range. It is possible that the situation may change with the implementation of a rehabilitation program.
Our findings indicate that younger patients tend to benefit more from SDR, which is consistent with previous studies demonstrating that patients below 6 years of age show better response to SDR compared to older patients (15).
Limitation
However, several limitations existed in this study. Firstly, the follow-up period was relatively short, and it remains uncertain whether the improvement in tibial torsion will be sustained in the long term. Secondly, the study was limited by the small number of cases, retrospective design, and the fact that it was conducted at a single center, which may impact the reliability of the results. Nevertheless, considering that this study aimed to preliminarily investigate the changes in tibial torsion after SDR, we believe that SDR can offer an alternative treatment option for tibial torsion in children with spastic CP. Further research is warranted to address these limitations and provide more comprehensive insights into the long-term outcomes of SDR in this population.
Conclusions
SDR can reduce muscle tone and improve joint mobility in children with spastic CP. Patients with older ages and higher spasticity in tibialis anterior and soleus are more likely to develop internal tibial torsion. SDR can improve tibial torsion in patients with spastic CP. Limbs classified as internal tibial torsion tended to improve after SDR if they presented with lower muscle tone of hamstring and derived from patients with younger age.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-339/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-339/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-339/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-339/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the relevant guidelines and the Declaration of Helsinki (as revised in 2013). Ethical approval for this study was obtained from the Ethics Committee of Shanghai Children’s Hospital (approval No. 2020R069-E02). Informed consent was taken from all the patients or patients’ legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Min JJ, Kwon SS, Kim KT, et al. Evaluation of factors affecting external tibial torsion in patients with cerebral palsy. BMC Musculoskelet Disord 2021;22:684. [Crossref] [PubMed]
- Stefko RM, de Swart RJ, Dodgin DA, et al. Kinematic and kinetic analysis of distal derotational osteotomy of the leg in children with cerebral palsy. J Pediatr Orthop 1998;18:81-7.
- Radler C, Kranzl A, Manner HM, et al. Torsional profile versus gait analysis: consistency between the anatomic torsion and the resulting gait pattern in patients with rotational malalignment of the lower extremity. Gait Posture 2010;32:405-10. [Crossref] [PubMed]
- Aktas S, Aiona MD, Orendurff M. Evaluation of rotational gait abnormality in the patients cerebral palsy. J Pediatr Orthop 2000;20:217-20.
- Theologis T. Lever arm dysfunction in cerebral palsy gait. J Child Orthop 2013;7:379-82. [Crossref] [PubMed]
- Staheli LT. Torsion--treatment indications. Clin Orthop Relat Res 1989;61-6.
- Er MS, Abousamra O, Rogers KJ, et al. Long-term Outcome of Internal Tibial Derotation Osteotomies in Children With Cerebral Palsy. J Pediatr Orthop 2017;37:454-9. [Crossref] [PubMed]
- Er MS, Bayhan IA, Rogers KJ, et al. Long-term Outcome of External Tibial Derotation Osteotomies in Children With Cerebral Palsy. J Pediatr Orthop 2017;37:460-5. [Crossref] [PubMed]
- Walton DM, Liu RW, Farrow LD, et al. Proximal tibial derotation osteotomy for torsion of the tibia: a review of 43 cases. J Child Orthop 2012;6:81-5. [Crossref] [PubMed]
- Tedroff K, Hägglund G, Miller F. Long-term effects of selective dorsal rhizotomy in children with cerebral palsy: a systematic review. Dev Med Child Neurol 2020;62:554-62. [Crossref] [PubMed]
- Morota N. Functional posterior rhizotomy for treatment of spasticity. No Shinkei Geka 2010;38:209-28.
- Dudley RW, Parolin M, Gagnon B, et al. Long-term functional benefits of selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg Pediatr 2013;12:142-50. [Crossref] [PubMed]
- Nordmark E, Josenby AL, Lagergren J, et al. Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr 2008;8:54. [Crossref] [PubMed]
- Tedroff K, Löwing K, Jacobson DN, et al. Does loss of spasticity matter? A 10-year follow-up after selective dorsal rhizotomy in cerebral palsy. Dev Med Child Neurol 2011;53:724-9. [Crossref] [PubMed]
- Zhan Q, Yu X, Jiang W, et al. Whether the newly modified rhizotomy protocol is applicable to guide single-level approach SDR to treat spastic quadriplegia and diplegia in pediatric patients with cerebral palsy? Childs Nerv Syst 2020;36:1935-43. [Crossref] [PubMed]
- Young K. Correlating nerve rootlet and spastic muscle activation intraoperatively during selective dorsal rhizotomy. Dev Med Child Neurol 2023;65:9-10. [Crossref] [PubMed]
- Graham D, Aquilina K, Cawker S, et al. Single-level selective dorsal rhizotomy for spastic cerebral palsy. J Spine Surg 2016;2:195-201. [Crossref] [PubMed]
- Jiang W, Jiang S, Yu Y, et al. Improvement of the gait pattern after selective dorsal rhizotomy derives from changes of kinematic parameters in the sagittal plane. Front Pediatr 2022;10:1047227. [Crossref] [PubMed]
- Xiao B, Constatntini S, Browd SR, et al. The role of intra-operative neuroelectrophysiological monitoring in single-level approach selective dorsal rhizotomy. Childs Nerv Syst 2020;36:1925-33. [Crossref] [PubMed]
- Russell DJ, Leung KM, Rosenbaum PL. Accessibility and perceived clinical utility of the GMFM-66: evaluating therapists’ judgements of a computer-based scoring program. Phys Occup Ther Pediatr 2003;23:45-58.
- Piscitelli D, Ferrarello F, Ugolini A, et al. Measurement properties of the Gross Motor Function Classification System, Gross Motor Function Classification System-Expanded & Revised, Manual Ability Classification System, and Communication Function Classification System in cerebral palsy: a systematic review with meta-analysis. Dev Med Child Neurol 2021;63:1251-61. [Crossref] [PubMed]
- Novak I, Morgan C, Fahey M, et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr Neurol Neurosci Rep 2020;20:3. [Crossref] [PubMed]
- Lee SH, Chung CY, Park MS, et al. Tibial torsion in cerebral palsy: validity and reliability of measurement. Clin Orthop Relat Res 2009;467:2098-104. [Crossref] [PubMed]
- Mooney JF 3rd. Lower extremity rotational and angular issues in children. Pediatr Clin North Am 2014;61:1175-83. [Crossref] [PubMed]
- Mishra D, Barik S, Raj V, et al. A systematic review of complications following selective dorsal rhizotomy in cerebral palsy. Neurochirurgie 2023;69:101425. [Crossref] [PubMed]


