
Pediatric spindle cell/sclerosing rhabdomyosarcoma with FUS–TFCP2 fusion: a case report and literature review
Highlight box
Key findings
• Spindle cell/sclerosing rhabdomyosarcoma (RMS) with FUS–TFCP2 fusion is a rare aggressive malignancy, and its unique biological and clinical characteristics lead to poor prognosis despite aggressive treatment.
What is known and what is new?
• Spindle cell/sclerosing RMS with EWSR1/FUS–TFCP2 fusion is almost exclusively intraosseous RMS and mostly involves the craniofacial skeleton, especially the mandible. Histologically, this subtype of RMS shows a common origin of epithelium and muscle.
• Almost all cases of spindle cell/sclerosing RMS with EWSR1/FUS–TFCP2 fusion were found to have a positive expression of anaplastic lymphoma kinase (ALK). It appears treatment guided by conventional risk stratification of RMS is not effective.
What is the implication, and what should change now?
• More in-depth research should be carried out on molecular profiling and targeted treatment of this subtype and whether it requires more aggressive treatment.
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in pediatric patients. Spindle cell/sclerosing RMS is a subtype of RMS that was not classified as a separate subtype until the fourth edition of the 2013 World Health Organization classification of soft tissue and bone tumors (1). Due to its low incidence, clinical studies on this subtype are limited. Current research indicates that spindle cell/sclerosing RMS is a heterogeneous neoplasm with distinct morphologies and clinical presentations due to its variability in molecular features (2). FET–TFCP2 fusion RMS (RMS with EWSR1/FUS–TFCP2 fusion) is the rarest subtypes with fewer than 40 cases being reported to date. Previous studies have shown that this subtype is almost exclusively intraosseous RMS, which has extremely strong invasiveness and poor prognosis (3,4). However, in most previous reports, the focus was on describing the clinical and pathological characteristics of tumors (4-6), with little detailed description of their treatment process, resulting in insufficient management experience and reference for this subtype.
To increase clinicians’ understanding of this subtype, herein, we share a case of pediatric spindle cell/sclerosing RMS with FUS–TFCP2 fusion that originated in the mandible and report the clinical characteristics, histology and molecular features. Through a detailed description of its bumpy treatment process, we hope to further improve the understanding of clinicians on its extremely aggressive nature, and emphasize the importance of early molecular detection of spindle cell/sclerosing RMS. By analyzing the treatment experience of this case and accumulating experience through additional studies, we are inclined to carry out more active treatment for such subtypes, and attempt targeted treatment with ALK inhibitors when conventional treatment is ineffective. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-603/rc).
Case presentation
A 13-year-old boy, who was previously healthy and had no family history of tumor, was first admitted to Beijing Children’s Hospital on March 22, 2021, with complaints of painful swelling in the right mandibular gum for 2 months. He had been treated with cephalosporins in another hospital, but the mass had further enlarged, resulting in an eating disorder. Physical examination showed a 90 mm × 80 mm × 60 mm-sized mass in the patient’s right mandible, which limited mouth opening. Vegetations with a diameter of about 2 cm can be seen in the right oral mucosa, accompanied by local surface ulceration and bleeding. Computed tomography (CT) and magnetic resonance imaging (MRI) of the nasopharynx revealed that the right mandible was occupied by a huge mixed-density mass measuring 99 mm × 90 mm × 105 mm with bone destruction (Figure 1). Positron emission tomography CT (PET-CT) showed that the mass had caused bony destruction of the right temporal bone and mandible, and the invasion involved the infratemporal fossa, palate, tongue, floor of the mouth, and parapharyngeal space. However, no metastasis was found in the lungs, bone marrow, or liver. The pathological results of the biopsy suggested malignant spindle cells, which were poorly differentiated. The immunohistochemical results showed that the tumor cells diffusely expressed desmin, MyoD1, vimentin, myogenin, and Mdm2. The cytokeratin was focally positive, and a high anaplastic lymphoma kinase (ALK) expression and catenin were found in the cytoplasm. However, smooth muscle antibody (SMA), endomysial antibody (EMA), calponin, Sox10, S-100, CD34, and factor VIII were negative. The maximum Ki67-labeling index was 15%. The final pathologic diagnosis was spindle cell/sclerosing RMS (Figure 2).


According to the examination results, the primary tumor was located at a site with a good prognosis on the basis of previous study (7), with a clinical TNM staging of T2bN0M0 and an IRS staging of III, according to which the case was initially classified into intermediate-risk group. After 2 cycles of chemotherapy of VAC (vincristine, actinomycin D, and cyclophosphamide) and VI (vincristine and irinotecan), meningeal involvement was discovered through radiological examination, so this patient was ultimately classified as a central-invasion group. Subsequently, the patients received 2 cycles of chemotherapy of VDE (vincristine, doxorubicin, and etoposide) and VACa (vincristine, actinomycin D, and carboplatin). Unfortunately, the child was chemotherapy insensitive, the tumor further increased in size (about 130 mm × 120 mm ×15 mm), and thoracic vertebra metastasis was suspected. The enhanced CT of the mandible was performed 2 months later (Figure 3).

As a consequence, next-generation gene sequencing (NGS) on tumor tissue was performed, and the results revealed a FUS (exon 6)–TFCP2 (exon 2) fusion and CDKN2A deletion (Figure 4). However, no molecular targets were identified After a multidisciplinary discussion, the patient received tumor interventional therapy and resection on June 10, 2021. The tumor was resected in its entirety, and the margins were negative. Chemotherapy of VAI (vincristine, actinomycin D, and ifosfamide) was continued postoperatively. During the interval of chemotherapy, the child experienced respiratory distress, and a CT examination was immediately performed (Figure 5). CT demonstrated right pleural effusion with right lung consolidation atelectasis. There were multiple adherent soft tissue thickening shadows on the right chest wall and diaphragmatic surface, multiple pulmonary nodules, and destruction of the T1 vertebral body and adnexal bone. Due to the further progression of the tumor, the child received chemotherapy of VTC (vincristine, topotecan, and cyclophosphamide) after closed thoracic drainage, but there was still no improvement. The pleural effusion increased, and the symptoms of dyspnea were further aggravated. Moreover, the patient complained of severe neck pain with restricted mobility, and thus cervical metastasis was suspected. The guardians of this patient decided to abandon the treatment, and he ultimately died within 1 month after discharge, more than 5 months after diagnosis. The timeline of this case is shown in Figure 6.


All procedures performed in this study were in accordance with the relevant ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
International multidisciplinary team (iMDT) discussion
Discussion among physicians from Beijing Children’s Hospital
Department of Medical Oncology
As the most common pediatric soft tissue sarcoma, RMS is classified into four main subtypes according to the fifth edition of WHO classification [2020]: embryonal, alveolar, pleomorphic, and spindle cell/sclerosing (8). Of these, spindle cell/sclerotic RMS represents only 5–10% of all RMS cases. Although it is classified into a separate subtype based on morphology, recent reports indicate that it can be classified into at least three subtypes: MYOD1 mutation, VGLL2–NCOA2 fusion, and EWSR1/FUS–TFCP2 fusion, each with their own distinctive morphological, clinical, and prognostic features (9). Among them, MYOD1 mutation is the most commonly reported mutation phenotype to date, and can occur at any age. MyoD1-mutated tumors tend to follow a more aggressive process, which is mostly associated with poor prognosis (2,6). VGLL2–NCOA2 fusion occur almost exclusively in infantile patients and are detected in up to 50% of spindle cell/sclerotic RMS in infancy. Children with this molecular subtype have a good prognosis, whose 5-year EFS and OS are 90% and 100%, respectively (10).
In 2018, Watson et al. (11) were first to describe a completely novel group of epithelioid and spindle-cell RMS characterized by EWSR1/FUS–TFCP2 fusion. Thus far, only 37 cases of this molecular subtype have been reported in the English-language literature (3-6,11-22). The clinical features, pathological, immunophenotypic, and molecular features of RMS with EWSR1/FUS–TFCP2 fusion previously reported are all summarized in Table 1. The disease has been reported at all ages (range, 11–86 years; median age 27 years), but most cases have occurred in young adults under 30 years of age (21/37; 56.8%), with essentially the same number of males and females (male to female ratio, 18:19). In contrast to those of other RMS subtypes, tumors of this subtype are almost exclusively intraosseous (32/37, 86.5%) and mostly involve the craniofacial skeleton (26/32, 81.3%), with the most common site being the mandible (14/32, 43.8%), followed by the maxilla (6/32, 18.8%), skull, and femur. Onset with pure soft tissue involvement has only been reported in 5 patients (5/37, 13.5%) (3,4,12,13), whose primary tumors were located in superficial soft tissue of neck and scalp, inguinal region, peritoneum, and maxillary gingiva, with no bone metastasis being observed throughout the disease course in these cases. Bone tumors typically appear at locally advanced stage, with a median size of 5.5–6.0 cm (4,14), and are often accompanied by infiltration of the surrounding soft tissue (4).
Table 1
| Author/year | Age/sex | Location | Tumor maximum diameter (mm) | Local invasion | Metastasis | Cytomorphology | ALK expression | Gene fusion | Treatment | Evolution | Survival/follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dashti et al., 2018 (21) | 72/M | Mandible | 40 | Soft tissue extension anterior and posterior to the mandible and surrounding the roots of multiple teeth | No | Spindle and epithelioid | Strong | FUS–TFCP2 | Mandibulectomy | No | ANED (2 months) |
| Tagami et al., 2019 (5) | 70/F | Lumbar vertebra | NA | Arch and spinous process of the fifth lumbar vertebra | No | Spindle | Strong | FUS–TFCP2 | Chemotherapy and radiotherapy | NA | AWD (6 months) |
| Agaram et al., 2019 (22) | 33/F | Maxilla | NA | NA | No | Spindle and epithelioid | + | EWSR1–TFCP2 | Surgical resection | No | ANED (108 months) |
| 20/M | Maxilla | NA | Masticator space with extensive extension into the maxillary and sphenoidal sinuses, orbit, and clivus | Femur | Spindle | + | EWSR1–TFCP2 | NA | NA | NA | |
| 37/F | Iliac bone | NA | NA | NA | Spindle | + | FUS–TFCP2 | NA | NA | NA | |
| Le Loarer et al., 2020 (4) |
16/F | Sphenoid bone | 90 | Dura, left orbit, and infratemporal fossa | No | Spindle and epithelioid | 50% | FUS–TFCP2 | Chemotherapy, surgical resection (R1), and radiotherapy | Progression and metastasis in right femur | DOD (15 months) |
| 26/F | Sacrum | 100 | Right sacroiliac joint and medullary involvement | Lung and mediastinal lymphadenopathy | Epithelioid | 50% | FUS–TFCP2 | Chemotherapy | Progression and pleural effusion | DOD (4 months) | |
| 38/F | Peritoneum | Multiple nodules | Pleural effusion | No | Spindled and epithelioid | 50% | EWSR1–TFCP2 | Chemotherapy | Local progression and lymph node metastasis | DOD (2 months) | |
| 32/M | Hard palate and upper lip |
Multiple confluent nodules | Hard palate and gingiva | Vertebrae, ribs, and pelvis | Spindled and epithelioid | NA | EWSR1–TFCP2 | Chemotherapy | Local progression | DOD (8 months) | |
| 20/M | Orbito-temporal-sphenoid mass | 150 | Left orbit, sphenoid and temporal bones, dura, left infratemporal fossa, soft tissue, and dermis of left temporal region | No | Spindled and epithelioid | <5% | FUS–TFCP2 | Chemotherapy, radiotherapy, and ALK inhibitor | Local progression | DOD (6 months) | |
| 86/M | Inguinal area | 65 | NA | NA | Spindled and epithelioid | 100% | EWSR1–TFCP2 | Resection (R1) | Local progression | DOD (6 months) | |
| 18/F | Right femur (neck and intertrochanteric area) | 51 | No | Lungs, L5 vertebrae, left ischiopubic branch, and ribs | Spindled and epithelioid | 100% | EWSR1–TFCP2 | Chemotherapy and ALK inhibitor | Local progression | DOD (8 months) | |
| 17/F | Cervico-occipital junction | 53 | Lysis of left occipital condyle, C1 vertebra, and left clivus; extension into to the left jugular vein, hypoglossal foramen, and paravertebral soft tissue | No | Round | 100% | FUS–TFCP2 | Chemotherapy, radiotherapy, and ALK inhibitor | Stable since anti-ALK therapy | AWD (15 months) | |
| 31/M | Left occipital bone | 118 | Extracranial: scalp soft tissue; intracranial: meningeal and left superior sagittal sinus | No | Epithelioid and spindled | 100% | FUS–TFCP2 | Fragmented resection (R1) and chemotherapy | Local progression and metastasis (lung and mediastinum) | DOD (6 months) | |
| 32/M | Mandible | 45 | Vestibular gingiva, mylohyoid, and hyoglossus muscles | No | Spindled | 70% | FUS–TFCP2 | Resection (R0) and chemotherapy | Local relapse at 12 months, lung met | AWD (14 months) | |
| 58/F | Mandible | 16 | Surrounding soft tissue (muscle) | No | Spindled and epithelioid | 70% | FUS–TFCP2 | Resection (R0), radiotherapy, and chemotherapy | No | ANED (21 months) | |
| 12/F | Mandible | 55 | Surrounding soft tissue (muscle) | No | Spindled and epithelioid | 50% | FUS–TFCP2 | Chemotherapy, resection (R0), and radiotherapy | No | ANED (21 months) | |
| 11/F | Maxilla | 60 | Surrounding soft tissue (muscle) | No | Epithelioid | <5% | EWSR1–TFCP2 | Chemotherapy and radiotherapy | Local progression | DOD (unknown) | |
| 25/M | Mandible | 34 | Surrounding soft tissue (muscle) | No | Epithelioid | 80% | EWSR1–TFCP2 | Resection (R0) and chemotherapy | No | ANED (20 months) | |
| Xu et al., 2021 (3) | 22/M | Mandible | NA | NA | Lymph node | Spindle and epithelioid | + | FUS–TFCP2; ALK: WT | NA | NA | NA |
| 34/M | Mandible | NA | NA | No | Spindle, epithelioid, and rhabdoid | − | FUS–TFCP2; ALK deletion | NA | NA | AWD (10 months) | |
| 16/M | Mandible | NA | NA | Bone, lung, and lymph node | Spindle, epithelioid, and rhabdoid | + | FUS–TFCP2; ALK deletion | NA | NA | DOD (20 months) | |
| 43/F | Mandible | NA | NA | NA | Spindle and epithelioid | + | FUS–TFCP2; ALK: ND | NA | NA | NA | |
| 74/F | Maxilla/gingiva | NA | NA | Lymph node | Spindle and epithelioid | + | FUS–TFCP2; ALK: WT | NA | NA | DOD (21 months) | |
| 27/F | Skull | NA | NA | Bone | Spindle and epithelioid | + | EWSR1–TFCP2; ALK: ND | NA | NA | AWD (1 months) | |
| 18/M | Skull | NA | NA | NA | Spindle and epithelioid | + | FUS–TFCP2; ALK: WT | NA | NA | NA | |
| 29/M | Skull (base) | NA | NA | Lung | Spindle and epithelioid | NA | EWSR1–TFCP2; ALK: WT |
NA | NA | AWD (2 months) | |
| 40/F | Neck (superficial soft tissue) | NA | NA | NA | Spindle, epithelioid, and round | + | FUS–TFCP2; ALK deletion | NA | NA | NA | |
| Chrisinger et al., 2020 (14) |
20-30s/F | Frontal bone | 50 | Dura | No | Spindle and epithelioid | Patchy | EWSR1–TFCP2 | Chemotherapy, radiotherapy, and resection | Metastasis (right acetabulum, left iliac bone, and lung) | DOD (17 months) |
| 20/F | Pelvic bones | 95 | Left posterior iliac, acetabular roof, sacroiliac joint, left sacral ala, gluteus medius; marrow-replacing lesions in the right supraacetabular iliac and right posterior iliac bone | L3 and L4 vertebral, lung, peri-portal lymph node | Spindle, epithelioid, and rounded | + | FUS–TFCP2 | Chemotherapy and radiotherapy | Local progression and metastases (liver, gluteal soft tissue nodules, bone) | DOD (11 months) | |
| Valério et al., 2023 (18) | 19/F | Mandible | NA | Surrounding soft tissue | No | Spindle and epithelioid | Diffuse | FUS–TFCP2, ALK deletion | Chemotherapy, radiotherapy, resection, and ALK inhibitor | Local recurrence and progression | DOD (>15 months) |
| Panferova et al., 2022 (17) |
16/F | Mandible | 64 | NA | No | Spindle | + | EWSR1–TFCP2 | Resection and chemotherapy | Local recurrence and metastases (lymph nodes in the neck, C1–C2 vertebrae, the iliac wing on the left) | AWD (2 months) |
| Ishiyama et al., 2022 (12) | 58/M | Scalp soft tissue | 32 | Periosteum | No | Round and spindle | >50% | FUS–TFCP2 | Resection (R0) | Local recurrence and metastasis (left lymph node of head and neck and supraclavicular fossa) | AWD (7 months) |
| Koutlas et al., 2021 (16) | 15/M | Mandible | 70 | NA | Ipsilateral cervical lymph nodes | Spindle, epithelioid, and round | − | EWSR1–TFCP2 | Resection, chemotherapy, and radiotherapy (proton beam therapy) | Metastases (T7 vertebra) | AWD (7 months) |
| Ochsner et al., 2022 (13) | 48/M | Maxillary gingiva | 27 | Perineural and skeletal muscle | No | Spindle, epithelioid, and round | + | FUS–TFCP2 | NA | NA | NA |
| Gallagher et al., 2023 (6) |
58/M | Maxilla | 50 | NA | NA | Spindle and epithelioid | + (focally) | FUS–TFCP2 | Chemotherapy | NA | DOD (3 months) |
| 22/M | Maxilla | NA | Maxillary sinus, nasal cavity, infratemporal fossa, and floor of the orbit | NA | Spindle and epithelioid | + | FUS–TFCP2 | NA | NA | NA |
ANED, alive, no evidence of disease; AWD, alive with disease; DOD, dead of disease; F, female; M, male; NA, not available.
Morphologically, most cases of RMS with TFCP2 rearrangement showed a mixed-spindle-and-epithelioid phenotype (21/37), whereas other cases appeared to be essentially pure epithelioid (2/37), pure round cell (1/37), pure spindle cell (5/37), or spindle, epithelioid, and round. Immunohistochemically, in addition to positivity for the muscle markers desmin, myogenin, and MyoD1, these RMS with TFCP2 rearrangement cases show staining for markers of epithelial origin such as cytokeratin (15,16). Interestingly, almost all RMS with TFCP2 rearrangement cases were found to have a positive expression of ALK (31/35), and all ALK-positive cases had mostly diffuse cytoplasmic overexpression.
The spindle cell/sclerosing RMS with TFCP2 rearrangement is an extremely aggressive tumor type, and the prognosis is extremely poor. Thus, few cases of patients with long-term follow-up have been reported. Among all 29 cases with follow-up data, the median overall survival time was 8 months (range, 1–108 months). Only five patients remained disease free at last follow-up (follow-up durations were 2, 108, 21, 21, and 20 months, respectively), and 31% of patients died within 1 year of diagnosis (Figure 7). The extremely poor prognosis may be related to the fact that the primary site of this subtype is the maxillofacial region and close to the central nervous system, and a massive initial tumor is likely to invade surrounding tissues. These factors make surgical total resection and radiotherapy challenging, resulting in insufficient local treatment. However, even after surgical total resection or postoperative combined radiotherapy, local recurrence cannot be avoided (4,11,15). In the case reported by Panferova et al., after undergoing total resection of the tumor, the patient developed a local recurrence even before starting chemotherapy (17). The highly invasive nature of this tumor type predisposes patients to metastasis. Gallagher et al. statistically analyzed the 27 previously reported cases of TFCP2-rearranged RMS and found that regional lymph node and/or distant metastases occurred in more than half of the cases (6). Distant metastatic sites are most commonly in the lung and bone (15/18, 83.3%). Unlike other RMS types, in spindle cell/sclerosing RMS with EWSR1/FUS–TFCP2 fusion, bone metastases seem to be more frequently than those in the lungs (9 vs. 8).
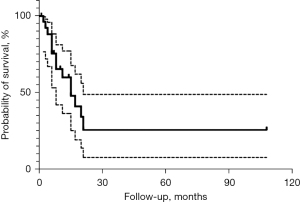
Our case was similar to previous reports that the tumor being located in the mandible, which is the typical primary site. Similarly, the initial tumor was large, measuring up to 10.5 cm in diameter, and had invaded adjacent bone and surrounding soft tissues. After being diagnosed with spindle cell/sclerosing RMS, we did not initially have the results of molecular testing, the patient was classified as intermediate risk according to the conventional criteria, and thus not administered the highest-intensity chemotherapy regimen. The tumor continued to progress after 4 cycles of multi drug combined chemotherapy. Although this subtype has its own unique clinical and biological characteristics, the key to its differential diagnosis mainly depends on the molecular testing results of tumor tissues. Therefore, it is very important to carry out molecular detection of spindle cell/sclerosing RMS in the early stage and definite diagnosis. In order to carry out early surgery, higher intensity chemotherapy or combined with radiotherapy and other more active management. According to the above, it has been proposed that although the clinical characteristics reasonably divide patients into a broad treatment cohort, genomic classification can more accurately guide the successful escalation or downgrade therapy (23). So whether the risk grouping in spindle cell/sclerosing RMS with EWSR1/FUS–TFCP2 fusion should be upgraded needs to be further researched. However, it should be noted that consistent with previous reports, chemotherapy insensitivity is also a major feature of this subtype. A number of reports have described progression and metastasis during chemotherapy, even when combined with surgery and radiotherapy (4,15,18). In our case, the patient initially had no distant metastasis but was highly refractory to chemotherapy, and primary lesion progression and thoracic metastasis occurred during chemotherapy. Even during the chemotherapy that was administered after primary resection, further neck and lung metastases continued to progress. Indeed, vertebral metastasis may be a frequent site of this subtype, and of the reported cases, five involved metastases to the vertebrae (4,14,16,17).
Because of its high aggressiveness and chemotherapy resistance, it is necessary to explore its targeted therapy. As mentioned above, the vast majority of RMS with TFCP2 rearrangement cases have a positive expression of ALK, the underlying mechanism of ALK upregulation does not involve translocation or amplification but may be linked to ALK deletion (4). As potential therapeutic targets, ALK inhibitors can suppress the kinase activity of ALK-expressing tumors despite the lack of potential fusions (19). Similarly, in our case, gene rearrangements or fusions of ALK were not identified on the NGS of the tumor tissue genes, but immunohistochemistry still revealed diffuse positivity for ALK. Unfortunately, we did not have the opportunity to administer ALK inhibitors in our patient. However, current data on the therapeutic effects of ALK inhibitors on spindle cell/sclerosing RMS are limited, and outcomes are not conclusive. Valério et al. reported a 19-year-old woman with spindle cell RMS with FUS–TFCP2 fusion and deletion of the ALK gene who was treated with ALK inhibitors, which resulted in a favorable response for 4 months (18). Brunac et al. reported that combination therapy of intensive radiotherapy and ALK inhibitors appeared to be effective (19). However, Panferova et al. and Lewin et al. described cases for whom ALK inhibitors were administered as salvage therapy, but disease progression still occurred (17,20). Therefore, for patients who fail in the conventional first-line treatment, it is an option to try ALK inhibitor treatment, but for its specific efficacy, further prospective research is needed to verify.
Interestingly, Chrisinger et al. (14) also found that among 5 patients who experienced disease-free survival, 4 had tumors originating from the mandible; in contrast, among 16 patients with progressive disease, only 1 had a tumor origin from this site. This seems to suggest that the primary lesion in the mandibular region is associated with better prognosis. However, in our case, the primary lesion was also in the mandible, but the patient still died fairly rapidly despite surgery and intensive chemotherapy, with less than half a year from diagnosis to death, which is inconsistent with the above findings. Therefore, for this subtype of tumor, collection of a larger number of cases is needed to determine if there is any association between primary site and prognosis. In addition, our cases are pediatric patients, and approximately one-fifth of the currently reported cases are children. Therefore, whether children with TFCP2-rearranged RMS have its unique clinical features, and treatment options focus on children need further research to explore.
Department of Pathology
RMS with TFCP2 rearrangement has its unique pathological features. First, almost all cases are primary in bone, and most cases of them showed a mixed-spindle-and-epithelioid phenotype morphologically. Secondly, immunohistochemically, in addition to positivity of muscle markers, they show staining for markers of epithelial origin also, the same goes for this case. Therefore, when making pathological diagnosis of RMS with the above characteristics, we should be vigilant and make further molecular diagnosis as soon as possible to clarify the final diagnosis.
Department of Otolaryngology Head and Neck Surgery
As mentioned above, RMS with TFCP2 rearrangement is not sensitive to multidrug combined chemotherapy. Like this case, 4 courses of chemotherapy before operation did not prevent the further enlargement of the primary tumor. So, after the diagnosis of this subtype, whether surgical resection should be performed immediately instead of neoadjuvant chemotherapy needs further research and discussion. However, this kind of tumor tends to occur in the head and neck, and is extremely aggressive. Most cases have huge tumor foci before surgery, which will hinder the total resection of tumor foci. Therefore, if there is an opportunity, it can be combined with tumor interventional therapy before surgery in order to reduce the tumor focus or prevent the further progression of the tumor focus together with neoadjuvant chemotherapy.
Several issues on the treatment of these patients were further discussed
Question 1: for spindle cell/sclerosing RMS with FUS–TFCP2 fusion, even if it is classified into the intermediate-risk group based on pathological subtype, IRS staging, and TNM staging, should more aggressive treatment than the traditional treatment be administered once the mutation is detected?
Expert opinion 1: Dr. Loretta M. S. Lau
Yes, given the very prognosis (median survival of 8 months based on cases reported in the literature) of spindle cell/sclerosing RMS with FUS–TFCP2 fusion, these tumors should be considered very high-risk, and patients be offered upfront intensive chemotherapy. Proton therapy may also be considered as a modality of radiotherapy to provide adequate local control, especially when proton offers additional benefits to photon. There is also a possibility that these tumours are intrinsically resistant to chemotherapy and neoadjuvant chemotherapy as a conventional RMS treatment approach may not be the best treatment strategy. Upfront surgery and radiotherapy, which is generally used for tumors which are not sensitive to chemotherapy, could potentially be a better option. However, more studies of this rare RMS subtype are required to answer this question.
Expert opinion 2: Dr. Michael D. Deel
This case highlights the difficulty of clinical decision making when treating a rare molecular subtype of an already rare tumor. Prospective study using NGS that incorporates molecular features in risk stratification (24) may help elucidate whether spindle cell/sclerosing RMS with TFCP2 fusions should be considered high-risk. For example, spindle cell/sclerosing RMS with MYOD1 or TP53 mutations are associated with an especially poor prognosis and are now considered high-risk features. Until NGS is routinely performed on all new RMS, an argument could be made that TFCP2 fusion testing should be performed for any intraosseous spindle cell/sclerosing RMS of craniofacial sites. Consideration for intensification of therapy can be considered if the fusion is detected, although it is not known whether intensification of therapy would improve local control and/or survival rates.
Question 2: in this case, the initial preoperative chemotherapy was ineffective, the tumor progressed locally, and distant metastasis occurred. In addition to emergency surgery, what other measures can be taken for this situation?
Expert opinion 1: Dr. Loretta M. S. Lau
In addition to surgery for the primary tumour, disease control for metastatic disease using radiotherapy and other chemotherapy regimen [e.g., ifosfamide/carboplatin/etoposide (ICE) or other chemotherapeutic agents including ifosfamide, doxorubicin, irinotecan/temozolomide, topotecan/cyclophosphamide] be considered.
Expert opinion 2: Dr. Michael D. Deel
Progression of any non-metastatic RMS tumor during neoadjuvant chemotherapy should prompt a re-evaluation of management. This could include verification that the correct initial diagnosis was made and could warrant testing for additional genetic or molecular features. In addition, re-staging should occur to determine the extent of progression and to evaluate for regional and/or metastatic disease. Prompt local control with either surgery or radiation is warranted for locally progressive non-metastatic tumors or metastatic tumors in which local control could be beneficial for palliation. If re-staging demonstrates distant metastatic disease, discussions around goals of care and alternative salvage regimens should be considered.
Question 3: for spindle cell/sclerosing RMS with FUS–TFCP2 fusion with a positive expression of ALK, can ALK inhibitors be used for targeted therapy without lack of potential fusions? When is it appropriate to apply ALK inhibitor therapy?
Expert opinion 1: Dr. Loretta M. S. Lau
Yes, given the extremely poor outcome of these tumors which have failed first line treatment, ALK inhibitors should be considered as an experimental therapy as part of second line therapy, either as monotherapy or in combination with chemotherapy. There are only five patients with spindle cell/sclerosing RMS with FUS–TFCP2 fusion treated with an ALK inhibitor in the literature with reported outcome. Based on these small number of patients, it appears that newer generation ALK inhibitors, e.g., alectinib and lorlatinib, could be more effective than first generation ALK inhibitor crizotinib. Two patients treated with alectinib (18,19) had a response, while of the three patients who were treated with crizotinib, two did not response (17,20) and one had stable disease (4).
Expert opinion 2: Dr. Michael D. Deel
A role for ALK inhibitors in ALK expressing spindle cell/sclerosing RMS with TFCP2 fusions has not been empirically studied. Outside of clinical trial, it may not be prudent to initiate an ALK inhibitor concurrently with conventional neoadjuvant chemotherapy at diagnosis. However, for refractory or relapsed disease in tumors that express ALK, treatment with an ALK inhibitor would not be unreasonable. In non-small cell lung carcinoma expressing ALK fusions, combining an ALK inhibitor with radiotherapy showed greater activity than either modality alone. That could be a reasonable strategy for refractory cases of spindle cell/sclerosing RMS with TFCP2 fusions.
Conclusions
In this paper, we report the clinical, pathologic, and molecular findings and treatment course of a rare pediatric case of spindle cell/sclerosing RMS with FUS–TFCP2 fusion and review similar cases published in the literature. This subtype has a predilection for skeletal involvement, in particular the mandibular bones, and usually has an aggressive clinical course, even with aggressive surgery combined with radiotherapy and chemotherapy, the prognosis is still very poor. Molecular detection is crucial in managing this subtype, once the diagnosis is clear, a more aggressive treatment plan is needed. Histologically, there is a common origin of the epithelium and muscle, and has been a positive expression of ALK in almost all cases, a fact which may guide the future direction of targeted therapy. Therefore, more case summaries and in-depth studies on management of this subtype are needed in the future.
Acknowledgments
Funding: The study was funded by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-603/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-603/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-603/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the relevant ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parham DM, Barr FG, Montgomery E, Nascimento AF. Skeletal muscle tumors. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. editors. WHO classification of tumours of soft issue and bone. 4th ed. Lyon: IARC Press; 2013: 123-35.
- Agaram NP, LaQuaglia MP, Alaggio R, et al. MYOD1-mutant spindle cell and sclerosing rhabdomyosarcoma: an aggressive subtype irrespective of age. A reappraisal for molecular classification and risk stratification. Mod Pathol 2019;32:27-36. [Crossref] [PubMed]
- Xu B, Suurmeijer AJH, Agaram NP, et al. Head and neck rhabdomyosarcoma with TFCP2 fusions and ALK overexpression: a clinicopathological and molecular analysis of 11 cases. Histopathology 2021;79:347-57. [Crossref] [PubMed]
- Le Loarer F, Cleven AHG, Bouvier C, et al. A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol 2020;33:404-19. [Crossref] [PubMed]
- Tagami Y, Sugita S, Kubo T, et al. Spindle cell rhabdomyosarcoma in a lumbar vertebra with FUS-TFCP2 fusion. Pathol Res Pract 2019;215:152399. [Crossref] [PubMed]
- Gallagher KPD, Roza ALOC, Tager EMJR, et al. Rhabdomyosarcoma with TFCP2 Rearrangement or Typical Co-expression of AE1/AE3 and ALK: Report of Three New Cases in the Head and Neck Region and Literature Review. Head Neck Pathol 2023;17:546-61. [Crossref] [PubMed]
- Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol 2001;19:3091-102. [Crossref] [PubMed]
- WHO Classification of Tumors Editorial Board. World Health Organization classification of tumors of soft tissue and bone. Fifth ed. Lyon: IARC Press; 2020.
- Chen S, Rudzinski ER, Arnold MA. Challenges in the Diagnosis of Pediatric Spindle Cell/Sclerosing Rhabdomyosarcoma. Surg Pathol Clin 2020;13:729-38. [Crossref] [PubMed]
- Whittle S, Venkatramani R, Schönstein A, et al. Congenital spindle cell rhabdomyosarcoma: An international cooperative analysis. Eur J Cancer 2022;168:56-64. [Crossref] [PubMed]
- Watson S, Perrin V, Guillemot D, et al. Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol 2018;245:29-40. [Crossref] [PubMed]
- Ishiyama T, Kato I, Ito J, et al. Rhabdomyosarcoma With FUS::TFCP2 Fusion in the Scalp: A Rare Case Report Depicting Round and Spindle cell Morphology. Int J Surg Pathol 2023;31:805-12. [Crossref] [PubMed]
- Ochsner AR, Foss RD. Epithelioid and Spindle Cell Rhabdomyosarcoma of the Oral Mucosa with FUS Rearrangement. Head Neck Pathol 2022;16:823-7. [Crossref] [PubMed]
- Chrisinger JSA, Wehrli B, Dickson BC, et al. Epithelioid and spindle cell rhabdomyosarcoma with FUS-TFCP2 or EWSR1-TFCP2 fusion: report of two cases. Virchows Arch 2020;477:725-32. [Crossref] [PubMed]
- Silva Cunha JL, Cavalcante IL, da Silva Barros CC, et al. Intraosseous rhabdomyosarcoma of the maxilla with TFCP2 fusion: A rare aggressive subtype with predilection for the gnathic bones. Oral Oncol 2022;130:105876. [Crossref] [PubMed]
- Koutlas IG, Olson DR, Rawwas J. FET(EWSR1)-TFCP2 Rhabdomyosarcoma: An Additional Example of this Aggressive Variant with Predilection for the Gnathic Bones. Head Neck Pathol 2021;15:374-80. [Crossref] [PubMed]
- Panferova A, Sinichenkova KY, Abu Jabal M, et al. EWSR1-TFCP2 in an adolescent represents an extremely rare and aggressive form of intraosseous spindle cell rhabdomyosarcomas. Cold Spring Harb Mol Case Stud 2022;8:a006209. [Crossref] [PubMed]
- Valério E, Furtado Costa JL, Perez Fraile NM, et al. Intraosseous Spindle Cell/Epithelioid Rhabdomyosarcoma with TFCP2 Rearrangement: A Recent Recognized Subtype with Partial Response to Alectinib. Int J Surg Pathol 2023;31:861-5. [Crossref] [PubMed]
- Brunac AC, Laprie A, Castex MP, et al. The combination of radiotherapy and ALK inhibitors is effective in the treatment of intraosseous rhabdomyosarcoma with FUS-TFCP2 fusion transcript. Pediatr Blood Cancer 2020;67:e28185. [Crossref] [PubMed]
- Lewin J, Desai J, Smith K, et al. Lack of clinical activity with crizotinib in a patient with FUS rearranged rhabdomyosarcoma with ALK protein overexpression. Pathology 2019;51:655-7. [Crossref] [PubMed]
- Dashti NK, Wehrs RN, Thomas BC, et al. Spindle cell rhabdomyosarcoma of bone with FUS-TFCP2 fusion: confirmation of a very recently described rhabdomyosarcoma subtype. Histopathology 2018;73:514-20. [Crossref] [PubMed]
- Agaram NP, Zhang L, Sung YS, et al. Expanding the Spectrum of Intraosseous Rhabdomyosarcoma: Correlation Between 2 Distinct Gene Fusions and Phenotype. Am J Surg Pathol 2019;43:695-702. [Crossref] [PubMed]
- Shern JF, Selfe J, Izquierdo E, et al. Genomic Classification and Clinical Outcome in Rhabdomyosarcoma: A Report From an International Consortium. J Clin Oncol 2021;39:2859-71. [Crossref] [PubMed]
- Haduong JH, Heske CM, Allen-Rhoades W, et al. An update on rhabdomyosarcoma risk stratification and the rationale for current and future Children's Oncology Group clinical trials. Pediatr Blood Cancer 2022;69:e29511. [Crossref] [PubMed]



