
Clinical features and prediction of risk factors for severe adenovirus pneumonia in children
Highlight box
Key findings
• Severe adenovirus pneumonia in children has a long duration, more serious clinical manifestations, and multiple complications, and fever duration >4.50 days, wheezing, neutrophil ratio (NEUT%) ≥47.60, serum ferritin (SF) ≥139.60 ng/mL are risk factors for severe adenovirus pneumonia.
What is known and what is new?
• Studies have shown that the high-risk factors of severe adenovirus pneumonia include premature birth, underlying diseases, long thermal duration, and significantly elevated C-reactive protein.
• This study found that wheezing, NEUT% ≥47.60, and SF ≥139.60 ng/mL were risk factors for severe adenovirus pneumonia.
What is the implication, and what should change now?
• For adenovirus pneumonia with high risk factors, early attention should be paid to prevention and reasonable treatment, so as to improve the cure rate and prognosis.
Introduction
Childhood adenovirus pneumonia accounts for about 4–10% of all childhood pneumonia cases (1-3). The disease has an abrupt onset and rapid progression, and it is not easy to distinguish it from other respiratory diseases in the early stage. It has a higher susceptibility to multiple complications, and a mortality rate of more than 50% (4-6). This study retrospectively analyzed the cases of children with adenovirus pneumonia who were hospitalized in Tianjin Children’s Hospital from January 2019 to December 2021, so as to explore the risk factors of severe adenovirus pneumonia, and provide clinical basis for early diagnosis and reasonable treatment. We present this article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-312/rc).
Methods
Children with adenovirus pneumonia (202 cases) admitted to Tianjin Children’s Hospital (Children’s Hospital of Tianjin University) from January 2019 to December 2021 were selected for the study, and their clinical records and laboratory examination data were collected retrospectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Tianjin Children’s Hospital (No. 2022-LXKY-013) and informed consent was obtained from the patients’ parents or legal guardians.
Diagnostic criteria for adenovirus pneumonia in children
Diagnosis was made according to the technical guidelines for prevention and control of human adenovirus (HAdV) respiratory infection and the treatment protocol for adenovirus pneumonia in children, meeting the diagnostic criteria for pneumonia and having evidence of adenovirus infection established diagnosis of adenovirus pneumonia (7,8). Adenovirus infection test: nasopharyngeal swab, sputum, and alveolar lavage fluid samples were collected and tested for adenovirus nucleic acid by real-time quantitative polymerase chain reaction (qRT-PCR), and a positive viral load of >l03 copies/mL for any of the above specimens was diagnosed as adenovirus infection.
Clinical grouping criteria
Children were divided into non-severe and severe groups according to the analysis of their condition according to the 2019 edition of the Guidelines for the Management of Community-Acquired Pneumonia in Children (9). Severe pneumonia was diagnosed when the diagnostic criteria for pneumonia were met and any of the following were present: worse general condition; signs of dehydration/refusal to eat; unconsciousness; cyanosis; rapid respiratory rate (≥70 times/min for less than 1 year old; ≥50 times/min for older than 1 year old); assisted breathing (moaning, wing flaps 3 concave sign); intermittent apnea; pulse oximetry <92%; 2/3 of the lungs’ infiltrate on 1 side, multilobed lungs, pleural effusion, pneumothorax, atelectasis, pulmonary necrosis, lung abscess, and extrapulmonary complications.
The inclusion criteria for adenovirus pneumonia patients in this paper were as follows: (I) age ≥28 days to <14 years, (II) cases diagnosed with pneumonia, (III) positive for adenovirus nucleic acid. The exclusion criteria were as follows: (I) incomplete medical history; (II) serious comorbidities not directly related to pneumonia, such as intracranial hemorrhage, multiple injuries, diseases requiring surgical intervention, and so on, in conjunction with adenovirus pneumonia.
The general conditions (gender, age), early symptoms (fever, cough, wheezing, etc.), results of the first laboratory test in the early days of admission [white blood cells (WBC), neutrophil ratio (NEUT%), C-reactive protein (CRP), etc.], and imaging tests [frontal and lateral chest films or lung computed tomography (CT)] of children with pneumonia who met the inclusion criteria were collected through the electronic medical record system of our hospital.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 (IBM Corp., Armonk, NY, USA). The measurement data conforming to the normal distribution was presented by mean ± standard deviation, and the comparison between groups was made by 2-sample t-test; the measurement data that were not normally distributed were represented by median (M) and interquartile range (P25, P75), and the comparison between groups was by the Mann-Whitney U test. Enumeration data were expressed as cases (%), and the comparison between groups was performed using the χ2 test. The prediction of risk factors was investigated by binary logistic regression analysis combined with receiver operating characteristic (ROC) curve. A P value of less than 0.05 was considered statistically significant.
Results
General information
Between 2019 and 2021, the annual HAdV deoxyribonucleic acid (DNA) detection rates were 12.22% (333/2,726), 7.82% (172/2,199), and 9.69% (897/9,253), respectively, and there was no statistical significance in the HAdV DNA detection rates between different years. If distributed by season, the highest HAdV DNA detection rate was 14.78% (354/2,395) in the summer of 2021, and the lowest was 5.11% (7/137) in the spring of 2020, which was statistically significant between different seasons (P<0.001) (Figure 1). Children with adenovirus pneumonia (202 cases) were selected for the study, including 77 cases (38.11%) in the severe group and 125 cases (61.88%) in the non-severe group; 108 cases (53.47%) in males and 94 cases (46.53%) in females, with a male to female ratio of 1.15:1; the age of the children ranged from 2 months of age to 13 years (Table 1).
Table 1
| Variables | Severe (n=77) | Non-severe (n=125) | χ2/Z | P value |
|---|---|---|---|---|
| Gender (male/female), N | 41/36 | 67/58 | 0.002 | 0.961 |
| Age (months) | 36 (36.00, 72.00) | 36 (12.00, 48.00) | −2.490 | 0.013 |
| Duration of fever (days) | 7.00 (6.00, 9.00) | 4.00 (4.00, 5.00) | −9.173 | <0.001 |
| Hospital stay (days) | 8.00 (5.00, 11.00) | 3.00 (1.00, 5.00) | −7.745 | <0.001 |
| Symptoms | ||||
| Fever | 75 (97.4) | 118 (94.4) | 0.427 | 0.513 |
| Wheezing | 19 (24.7) | 15 (12) | 5.469 | 0.019 |
| Rales | 69 (89.6) | 107 (85.6) | 0.638 | 0.408 |
| Complication | ||||
| Respiratory failure | 2 (2.6) | 0 (0) | 0.144 | 0.144 |
| Liver injury | 8 (10.4) | 0 (0) | 0.001 | 0.001 |
| Myocardial damage | 4 (5.2) | 2 (1.6) | 0.204 | 0.151 |
| Electrolyte disturbances | 16 (20.8) | 0 (0) | 28.208 | 0.001 |
| Abdominal pain | 8 (10.4) | 8 (6.4) | 1.040 | 0.308 |
| Vomiting | 15 (19.5) | 16 (12.8) | 1.637 | 0.201 |
| Diarrhea | 5 (6.5) | 6 (4.8) | 0.038 | 0.845 |
| Coagulation disorders | 18 (23.4) | 0 (0) | 32.079 | 0.001 |
| Toxic encephalopathy | 3 (3.9) | 1 (0.8) | 0.156 | 0.156 |
| With two or more comorbidities | 9 (11.7) | 4 (3.2) | 5.702 | 0.017 |
Data are presented as median (P25, P75) or number (percentage).
Adenovirus pathogenic examination
Among 202 children, 114 (56.44%) were positive for adenovirus nucleic acid in nasopharyngeal swabs, 71 (35.15%) were positive for adenovirus nucleic acid in sputum, and 24 (11.88%) were positive for adenovirus nucleic acid in alveolar lavage fluid. Regarding positivity across multiple tests, 5 children were positive for nasopharyngeal swabs and alveolar lavage fluid specimens, and 2 were positive for sputum and alveolar lavage fluid specimens.
Clinical features
193 of 202 children (95.54%) had fever, mostly continued fever or remittent fever, with fever peaks of 37.5–42.0 ℃. All children had cough, the severe group had significantly more wheezing than the non-severe group, and the difference was statistically significant (P<0.05) (Table 1).
Comorbidities
Liver injury, electrolyte disturbance, and coagulation dysfunction were significantly higher in the severe group than in the non-severe group, with statistically significant differences (P<0.05). The incidence of children with severe pneumonia combined with 2 or more co-morbidities was significantly higher than that in the non-severe disease group, and the difference was statistically significant (P<0.05) (Table 1).
Radiographic examination
Eighty-five children underwent chest X-ray examination, 51 cases (60.00%) had increased of lung markings, 28 cases (32.94%) had flaky shadow, 12 cases (14.11%) had patchy consolidation, 5 cases (5.88%) had lung consolidation, and 1 case (1.17%) had atelectasis with emphysema and pleural effusion. On Lung CT examination in 117 children, there were 3 cases (2.56%) of increased lung markings, 1 case (0.85%) of flaky shadow, 1 case (0.85%) of patchy consolidation, 94 cases (80.34%) of lung consolidation, 13 cases (11.11%) of pleural effusion, and 19 cases (16.24%) of pulmonary atelectasis.
Laboratory tests
Platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase MB isoenzyme (CK-MB), blood lactate (LA), procalcitonin (PCT), immunoglobin E (IgE), complement C3 (C3), and complement C4 (C4) were not statistically significant in the 2 groups of children, but WBC, NEUT%, CRP, interleukin-6 (IL-6), serum ferritin (SF), erythrocyte sedimentation rate (ESR), immunoglobin G (IgG), immunoglobin A (IgA), and immunoglobin M (IgM) were higher in the severe disease group than in the non-severe disease group, and the differences were statistically significant (P<0.05) (Table 2).
Table 2
| Laboratory index | Severe (n=77) | Non-severe (n=125) | χ2/Z | P value |
|---|---|---|---|---|
| WBC (×109/L) | 8.97 (6.74, 12.80) | 7.09 (5.47, 9.77) | −3.280 | 0.001 |
| NEUT% | 57.99±17.15 | 43.64±16.81 | 5.831 | <0.001 |
| PLT (×109/L) | 333.00 (239.00, 426.00) | 300.00 (238.00, 353.50) | −1.885 | 0.059 |
| ALT (IU/L) | 14.00 (10.00, 21.00) | 13.00 (10.00, 17.00) | −0.883 | 0.377 |
| AST (IU/L) | 35.00 (26.50, 44.00) | 33.00 (28.00, 41.00) | −0.167 | 0.867 |
| LDH (IU/L) | 383.00 (306.50, 493.50) | 363.00 (304.00, 422.50) | −1.406 | 0.160 |
| CK (U/L) | 88.00 (58.00, 128.00) | 93.00 (64.00, 137.50) | −1.252 | 0.211 |
| CK-MB (U/L) | 4.00 (4.00, 8.00) | 4.00 (4.00, 10.00) | −0.645 | 0.519 |
| LA (U/L) | 2.79 (2.17, 3.31) | 2.73 (2.24, 3.48) | −0.120 | 0.904 |
| CRP (mg/L) | 21.80 (7.00, 77.35) | 11.07 (3.33, 27.4) | −2.941 | 0.003 |
| PCT (ng/mL) | 0.15 (0.08, 0.78) | 0.15 (0.06, 0.40) | −1.846 | 0.065 |
| IL-6 (pg/mL) | 33.25 (10.79, 67.95) | 18.73 (8.48, 42.16) | −2.969 | 0.003 |
| SF (ng/mL) | 168.20 (101.25, 271.50) | 114.60 (77.73, 167.85) | −3.845 | <0.001 |
| ESR (mm/h) | 35.00 (17.50, 47.00) | 23.00 (15.00, 39.50) | −2.191 | 0.028 |
| IgG (g/L) | 8.53±2.65 | 7.36±2.27 | 3.325 | 0.001 |
| IgA (g/L) | 0.95 (0.55, 1.46) | 0.73 (0.35, 1.18) | −2.594 | 0.009 |
| IgM (g/L) | 1.18 (0.82, 1.78) | 1.07 (0.76, 1.35) | −2.439 | 0.015 |
| IgE (IU/mL) | 74.71 (43.99, 324.80) | 76.79 (33.94, 145.55) | −1.294 | 0.196 |
| C3 (g/L) | 1.25±0.24 | 1.22±0.21 | 1.117 | 0.265 |
| C4 (g/L) | 0.33 (0.24, 0.38) | 0.32 (0.27, 0.39) | −0.185 | 0.853 |
Data are presented as median (P25, P75) or mean ± SD. WBC, white blood cells; NEUT%, neutrophil ratio; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; CK-MB, creatine kinase MB isoenzyme; LA, blood lactate; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin-6; SF, serum ferritin; ESR, erythrocyte sedimentation rate; IgG, Immunoglobin G; IgA, Immunoglobin A; IgM Immunoglobin M; IgE, Immunoglobin E; C3, Complement C3; C4, Complement C4; SD, standard deviation.
Risk factors for severe adenovirus pneumonia
Logistic regression analysis combined with ROC curve showed that fever >4.50 days, wheezing, NEUT% ≥47.60, and SF ≥139.60 ng/mL were risk factors for severe adenovirus pneumonia in children (P<0.05), and the area under the curve (AUC) from high to low was as follows: fever duration, NEUT%, SF. The specificity and sensitivity of fever duration and NEUT% were greater than 0.70 (Figure 2, Tables 3,4).
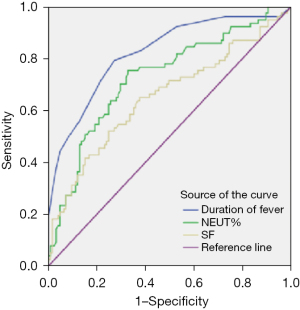
Table 3
| Variables | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Wheezing | 1.301 | 0.552 | 5.562 | 0.018 | 3.673 | 1.246–10.828 |
| Duration of fever | 0.333 | 0.064 | 26.953 | 0.000 | 1.394 | 1.230–1.581 |
| NEUT% | 0.033 | 0.016 | 4.205 | 0.040 | 1.034 | 1.001–1.067 |
| SF | 0.004 | 0.002 | 5.840 | 0.016 | 1.004 | 1.001–1.008 |
SE, standard error; OR, odds ratio; 95% CI, 95% confidence interval; NEUT%, neutrophil ratio; SF, serum ferritin.
Table 4
| Variables | Cut-off | Sensitivity | Specificity | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| Duration of fever | 4.50 days | 0.792 | 0.728 | 0.823 (0.762–0.884) | <0.001 |
| NEUT% | 47.60 | 0.701 | 0.704 | 0.728 (0.655–0.801) | <0.001 |
| SF | 139.60 ng/mL | 0.662 | 0.632 | 0.661 (0.580–0.742) | <0.001 |
ROC, receiver operating characteristic; AUC, area under the curve; NEUT%, neutrophil ratio; SF, serum ferritin; CI, confidence interval.
Discussion
Adenovirus pneumonia is the most common respiratory severe pneumonia in children (10). At the same time, HAdV infection is also an important factor leading to death caused by and poor prognosis of severe pneumonia in children (11). About 14–60% of adenovirus pneumonia will incur different degrees of sequelae (12). This study shows that children with adenovirus infection fever duration greater than 4.50 days, accompanied by wheezing symptoms, and NEUT% ≥47.60, SF ≥139.60 ng/mL should be vigilant about the trend of critical severity. So far, there is no specific drug treatment for severe HAdV pneumonia (13,14). Therefore, only early identification and diagnosis can enable early intervention and comprehensive treatment to improve prognosis.
The prevalence of male to female was 1.15:1, and the difference was not statistically significant. There was no significant difference in prevalence between men and women, which was consistent with the results of recent studies (1,2,15). However, it has also been reported that boys are comparatively more susceptible to adenovirus (16). Therefore, whether there is a gender difference in adenovirus infection needs to be further studied with large samples. Humans are generally susceptible to HAdV, and adenovirus pneumonia occurs most often in children between 6 months and 5 years of age (15,17). In this study, children under 5 years old accounted for 84.20%, which was consistent with local Chinese and international reports (1,15). However, there were a total of 20 cases (26.0%) in the severe disease group >5 years of age ,which was significantly higher than the 12 cases (9.6%) in the non-severe disease group, suggesting that children above 5 years of age are at high risk of developing critical illness from adenovirus pneumonia, so children of preschool and school age should be alerted to their tendency to become critically ill, which is not consistent with the study by Huang et al. (18). However, the age distribution of high risk for severe adenoviral pneumonia needs to be further investigated in a large sample and multicenter study due to the sample size and age limit of this study.
The incubation period of HAdV infection is on average 3–8 days and the infection is mainly transmitted by contact, droplets, feces, and oral cavity. Patients tend to develop a high fever above 39 ℃ within 1–2 days of onset, usually accompanied by coughing and wheezing, and the duration of symptoms varies with the disease (9,15). All children included in this study had cough symptoms from the onset of the disease and 193 cases (95.5%) had fever, mostly indolent or flaccid. The fever duration was significantly longer in the severe group than in the non-severe group, and dichotomous logistic regression analysis suggested that fever duration >4.5 days was a high-risk factor for severe disease and a valid predictor of whether adenovirus pneumonia was heading toward severe disease. Previous literature (15) has suggested that the duration of fever (≥7 days) was an independent risk factor for severe adenoviral pneumonia, and the present study advanced the warning range of fever duration further to provide some reference for early clinical assessment of the extent of the disease and prognosis.
HAdV infection and inflammatory mediators can cause bronchial and fine bronchial mucosa edema, congestion, and shedding of necrotic material, and so on. In this study, 19 cases (24.76%) of severe patients had continuous wheezing, and the difference was statistically significant. It is suggested that wheezing is a risk factor for severe adenovirus pneumonia; especially, continuous wheezing may suggest poor prognosis. In addition to causing pulmonary infection, HAdV can manifest as viremia and then systemic lesions in the early stages of infection, leading to extrapulmonary damage and dysfunction of the central nervous system, circulatory system, and hematological system. A study (19) reported a global outbreak of childhood adenoviral hepatitis in the coronavirus disease 2019 (COVID-19) pandemic, which may be related to the lack of exposure to adenoviruses and other common childhood viral diseases during the COVID-19 pandemic, which resulted in these viruses providing some immune protection to children during their frequent/mild illnesses, although the exact cause still needs to be explored. In this study, the occurrence of liver injury, electrolyte disorders, coagulation dysfunction, and the combination of 2 or more complications was significantly higher in the severe group than in the non-severe group, and the difference was statistically significant (P<0.05). It is suggested that extensive lesions and multiple organ damage are important factors in triggering critical illness and have decisive significance for disease regression.
The inconsistency between the late appearance of pulmonary rales and the earlier appearance of imaging changes in pulmonary signs in children with adenoviral pneumonia makes imaging particularly important for the diagnosis and determination of severe adenoviral pneumonia. The current imaging methods for adenoviral pneumonia are mainly X-ray and CT examinations. In this study, X-ray examination revealed that 51 cases (67.10%) of pulmonary texture thickening occurred in the non-severe disease group, which was significantly higher than the 0 cases in the severe disease group, and the difference was statistically significant. Chest CT examination revealed that the occurrence of pulmonary solidity, pleural effusion, and pulmonary atelectasis were significantly higher in the severe group with 66 cases (97.1%), 12 cases (17.6%), and 17 cases (25%) than in the non-severe group in 28 cases (57.1%), 1 case (20.4%), and 2 cases (40.8%), respectively, and the differences were statistically significant. It is suggested that X-ray is more suitable for the initial diagnosis and screening of adenovirus pneumonia in children; CT examination is recommended in severe cases, combined complications or when the images are inconsistent with clinical manifestations (20), and is an important examination tool for the diagnosis, disease assessment, and prognosis of severe adenovirus pneumonia and complications in children.
In this paper, the laboratory indicators of children with adenovirus pneumonia were analyzed, and it was found that the levels of biochemical indicators such as WBC, NEUT%, CRP, IL-6, SF, ESR, IgG, IgA, and IgM in the severe group were significantly higher than those in the non-severe group, suggesting that severe adenovirus pneumonia causes the body’s immune response to produce a large number of cytokines and inflammatory mediators. Further analysis found that NEUT% ≥47.60 and SF ≥139.60 ng/mL were independent risk factors. For children with adenovirus pneumonia with persistent fever and wheezing, laboratory tests suggest that severe adenovirus pneumonia should be considered when NEUT%, SF, and other indicators are significantly increased, and adequate attention should be paid and active treatment should be given to improve the prognosis.
This study found that fever duration >4.50 days, wheezing, NEUT% ≥47.60, and SF ≥139.60 ng/mL were risk factors for severe adenovirus pneumonia. A study (16) found that longer severe adenoviral pneumonia was more likely to develop with a fever course, co-infection, pleural effusion, decreased hemoglobin concentration, and increased procalcitonin and lactate dehydrogenase concentrations. Wu et al. (21) asserted that high serum LDH level and low lymphocyte count could be used as predictors for the severity of adenovirus respiratory infection in children. Reports (15) have stated that duration of fever (≥7 days) and high IgE are independent risk factors for severe adenovirus pneumonia. A study (22) also showed that the high risk factors of severe adenovirus pneumonia include premature birth, underlying diseases, prolonged febrile duration, and significantly elevated CRP. The different risk factors in different studies may be related to the population and grouping of the included cases.
This paper has some limitations. First, it is a single-center study with limited sample size. Second, serotype analysis of adenovirus was not performed, and the relationship between adenovirus type and clinical disease could not be further elaborated. Third, the participants were hospitalized patients, and the selection of cases may have caused admission bias.
Conclusions
Severe adenoviral pneumonia is prone to severe clinical manifestations and multiple extrapulmonary complications, and children of preschool and school age should be alerted to its tendency to become critically ill. Indicators such as duration of fever >4.50 days, with wheezing, NEUT% ≥47.60, and SF ≥139.60 ng/mL to predict severe adenovirus pneumonia in children have good differentiation and accuracy. For children with adenovirus pneumonia with the above characteristics, clinical management should be strengthened, and rational treatment should be provided to prevent and control the inflammatory storm caused by adenovirus, improve the cure rate, and actively improve the prognosis. In addition, multicenter and large-sample experiments need to be conducted to confirm our findings.
Acknowledgments
We are very grateful to the parents and participants who participated in this study.
Funding: This study was funded by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-312/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-312/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-312/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-312/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Tianjin Children’s Hospital (No. 2022-LXKY-013) and informed consent was obtained from the patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu X, Ma Y, Gao Y, et al. Epidemiology of Adenovirus Pneumonia and Risk Factors for Bronchiolitis Obliterans in Children During an Outbreak in Jilin, China. Front Pediatr 2021;9:722885. [Crossref] [PubMed]
- Zou L, Yi L, Yu J, et al. Adenovirus infection in children hospitalized with pneumonia in Guangzhou, China. Influenza Other Respir Viruses 2021;15:27-33. [Crossref] [PubMed]
- Zhu Y, Xu B, Li C, et al. A Multicenter Study of Viral Aetiology of Community-Acquired Pneumonia in Hospitalized Children in Chinese Mainland. Virol Sin 2021;36:1543-53. [Crossref] [PubMed]
- Xu N, Chen P, Wang Y. Evaluation of Risk Factors for Exacerbations in Children with Adenoviral Pneumonia. Biomed Res Int 2020;2020:4878635. [Crossref] [PubMed]
- Fu Y, Tang Z, Ye Z, et al. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect Dis 2019;19:36. [Crossref] [PubMed]
- Radke JR, Cook JL. Human adenovirus infections: update and consideration of mechanisms of viral persistence. Curr Opin Infect Dis 2018;31:251-6. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China, State Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of adenovirus pneumonia in children (2019 version). Chinese Journal of Clinical Infectious Diseases 2019;12:161-6.
- Expert Writing Group of Technical Guidelines for Prevention and Control of Human Adenovirus Respiratory Infection. Technical guidelines for prevention and control of human adenovirus respiratory infection (2019 edition). Chinese Journal of Preventive Medicine 2019;53:1088-93. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China, State Administration of Traditional Chinese Medicine. Guideline for diagnosis and treatment of community-acquired pneumonia in Children (2019 version). Chinese Journal of Clinical Infectious Diseases 2019;12:6-13.
- Lu MP, Ma LY, Zheng Q, et al. Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J Pediatr 2013;9:346-9. [Crossref] [PubMed]
- Shieh WJ. Human adenovirus infections in pediatric population - An update on clinico-pathologic correlation. Biomed J 2022;45:38-49. [Crossref] [PubMed]
- Park JW, Lee KJ, Lee KH, et al. Hospital Outbreaks of Middle East Respiratory Syndrome, Daejeon, South Korea, 2015. Emerg Infect Dis 2017;23:898-905. [Crossref] [PubMed]
- Lynch JP 3rd, Kajon AE. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin Respir Crit Care Med 2016;37:586-602. [Crossref] [PubMed]
- Gu J, Su QQ, Zuo TT, et al. Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection 2021;49:1-13. [Crossref] [PubMed]
- Zhong H, Dong X. Analysis of Clinical Characteristics and Risk Factors of Severe Adenovirus Pneumonia in Children. Front Pediatr 2021;9:566797. [Crossref] [PubMed]
- Lou Q, Zhang SX, Yuan L. Clinical analysis of adenovirus pneumonia with pulmonary consolidation and atelectasis in children. J Int Med Res 2021;49:300060521990244. [Crossref] [PubMed]
- Li M, Han XH, Liu LY, et al. Epidemiological characteristics, clinical characteristics, and prognostic factors of children with atopy hospitalised with adenovirus pneumonia. BMC Infect Dis 2021;21:1051. [Crossref] [PubMed]
- Huang H, Chen Y, Ma LY, et al. Analysis of the clinical features and the risk factors of severe adenovirus pneumonia in children. Zhonghua Er Ke Za Zhi 2021;59:14-9. [Crossref] [PubMed]
- Gutierrez Sanchez LH, Shiau H, Baker JM, et al. A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N Engl J Med 2022;387:620-30. [Crossref] [PubMed]
- Wang Y, Peng Y. Imaging features of adenovirus pneumonia in children. Chinese Pediatric Emergency Medicine 2019;26:725-8.
- Wu PQ, Zeng SQ, Yin GQ, et al. Clinical manifestations and risk factors of adenovirus respiratory infection in hospitalized children in Guangzhou, China during the 2011-2014 period. Medicine (Baltimore) 2020;99:e18584. [Crossref] [PubMed]
- Gu Y, Huang RW, Wang M, et al. Epidemiological characteristics of adenovirus infection in hospitalized children with acute respiratory tract infection in Kunming during 2019. Zhonghua Er Ke Za Zhi 2021;59:772-6. [Crossref] [PubMed]
(English Language Editor: J. Jones)



