
The efficacy of extracted Tanshinone compounds for infantile hemangiomas of the skin: results from a pilot study
Highlight box
Key findings
• The study firstly applied the Tanshin extract in treating superficial infantile hemangiomas (IHs), rendering the application of Tanshinone on IHs an innovation.
What is known and what is new?
• The past few decades have witnessed extensive exploration of diverse treating methods in IHs, varying from beta blockers, injections, and laser to surgical interventions, based on the condition’s severity. Oral or topical use of beta blockers has become first-line therapy of IHs.
• Our preclinical studies suggested that Tanshinone, extracted from Tanshin, exerted a benefit on IHs. Thus, we conducted a pilot clinical study to evaluate the safety and efficacy of topical Tanshinone compounds on superficial IHs, suggesting that Tanshinone compounds might be a potentially effective and noninvasive therapy in treating IHs.
What is the implication, and what should change now?
• Chemical improvement of plant extracts has demonstrated enhanced clinical applications, emphasizing the necessity of further research in this area.
Introduction
Background
Infantile hemangiomas (IHs), with a demonstrated frequency of between 5% and 10%, are the most frequently occurring benign tumors during infancy (1). These tumors typically appear within the first few weeks after birth and follow a unique pattern of growth, followed by natural involution. However, even smaller and superficial IHs that develop in aesthetically crucial areas often lead to undesirable cosmetic complications (2). Inadequately treated or untreated tumors, after undergoing regression, usually incur consequences such as local telangiectasia, remaining fibrofatty tissue, and skin laxity, adversely affecting the child’s physical appearance (3). As a result, superficial hemangiomas that are situated on aesthetically sensitive areas such as the face and exposed skin require more assertive, timely, and suitable intervention (4). Pulsed dye laser (PDL) is a conventional treatment for these lesions; however, Asian individuals tend to experience residual complications post-PDL and other similar treatments (5). At present, external preparations of beta blockers are often used as the first choice of treatment for superficial and small-scale IHs (6). In China, the primary treatments are wet compresses using 0.5% timolol eye drops and 2% carteolol eye drops (7). These are, however, constrained by the complicated preparation process, sometimes leading to skin irritation, maceration, and other local side effects such as skin flushing, desquamation, and eczema (8).
Rationale and knowledge gap
The past few decades have witnessed extensive exploration of diverse treatment methods, varying from oral or topical beta blockers, injections, and laser to surgical interventions, based on the condition’s severity (2,9-11). Our preclinical studies suggested that Tanshinone, extracted from Salvia miltiorrhiza (Tanshin), exerted a benefit on IHs (12,13). Thus, we have managed to formulate an external preparation composed of Tanshin extract for treating IHs and conducted a pilot clinical study to evaluate the safety and efficacy of topical Tanshinone compounds on superficial IHs.
Objective
We aimed to assess the clinical efficacy and safety of Tanshinone compounds, extracted from Tanshin, in the treatment of IHs. We present this article in accordance with the TREND reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-555/rc).
Methods
Study design and participants
This study was a single-center, open-label, single-arm pilot study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this study was reviewed and approved by the Xinhua Hospital Research Ethics Committee (No. XHEC-C-2018-006-3) and informed consent was taken from all the patients’ legal guardians. Patients were recruited from the Department of Pediatric Surgery, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. After patient consent was obtained, we collected demographic information, study participants were instructed in diary completion, and the details were collected at 1-, 3-, 6- and 9-month as long-term follow-up by a researcher.
The participants of this study were infants (aged less than 6 months) exhibiting proliferating superficial IHs. Participants had to meet the following criteria: (I) children with a confirmed diagnosis of superficial IHs in line with the International Society for the Study of Vascular Anomalies (ISSVA) classification and nomenclature criteria, identified by strawberry-like erythema on the skin surface with no apparent underlying cyanosis; (II) children who had a single hemangioma in the proliferative phase (i.e., child ≤6 months and the tumor was still exhibiting significant recent growth) with no restrictions on the child’s gender; and (III) parents who were fully aware that this was a clinical trial and willingly signed the informed consent as legal guardians, promising to cooperate with the follow-up examinations within the specified timeframe. Infants were excluded if they were (I) older than 6 months and their hemangioma had ceased to proliferate; (II) being treated with other medications; (III) having local ulcers, infections, or eczema that could interfere with the use of topical medications; (IV) experiencing severe congenital diseases such as congenital heart disease and asthma; and (V) participating in other clinical studies.
Intervention
A topical Tanshinone ointment was applied 3 times daily on the lesions of the children, ensuring an interval of 6–8 hours between applications. Parents were trained to apply a thin layer of the ointment onto the surface of the red tumor (sufficient to cover the lesion) and observe for at least 10 minutes to ensure absorption and prevent the ointment from being wiped off by clothes. The previous layer of the ointment had to be cleaned off before reapplication to prevent accumulation and any potential impact on absorption. The follow-ups were scheduled at the outpatient department at intervals of 1-, 3-, and 6-month, along with a total follow-up duration of 9 months. Family members were guided to capture weekly photographs for observation.
Outcomes
The main goal of the treatment is to decrease cosmetic impairment caused by IHs. Therefore, response to treatment was mainly assessed according to cosmetic improvement by the skin erythema index using SkinColorCatch (previously named Dermacatch; Delfin, Kuopio, Finland) (14) before and after treatment. The Achauer score (15) was evaluated by another two independent physicians after reviewing all clinical photographs of IHs at baseline and after the full course of treatment. All outcomes were assessed at baseline and at 1, 3, and 6 months. The parental satisfaction was evaluated with an assessment score range from 1 to 5, with 5 meaning very satisfactory, and 1 meaning unsatisfactory. Safety analysis primarily emphasized on the incidence of skin local adverse events. The researchers evaluated adverse events in every follow-up and questionnaires were answered by parents of the participants at the end of the long-term follow-up together.
Statistical analyses
Data with normal distribution were expressed as mean ± standard deviation (SD), whereas those with skewed distribution were presented as median (interquartile range). Differences in parameters before and after treatment were analyzed using the paired t-test. Comparisons of categorical variables between groups were carried out using McNemar’s chi-square test. All statistical analyses were performed using the software SPSS 28.0 (IBM Corp., Armonk, NY, USA). A two-sided P value <0.05 was considered statistically significant.
Results
A total of 36 patients underwent the screening process; two patients did not meet the inclusion criteria, and five participants failed to continue the intervention on the first follow-up of the study. Consequently, 29 participants completed the study (Figure 1). The individual characteristics of each participant are detailed in Table 1. At baseline, 7 males and 22 females participated in the study. Their mean age on enrollment was 60 days (SD: 60 days; interquartile range, 45–99 days). The hemangioma locations included across the trunk (44.8%), head (34.5%), and limbs (20.7%), as displayed in Table 2.
Table 1
| No. | Gender† | Age (days) | Lesion site | Baseline skin erythema index | Satisfaction of treatment from parents‡ | Adverse effects |
|---|---|---|---|---|---|---|
| 1 | Female | 37 | Left face | 579 | 3 | Eczema |
| 2 | Female | 51 | Ear | 593 | 5 | Dry skin |
| 3 | Female | 48 | Right thoracic wall | 586 | 5 | – |
| 4 | Male | 60 | Finger | 585 | 5 | – |
| 5 | Male | 50 | Anterior chest | 559 | 5 | – |
| 6 | Female | 57 | Right neck | 568 | 5 | – |
| 7 | Female | 68 | Scalp | 503 | 5 | – |
| 8 | Male | 145 | Left knee | 526 | 3 | – |
| 9 | Female | 78 | Scalp | 555 | 5 | – |
| 10 | Female | 118 | Left thoracic wall | 604 | 5 | – |
| 11 | Female | 120 | Right eyebrow | 566 | 5 | – |
| 12 | Female | 123 | Right thoracic wall | 571 | 5 | – |
| 13 | Female | 44 | Right neck | 516 | 4 | – |
| 14 | Male | 136 | Right shoulder | 553 | 5 | – |
| 15 | Female | 137 | Buttock | 575 | 5 | – |
| 16 | Female | 43 | Back | 591 | 5 | – |
| 17 | Female | 88 | Axillary | 579 | 5 | Dry skin |
| 18 | Female | 36 | Scalp | 506 | 5 | – |
| 19 | Female | 34 | Finger | 618 | 5 | – |
| 20 | Male | 99 | Back | 597 | 5 | – |
| 21 | Female | 108 | Right chest | 593 | 5 | – |
| 22 | Female | 45 | Abdomen | 591 | 4 | Dry skin |
| 23 | Male | 61 | Right knee | 569 | 5 | – |
| 24 | Female | 36 | Frontal part | 599 | 3 | – |
| 25 | Female | 64 | Right waist | 551 | 5 | – |
| 26 | Female | 60 | Right forearm | 532 | 4 | – |
| 27 | Male | 33 | Left face | 566 | 5 | – |
| 28 | Female | 47 | Abdomen | 560 | 5 | – |
| 29 | Female | 63 | Right calf | 546 | 5 | – |
†, gender: males or females; ‡, satisfaction of parents: total score of 5 represents great satisfaction, 1 represents not satisfied at all.
Table 2
| Characteristics | Total (n=29) |
|---|---|
| Males | 7 (24.14) |
| Age (days) | 60 [45, 99] |
| Position | |
| Head face neck | |
| Scalp | 3 (10.34) |
| Forehead | 1 (3.45) |
| Glabella | 1 (3.45) |
| Face | 2 (6.90) |
| Neck | 2 (6.90) |
| Ear | 1 (3.45) |
| Trunk | |
| Shoulder | 1 (3.45) |
| Axilla | 1 (3.45) |
| Back | 2 (6.90) |
| Chest | 5 (17.24) |
| Waist | 1 (3.45) |
| Abdomen | 2 (6.90) |
| Hip | 1 (3.45) |
| Limbs | |
| Knee | 2 (6.90) |
| Calf | 1 (3.45) |
| Forearm | 1 (3.45) |
| Palm | 2 (6.90) |
Data are presented as n (%) or median [interquartile range].
Efficacy assessments of Tanshinone ointment
The skin erythema index was significantly decreased after treatment, which was 566.79±854.67 at baseline (vs. 419.76±328.4 for normal skin), 552.52±645.62 at 1 month of treatment, 513.93±709.42 at 3 months of treatment, and 467.97±1,118.39 at 6 months of treatment (Figure 2). Compared with the baseline, all P values were less than 0.05. However, the skin erythema index at 6 months was still significantly higher than that of normal skin (P<0.001, Table 3).

Table 3
| Outcomes | Effects | P value | Reference |
|---|---|---|---|
| Skin erythema index | |||
| Baseline | 566.79±854.67 | – | – |
| 1-month treatment | 552.52±645.62 | 0.0013 | vs. baseline |
| 3-month treatment | 513.93±709.42 | <0.001 | vs. baseline |
| 6-month treatment | 467.97±1,118.39 | <0.001 | vs. baseline |
| Normal skin | 419.76±328.4 | <0.001 | vs. 6-month |
| Achauer score at 6-month (n=29) | |||
| 0–25% | 1 (3.45) | – | – |
| 26–50% | 13 (44.83) | – | – |
| 51–75% | 15 (51.72) | – | – |
| Achauer score at 9-month follow-up (n=29) | |||
| 51–75% | 2 (6.90) | – | – |
| 76–100% | 27 (93.10) | – | – |
| Follow-up status (n=29) | |||
| Dose addition | 1 (3.45) | – | – |
| Operation | 2 (6.90) | – | – |
| Medication change | 2 (6.90) | – | – |
| Parents’ satisfaction of treatment† (n=29) | |||
| 3 | 3 (10.34) | – | – |
| 4 | 3 (10.34) | – | – |
| 5 | 23 (79.31) | – | – |
| Adverse effects (n=4) | |||
| Eczema | 1 (25.00) | – | – |
| Dry skin | 3 (75.00) | – | – |
Data are presented as mean ± SD or n (%). †, satisfaction of parents: total score of 5 represents very satisfied, 1 represents not satisfied at all. SD, standard deviation.
After 6 months of treatment, 96.55% (n=28) of children had Achauer score improvement higher than 25% and 15 (51.72%) had Achauer score improvement higher than 50%. At 9 months after treatment, 27 (93.10%) children had an Achauer score improvement higher than 75%. A case of IHs before and after treatment with Tanshinone ointment is shown in Figure 3. During 6 months of treatment, 27 (93.10%) parents expressed their satisfaction with a satisfactory score of 5, indicative of high levels of satisfaction.
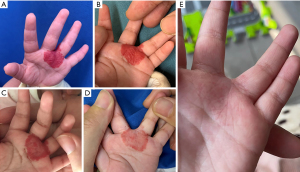
Adverse events
Reported adverse events included one case of eczema and three cases of dry skin recorded by observers on follow-up; no other self-reported adverse events were documented.
Discussion
Key findings
This study proposed a potential new drug to treatment for IHs. We evaluated the efficacy and safety of topical Tanshinone on superficial IHs and found that the skin erythema index was significantly decreased and the satisfaction of the parents was relatively high. Few adverse effects were reported.
Limitations
There are several limitations of this study should be acknowledged. Given that this is a pilot study, the sample size is small. We only aimed to evaluate the potential safety and efficacy of Tanshinone. Further larger-scale, randomized controlled clinical trial research should be conducted to confirm the efficacy and safety of Tanshinone. Additionally, as the study was conducted in a single center, the results may not be universally applicable to other populations. We only included the superficial IHs and the patients ages were within 6 months. Further study is warranted to evaluate the efficacy and safety of topical Tanshinone on other types of IHs.
Comparison with similar studies
Non-selective beta blocker has been proved by the Food and Drug Administration (FDA) as the first-line agent for IHs with dramatic response. To reduce the side effects caused by systemic administration of propranolol, timolol maleate treatment has been increasingly used as an alternative to systemic beta blockers and watchful waiting for many IH patients in recent years. Topical timolol maleate 0.5% ointment with a maximum dose of 0.5 mg per day is a safe and effective option for small superficial lesions (16,17). A large cohort study demonstrated that some children who do not receive treatment are at risk for changes that require early intervention. The treatment paradigm for hemangiomas should be changed (18). Our study firstly applied topical use of Tanshinone in treating superficial IHs, which can be rendered as an innovation.
Explanations of findings
The therapeutic evaluation of this study combines both subjective scores and the objective erythema index. The results indicate that the topical application of Tanshin extract can control hemangioma growth at an early stage, promoting regression and preventing complications in certain children. We assume that the treatment was more effective on lesions located on the palm and between the fingers, yet less effective on the face, forehead, and scalp, possibly due to the local retention of the drug. But the hypothesis was only based on the subjective feelings of the observers, which are not statistically significant, and will be further verified when the sample is expanded. Parents expressed high satisfaction with the treatment, particularly with its long-term effects. Hemangiomas affecting cosmetic areas can induce negative emotions, such as anxiety and low self-esteem in parents and children, thereby accentuating the importance of psychological factors in the treatment of IHs (19). Timely topical drug use can prevent overtreatment driven by anxiety. The drawbacks of this study include the lack of a control group and limited case number, emphasizing that this was only a pilot study.
In the early laboratory work with Tanshinone extracts, we discovered that eight Tanshinone monomers could significantly inhibit the proliferation of hemangiomas. Cytological and animal experiments showed that the potency of dihydrotanshinone I (DHTS) was 20 times that of propranolol, and that it differs from the possible mechanism of propranolol (20). We discovered for the first time that a series of Tanshinone compounds isolated from the fat-soluble extract of Tanshin (Tanshinone extract) have a significant inhibitory impact on the proliferation of hemangioma cells (positive control: propranolol). DHTS had the most substantial effect, significantly superior to the inhibitory effect of the control propranolol on hemangioma cells. Investigation of the action mechanism revealed that Tanshinone extract significantly promotes hemangioma cell apoptosis, which is time- and concentration-dependent. The results of western blot and tube formation demonstrated that Tanshinone extract acted on hemangioma cells through the FAS/FASL and mitochondrial pathways simultaneously, inducing apoptosis and inhibiting the angiogenesis process of the hemangioma endothelium (12). In order to explore the potential mechanism of DHTS, our results suggest that DHTS downregulates HIF-1α expression by interfering with its post-transcriptional processing, and that the RNA-binding protein HuR is involved in this mechanism (13). Our findings provide a theoretical basis for the clinical translation of DHTS and insight into the pathogenic mechanism of IHs.
Thus, in order to develop an original treatment of IHs, we attempted to administer Tanshin extract, in the form of a Tanshinone topical preparation. Conventional treatment options for IHs, such as surgical resection or laser treatment, have notable limitations. Topical medication for hemangioma is currently becoming the first line of treatment in superficial IHs (6,9,16,17). Reported local adverse reactions include erythema, peeling, ulceration, crusting, and superficial scars. If these are administered topically on mucosal surfaces, thinner skin, or the ulcerated area of hemangioma, it may amplify the systemic absorption of the drug, potentially inducing systemic adverse reactions such as decreased heart rate, hypotension, allergies, diarrhea, vomiting, and hypoglycemia (8,10). Given this backdrop, our team began with a topical Tanshinone preparation focused on treating local lesions, accelerating hemangioma resolution effectively, and avoiding systemic side effects. In comparison to timolol and carteolol eye drops, it may offer convenient administration, establishing itself as a safe and well-tolerated innovative clinical method.
Implications and actions needed
Considering the discrepancy between the clinical effects and laboratory conclusions, which could be attributed to variations in cell line characteristics and drug dosage forms, further refinement is required. Improvements in the topical preparations, such as enhanced formulation, increased drug concentration, and improved transdermal properties, will be the focus of subsequent research.
Conclusions
The pilot study indicated that in certain cases, topical use of Tanshinone compounds might control the growth of superficial IHs and promote lesion regression at an early stage with few complications. Topical use of Tanshinone compounds might be a potentially effective and noninvasive therapy in treating IHs. A multi-centered randomized controlled clinical trial is needed to further evaluate the safety and efficacy of the therapy in a larger scale.
Acknowledgments
We would like to thank all the patients and their parents participated in the prospective study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-555/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-555/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-555/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-555/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol of this study was reviewed and approved by the Xinhua Hospital Research Ethics Committee (No. XHEC-C-2018-006-3) and informed consent was taken from all the patients’ legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Surlis T, De Sa Reilly H, Sadlier M, et al. Infantile haemangiomas. BMJ 2022;378:e068734. [Crossref] [PubMed]
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019;143:e20183475. [Crossref] [PubMed]
- Bauland CG, Lüning TH, Smit JM, et al. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg 2011;127:1643-8. [Crossref] [PubMed]
- Brennan TE, Waner M. O TM. The Tissue Expander Effect in Early Surgical Management of Select Focal Infantile Hemangiomas. JAMA Facial Plast Surg 2017;19:282-6. [Crossref] [PubMed]
- Nakazono M, Kagimoto S, Koike T, et al. Clinical Outcomes of Small Infantile Hemangiomas Treated With Pulsed Dye Laser. Dermatol Surg 2022;48:833-7. [Crossref] [PubMed]
- Muñoz-Garza FZ, Ríos M, Roé-Crespo E, et al. Efficacy and Safety of Topical Timolol for the Treatment of Infantile Hemangioma in the Early Proliferative Stage: A Randomized Clinical Trial. JAMA Dermatol 2021;157:583-7. [Crossref] [PubMed]
- Gan LQ, Wang H, Ni SL, et al. A prospective study of topical carteolol therapy in Chinese infants with superficial infantile hemangioma. Pediatr Dermatol 2018;35:121-5. [Crossref] [PubMed]
- Frommelt P, Juern A, Siegel D, et al. Adverse Events in Young and Preterm Infants Receiving Topical Timolol for Infantile Hemangioma. Pediatr Dermatol 2016;33:405-14. [Crossref] [PubMed]
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: Epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol 2021;85:1379-92. [Crossref] [PubMed]
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med 2015;372:735-46. [Crossref] [PubMed]
- Zheng JW, Wang XK, Qin ZP, et al. Chinese expert consensus on the use of oral propranolol for treatment of infantile hemangiomas (version 2022). Front Oral Maxillofac Med 2022;4:32.
- Cai Y, Lv F, Kaldybayeva N, et al. 15, 16-Dihydrotanshinone I Inhibits Hemangiomas through Inducing Pro-apoptotic and Anti-angiogenic Mechanisms in Vitro and in Vivo. Front Pharmacol 2018;9:25. [Crossref] [PubMed]
- Duan P, Huang Y, Chen K, et al. 15,16-dihydrotanshinone I inhibits EOMA cells proliferation by interfering in posttranscriptional processing of hypoxia-inducible factor 1. Int J Med Sci 2021;18:3214-23. [Crossref] [PubMed]
- Sepaskhah M, Karimi F, Bagheri Z, et al. Comparison of the efficacy of cysteamine 5% cream and hydroquinone 4%/ascorbic acid 3% combination cream in the treatment of epidermal melasma. J Cosmet Dermatol 2022;21:2871-8. [Crossref] [PubMed]
- Achauer BM, Chang CJ, Vander Kam VM. Management of hemangioma of infancy: review of 245 patients. Plast Reconstr Surg 1997;99:1301-8. [Crossref] [PubMed]
- Nagata E, Kashiwagura Y, Okada E, et al. Efficacy and safety of propranolol cream in infantile hemangioma: A prospective pilot study. J Pharmacol Sci 2022;149:60-5. [Crossref] [PubMed]
- Ji Y, Chen S, Yang K, et al. Efficacy and Safety of Propranolol vs Atenolol in Infants With Problematic Infantile Hemangiomas: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2021;147:599-607. [Crossref] [PubMed]
- Baselga E, Roe E, Coulie J, et al. Risk Factors for Degree and Type of Sequelae After Involution of Untreated Hemangiomas of Infancy. JAMA Dermatol 2016;152:1239-43. [Crossref] [PubMed]
- Peng W, Liu H, Chen J, et al. Development and validation of psychological status questionnaire for parents of infantile hemangiomas. Transl Pediatr 2021;10:3261-72. [Crossref] [PubMed]
- Wu ZB, Shi SL, Pan FJ, et al. Propranolol inhibits infantile hemangioma by regulating the miR-424/vascular endothelial growth factor-A (VEGFA) axis. Transl Pediatr 2021;10:1867-76. [Crossref] [PubMed]



