Narrative review: precision medicine applications in neuroblastoma—current status and future prospects
Introduction
Neuroblastoma (NB), which arises from undeveloped neural crest cells in the sympathetic nervous system, is the most common solid tumor found in children. It is responsible for approximately 15% of all pediatric cancer-related deaths (1). Based on clinical and biological prognostic factors such as the standard International NB Risk Group staging, age, histological category, tumor differentiation grade, MYCN status, presence of 11q aberrations, and tumor cell ploidy, the Children’s Oncology Group (COG) has established a risk classification system for NB. This system categorizes NB into very low-, low-, intermediate-, and high-risk groups (2). For the majority of patients with low-risk NB, surgery alone constitutes a curative treatment, with the use of chemotherapy being limited to specific circumstances (3). In the case of the intermediate-risk group, the duration and dosage of chemotherapy treatment have been significantly reduced compared to the regimens used in early clinical trials (4). The survival rates for patients with low- and intermediate-risk NB are nearly 100%, whereas those with high-risk NB have survival rates below 50% (5-7). Treatment for high-risk patients includes five to six stages of induction chemotherapy, surgery, high-dose therapy with autologous hematopoietic stem cell transplantation, consolidation therapy with radiotherapy, and subsequent therapy to manage minimal residual disease (7). The majority of patients with high-risk NB do not respond to initial treatments or experience relapse within 2 years of treatment initiation. Survival rates are notably low for patients with recurrent or refractory NB (8-10). Most cancer therapies are based on a “one-size-fits-all” strategy that works for a subset of patients. The concept of precision medicine relies on molecular profiling of genetics, focusing on individual differences, adapting treatment and follow-up according to the individual patient, and tailoring diagnosis and treatment (11). The application of precision medicine to solid tumors was initiated through the use of imatinib in gastrointestinal mesenchymal tumors (12). The core of precision medicine lies in the application of genomics technology, which integrates treatment strategies that take into account individual genetic, environmental, and lifestyle differences (13). The precision medicine approach relies on the selection of appropriate biomarkers to predict the efficacy of targeted therapies in specific patient populations (14). Despite the remarkable advances in genomic precision medicine in adult cancers, we still face many challenges in pediatric oncology. The genetic characteristics of pediatric tumors are significantly different from those of adult tumors, and in-depth studies are needed to address the specificities of pediatric tumors. In this paper, we will discuss several aspects of targeted therapy, molecular biomarkers, liquid biopsy, and immunotherapy for NB. We present this article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-557/rc).
Methods
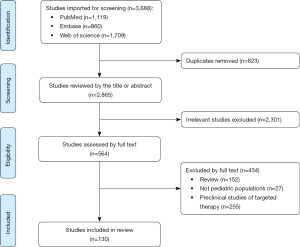
We conducted extensive searches in PubMed, EMBASE, and Web of Science using key terms and database-specific strategies, and filtered for time and language to ensure a comprehensive collection of literature related to precision medicine for NB (Figure 1). The main keywords included neuroblastoma, precision medicine, pediatrics and targeting, and articles were searched from 1985 to the present, with no restrictions on article type. The synthesis evaluated pre-treatment-related studies of NB as well as studies regarding long-term prognosis. If additional data were required for the review and these were considered to be time-insensitive the search was extended to an earlier time. The methodology of the studies used in this narrative review is detailed in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 June 2023 to 31 July 2023 |
| Databases and other sources searched | PubMed, EMBASE, and Web of Science |
| Search terms used | “Neuroblastoma”, “precision medicine”, “Targeted therapy”, “molecular biomarker” |
| Timeframe | 1985-present |
| Inclusion and exclusion criteria | Inclusion: human studies, randomized control trials, cohort studies, case control studies, reviews, care guidelines; exclusion: non-English language |
| Selection process | Identification of articles by the first author; consensus was obtained after discussion |
Targeted therapy
Traditional therapies such as chemotherapy and radiotherapy are effective against NB but often come with a range of side effects. In contrast, targeted therapy offers a more refined treatment approach, utilizing specific drugs or substances to identify and further attack cancer cells, while minimizing harm to normal human cells. This is particularly important in the treatment of pediatric tumors, as the subsequent growth and development of children are also a crucial aspect of treatment. Additionally, targeted therapy is quite effective for high-risk cases that are resistant to traditional treatments. With more and more targets being identified, a variety of new therapies have been developed.
MYCN inhibitors
The MYCN oncogene encodes transcription factors that regulate multiple cellular processes. MYCN amplification is observed in 20–30% of NB cases, and this amplification is strongly correlated with disease stage and aggressiveness (15,16). Multiple studies conducted on pediatric NB patients have consistently shown that those with MYCN-amplified tumors experience significantly lower event-free survival (EFS) and overall survival (OS) (17,18). In an analysis of 6,000 patient samples, it was found that patients with wild-type MYCN had a better prognosis compared to those with homozygous or heterozygous MYCN amplification (16). While there is a clear clinical significance in NB, the development of small molecules directly targeting MYCN is challenging due to the lack of stable and specific binding pockets. The most effective approach for inhibiting MYCN or controlling its regulation seems to be through indirect targeted therapies.
Aurora kinase inhibitors
AURKA is a therapeutic target in a variety of malignant tumors, including NB, and elevated levels of its expression correlate with lower OS and EFS in NB patients. The expression of AURKA is strongly correlated with the state of MYCN amplification, which plays a key role in NB cell growth (19,20). Current studies focus on indirectly interfering with MYCN activity, using drugs or interaction partners of AURKA to promote the stability of MYCN protein (21,22). A phase I clinical trial and pharmacokinetic study in pediatrics evaluated the safety, tolerability, and pharmacokinetic properties of alisertib in pediatric patients, and determined its recommended dosage and preliminary efficacy in pediatric cancer therapy (23). Although alisertib showed compelling pharmacokinetic-pharmacodynamic relationships in preclinical models and adults and was active in pediatric xenograft models, objective remission rates in children and adolescents treated with alisertib alone were less than 5% in a phase 2 trial of pediatric patients with refractory or recurrent solid tumors or acute leukemia. Therefore, we should explore novel combinatorial strategies that include targeting other oncogenic signaling pathways in order to exploit this pathway while minimizing toxicity (24,25). A clinical study demonstrated that combining the Aurora kinase inhibitor alisertib with irinotecan and temozolomide exceeded the expected anti-tumor activity compared to using irinotecan and temozolomide alone. This combination therapy showed a good response rate and progression-free survival (26,27). Key research indicates that the overexpression of AURKB in NB cells is closely linked to poor prognosis and acquired resistance to carboplatin, a commonly used chemotherapy drug for treating NB. Therefore, inhibiting the AURKB-ERK axis may offer a potential therapeutic strategy to overcome carboplatin resistance in NB patients (28).
Cyclin-dependent kinase (CDK) inhibitor
In eukaryotes, cell CDK plays a key role as a serine/threonine-specific protein kinase responsible for regulation at different stages of the cell cycle, and plays a crucial role in a number of key processes such as cell proliferation, transcription, differentiation, metabolism, and apoptosis. CDKs are divided into two classes: those that regulate the cell cycle, including CDK 1, 2, 4, and 6; and those involved in phosphorylating transcriptional regulators, including CDK 7–9, 12, and 13 (29-31).
The FDA has approved four such drugs, including palbociclib, ribociclib, abemaciclib, and trilaciclib. As the prototypical drug among them, palbociclib has been approved for the treatment of a specific type of advanced breast cancer, namely, estrogen receptor-positive (ER+) and human epidermal growth factor receptor 2-negative (HER2−) cases (32,33).
The first subsequent clinical trial exploring CDK4/6 inhibitors in pediatric tumors evaluated the maximum tolerated dose of the CDK4/6 inhibitor ribociclib in the treatment of diseases such as NB in children and the recommended dose for phase II trials (34).
Anaplastic lymphoma kinase (ALK) inhibitors
ALK is an oncogene, with mutations including copy number variations, amplifications, and point mutations. ALK activating mutations account for approximately 6–10% of NB cases, and an additional 3–4% carry high-risk ALK mutations (35). The most common ALK mutations in sporadic NB cases are found at the R1275, F1174, and F1245 sites, which are located in the critical regulatory region of the receptor tyrosine kinases structural domain and possess both in vitro and in vivo transforming abilities (36). The incidence of ALK gene mutations increases in recurrent NB, occurring in approximately 20% of cases (37,38). In a high-risk NB cohort, the presence of abnormal ALK copy number status is highly associated with clinical phenotypes, such as metastasis at diagnosis and disease-related mortality (39).
Crizotinib is the most extensively studied ALK inhibitor in the treatment of NB. In pediatric clinical trials, it has demonstrated good tolerability, especially in patients with relapsed or refractory cancer, when administered at about double the adult recommended dose (approximately 280 mg/m2) (40). Additionally, crizotinib has shown success in treating children with anaplastic large cell lymphoma and inflammatory myofibroblastic tumors (41). The ADVL0912 study investigated the application of crizotinib in patients with ALK-abnormal relapsed or refractory NB. This study found that crizotinib’s effectiveness was limited in these cases, primarily due to its inability to achieve high enough concentrations to counteract ATP affinity competition (42). Furthermore, a phase II trial (NCT00939770) by the COG evaluated crizotinib’s effectiveness in pediatric patients with relapsed or refractory ALK-driven NB. This trial noted that only a minority of patients showed an objective response, which was mainly attributed to the intrinsic resistance of certain ALK hotspot mutations to crizotinib (40). The relative resistance of children with NB carrying F1174 and F1245 residue mutations may be due to enhanced adenosine triphosphate (ATP)-binding affinity, which can be compensated by higher doses of ALK inhibitors or alternatives (43). The focus has shifted to enhancing crizotinib’s effectiveness through combination therapies. When crizotinib was combined with chemotherapy agents typically used in high-risk NB, a synergistic effect was observed. This synergy is partly why crizotinib has been incorporated into the treatment protocol for high-risk NB patients with ALK mutations in the COG phase 3 ANBL1531 trial.
Meanwhile, second- and third-generation ALK inhibitors have been tested in phase I/II. The next-generation ALK inhibitors, including ceritinib, ensartinib, entrectinib, lorlatinib, and alectinib, have shown efficacy against crizotinib-resistant mutations. Ceritinib can overcome crizotinib resistance in non-small cell lung cancer (NSCLC) carrying ALK rearrangements (44,45). An open-label, multicenter phase I clinical study detailed the dose escalation and expansion of ceritinib in pediatric patients with ALK-positive, focusing on the drug’s safety, tolerability, maximum tolerated dose, and anti-tumor activity (46). A subsequent case reported the successful and sustained remission of refractory metastatic NB in an infant carrying a genetic variant of the ALKAL2 gene who was in good condition after more than 4 years of continuous and ongoing treatment with entrectinib (47). A phase 1/2 study of entrectinib in pediatric patients with advanced or metastatic solid central nervous system tumors without great treatment options (NCT02650401) is also ongoing another inhibitor, lorlatinib, has been extensively studied to demonstrate its use as an alternative to crizotinib in newly diagnosed high-risk NB patients with ALK genetic alterations (48,49). The results of phase I clinical trials have shown that lorlatinib, either as a stand-alone treatment or in combination with chemotherapy, has demonstrated the potential to be a safe and effective treatment for ALK-driven refractory or recurrent high-risk NB (50). Moreover, Lorlatinib has shown promise in the treatment of adult-onset NB, with 69% of patients presenting durable primary responses and nearly all patients receiving this treatment having objective efficacy responses (51).
Tropomyosin receptor kinase (TrK) inhibitors
Elevated TrkB expression is associated with high-risk NB and low survival rates, whereas increased TrkA expression is associated with low-risk NB and tumors prone to spontaneous regression (52). TrkB has been reported to increase angiogenesis and metastasis. Therefore, Trk is a target for NB therapy (53). Lestaurtinib, entrectinib, larotrectinib are multi-target Trk kinase inhibitors. Initial clinical trials in paediatric patients with refractory, high-risk NB, demonstrated that Lestaurtinib was well tolerated by patients, and effective doses were identified. At higher doses, these drugs not only demonstrated a favourable safety profile, but also showed initial efficacy. This provides an important basis for further research and treatment of NB (54). In phase 1 clinical trials in adults, entrectinib is currently being used as a monotherapy for patients with molecular alterations in neurotrophic tropomyosin kinase receptors (NTRK), ALK, and ROS1 genes (ALKA-372-001, STARTRK-1). Furthermore, when entrectinib is used in combination, it significantly enhances the efficacy of chemotherapy without additional toxicity (55). In numerous clinical trials, larotrectinib has shown quick and sustained effects, substantial disease management success, and a positive safety record in individuals with TRK fusion-positive central nervous system tumors, irrespective of the patient’s age or the kind of tumor (56-60).
Angiogenesis inhibitors
Angiogenesis inhibitors play a crucial role in the sustained growth and metastasis of NB, with a high vascular index being associated with poor disease prognosis. These include vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), fibroblast growth factor-2 (FGF-2), transforming growth factor-alpha (TGF-α), platelet-derived growth factor A (PDGF-A), erythropoietin, and angiopoietin (61). High levels of expression of angiogenic factors are strongly associated with high risk and high stage NB, making them sensitive targets for anti-angiogenic therapy (62,63). Antiangiogenic agents include single-pathway inhibitors and multi-pathway inhibitors. Bevacizumab, a single-pathway anti-angiogenic antibody, specifically targets VEGF and inhibits its binding to receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2) (64). Following the completion of the COG Phase 1 clinical trial evaluating the safety of bevacizumab in paediatric patients with drug-resistant solid tumours, the efficacy of incorporating bevacizumab into the chemotherapy combination of irinotecan and temozolomide was further investigated. However, this new chemotherapy combination did not significantly improve remission rates, so the current findings do not support the inclusion of bevacizumab in the standard chemotherapy regimen for patients with high-risk NB (64,65). Cabozantinib is a small molecule inhibitor targeting a number of tyrosine kinases including MET, VEGFR2, RET and AXL. Initial Phase 1 studies have shown it to be safe and tolerable in the treatment of recurrent or refractory solid tumours in children. Subsequent single institution case studies have also confirmed the therapeutic potential of cabozantinib. However, additional follow-up studies are still needed to fully evaluate its long-term use (66,67).
Mammalian target of rapamycin (mTOR) inhibitors
mTOR, a protein kinase known to be dysregulated in cancer and metabolic disorders, has been demonstrated through numerous preclinical studies to play a significant role in the occurrence and development of NB (68,69). Therefore, targeting key protein activities in the mTOR signaling pathway can be a potential therapeutic approach for NB. mTOR inhibitors, mTOR kinase inhibitors, and mTOR regulatory factor inhibitors have been studied for their safety and efficacy in patients with cancer, including NB. Combining mTOR inhibitors with other anticancer drugs may sensitize tumor cells to these treatments. These combinations have the potential to produce additional activity or may delay or prevent the development of resistance to these drugs (69,70). In the treatment of pediatric solid tumors, mTOR inhibitors such as ridaforolimus, sirolimus, temsirolimus, everolimus, and vistusertib have been proven to have good tolerability. Clinical trials have demonstrated their effectiveness, whether used alone or in combination with other drugs. This indicates significant potential for further research in this field (71-75).
Immunotherapy
Immunotherapy has a powerful effect on immunity and immunosuppression and has a greater role in the treatment of high-risk NB and other aggressive solid tumors.
Anti-GD2 antibodies
The bis-sialic acid ganglioside GD2, a glycosphingolipid containing sialic acid, has significant clinical and pathological implications. GD2 is involved in cell–cell adhesion and signal transduction on the cell surface, playing a crucial role in proliferation, angiogenesis, immune evasion, and invasion in both physiological and pathological processes. It is predominantly expressed on the cell surface and is mainly found in the central nervous system and rarely in peripheral nerves and skin melanocytes (76). When GD2 binds to the antibody, immune cells such as macrophages and granulocytes can recognize the tumor cells and initiate an active attack (77). The anti-GD2 monoclonal antibody dinutuximab has been approved as a first-line treatment for high-risk pediatric NB (78-80).
Based on preclinical data and studies of anti-HER2 monoclonal antibodies in combination with chemotherapy for the treatment of breast cancer, a follow-up study found that the addition of Dinutuximab beta to the chemoimmunotherapy combination resulted in significantly better EFS and OS than chemotherapy alone (81-84). However, the further addition of subcutaneously administered IL-2 to dinutuximab beta treatment did not achieve the expected results (85). There is substantial evidence that GM-CSF with anti-GD2 antibodies has demonstrated safety and efficacy in both the consolidation/maintenance phase and the relapsed/refractory disease setting in NB patients (86,87).
Chimeric antigen receptor (CAR) T cells
In the field of treatment of advanced malignancies, despite significant advances, patient survival remains suboptimal. The potential of emerging cellular immunotherapies, such as CAR-T cells, is immense. CAR-T therapy uses recombinant antigen receptors to re-target T-lymphocytes and other immune cells with the aim of improving specificity and functionality against tumours (88). Its main advantage is the ability to rapidly generate T cells that target tumours, bypassing the limitations of natural immunity and thus improving the kinetics of the immune response. Although CAR-T cell therapy is currently used primarily for haematological malignancies, it is being progressively researched in other cancer types such as NB (89).
The latest clinical trials have shown that administering the right amount of GD2 CAR-T cell therapy to patients significantly shrinks tumours. In the group of patients receiving the recommended dose, a 3-year OS rate of 60% and an EFS rate of 36% were observed (90,91). This suggests that GD2 CAR-T cell therapy has great potential for future applications. Nonetheless, this therapeutic approach still faces challenges, including insufficient T-cell persistence, target antigen selection challenges, and the inhibitory effects of the tumour microenvironment. Therefore, more in-depth exploration and improvement in these areas are needed.
Molecular biomarker
Biomarkers can enable specific early detection and prognostic assessment of tumors, allowing for the categorization of NB into different groups to enhance treatment and prognosis. Therefore, the discovery of novel biomarkers is crucial for the advancement of NB detection, prediction, and the development of new targeted therapeutic strategies.
Paired-like homeobox 2B (PHOX2B)
PHOX2B protein is encoded by the PHOX2B gene, which is located on chromosome 4p13. As a major regulator of neuronal, ganglion cell, and neural crest-derived cell development, it plays an important role in the differentiation and maturation of the nervous system. A study suggest that mutations in the PHOX2B gene, found in familial and syndromic peripheral NB, may play a role in the development of NB, thereby clarifying the gene’s relevance to this condition (92). Warren et al. showed that PHOX2B has a high (approximately 93–100%) sensitivity in NB tumors and is strongly expressed in undifferentiated and poorly differentiated NBs (93). In addition, a study on PHOX2B expression and its mimics in NB revealed exciting results. This study found that PHOX2B expression was both sensitive and specific for the diagnosis of NB in tissue specimens as well as in smaller specimens such as cytology specimens (94).
Programmed cell death ligand-1 (PD-L1)
Multivariable Cox regression analysis has indicated that the combination of PD-L1 and HLA class I tumor cell density can serve as a prognostic biomarker for predicting OS in NB patients. The expression of PD-L1 in NB cells can effectively assess the patients’ risk of survival and guide the selection of immunotherapy for NB (95,96).
Proviral integration site for Moloney murine leukemia virus (PIM) kinase
PIM serine/threonine kinase is significantly associated with poor OS in patients with solid tumors such as NB and many hematologic malignancies (97). Consequently, the expression of PIM has been identified as a promising prognostic indicator and novel therapeutic target in the treatment of NB (98).
Aryl hydrocarbon receptor (AHR)
The AHR, a ligand-activated transcription factor, influences migration genes through pathways affecting tumor migration (99,100). Studies based on a small group of patient samples have shown a negative correlation between AHR expression and MYCN as well as histological grading in NB tumors. Survival analysis has also demonstrated that positive AHR expression is associated with better prognosis (101,102). It has also been demonstrated in vitro that AhR can mediate the directional migration of human NB cells induced by low concentrations of a potent agonist of AhR. However, further research is needed to understand its in vivo effects (103).
Ubiquitin C-terminal hydrolase L1 (UCHL1)
UCH is a class of thiol proteases involved in the hydrolysis of polymerized ubiquitin (104). Based on tissue microarray and validation datasets, UCHL1 has been reported as a biomarker for detecting minimal residual disease in the bone marrow and peripheral blood of NB patients, with its expression positively correlated with differentiation markers. Therefore, UCHL1 can serve as a prognostic marker for better clinical outcomes in NB (105,106).
Tropomodulin (TMOD)
TMOD is a conserved protein family that cover the ends of actin filaments, stabilizing them and inhibiting their disassembly and turnover (107). Both TMOD1 and TMOD2 are highly expressed in NB, and the strong association between high expression of these 2 genes and favorable prognosis in NB makes them independent prognostic markers (108).
The PTPN11 gene
Through exome, genome and transcriptome sequencing, it was found that PTPN11 has a high frequency of mutations in the RAS/ERK pathway (35,109). These mutations can activate the Ras-Erk signaling pathway, leading to the development of Noonan syndrome (NS), an autosomal dominant disorder. Of note, NS patients are more likely to develop NB (110). The tyrosine phosphatase SHP2 encoded by PTPN11 acts as an activator of the RAS pathway. It has been pointed out that the combined inhibition of SHP2, MEK, or ERK within the RAS-MAPK pathway can effectively treat drug-resistant recurrent NBs (111,112). Multiple cases have already shown that NS patients develop multiple NBs due to PTPN11 mutations (113,114).
Liquid biopsy
A diagnostic concept known as “liquid biopsy” has emerged, primarily involving the detection of tumor fragments that have shed into the circulatory system through the analysis of body fluids such as blood and urine. These fragments can be identified and isolated from the blood using high-throughput methods, allowing for subsequent analysis at the single-cell level (115,116). Compared to traditional diagnostic techniques, liquid biopsy is an attractive non-invasive method for tracking disease progression in real time (117,118).
Circulating tumor DNA (ctDNA)
Analysis of genomic ctDNA reveals the clonal evolutionary dynamics within somatic alterations, which increase upon recurrence and can be targeted for therapy (119). Pediatric tumors carry relatively fewer mutations at diagnosis compared to many adult malignancies, but they often exhibit enriched potential targetable mutations upon relapse. Tissue biopsies do not allow for continuous monitoring, whereas continuous sampling of ctDNA provides us with the ability to assess potential tumor heterogeneity and its evolution, as well as detect genetic changes conferring resistance during treatment (49). Less ctDNA is released into the bloodstream at NB recurrence than at diagnosis. Alterations in ctDNA are prevalent in children with high-risk NB and are valuable for follow-up during treatment (120-122).
Circulating tumor cells (CTCs)
Cell-free DNA (cfDNA) interpretation reveals that aggressive tumors, often associated with areas of necrosis, result in higher DNA release. Its levels are related to staging and tumor burden, increasing up to 50 times higher than normal levels. Rapid reduction in cfDNA concentration in the blood of high-risk NB patients is indicative of a better response to cancer treatment (123). Detection of 17q gain in cfDNA increases with increasing stages of NB. Moreover, the detection of unique markers in cfDNA aligns with tumor load, reducing during initial treatment, vanishing with full remission, and re-emerging at the time of relapse (124,125).
Circulating RNA
Tyrosine hydroxylase messenger RNA (mRNA) demonstrates high sensitivity and specificity in identifying hidden NB cells in both bone marrow and peripheral blood. It can detect NB cells in the bone marrow even in the absence of cytologic evidence of the tumor, with stage 4 children being significantly more likely to be diagnosed than those in stages 1–3 (126,127).
Lactate dehydrogenase (LDH)
LDH is often released following tissue injury and is therefore considered a marker of tissue damage and disease. High levels of LDH have been found to be associated with poor prognosis in a review of outcome studies of melanoma, prostate cancer, and renal cell carcinoma, among others (128). Recent studies have investigated both LDH and serum ferritin as prognostic markers of NB, and they can also be used as factors in NB risk stratification (129,130).
Conclusions
Over the years, there has been a continuous search for more comprehensive and effective methods for diagnosing and treating NB. Precision medicine has demonstrated significant potential in the field of NB, offering diagnostic and therapeutic value. The discovery of biological markers has provided clinicians with a convenient tool for the diagnosis and treatment of NB. However, only a few mutations have been shown to have therapeutic value in NB, and research on NB remains relatively limited. In this context, the development of precision medicine for NB becomes particularly important as it can enhance the accuracy of diagnosis and treatment for patients.
Currently, there is still inconsistency in research findings regarding the treatment of NB, and combination therapies aimed at known treatment targets are continuously being improved. It is necessary to continue conducting research and strive to enhance treatment efficacy while reducing toxicity. The continuous progress of precision medicine has laid a solid foundation for innovation and validation in NB and has paved the way for its future development.
Acknowledgments
Funding: This study was support by National Key R&D Program of China: Reproductive Health and Women’s and Children’s Health Protection (No. 2022YFC2705002); National Science and Technology of Liaoning Province (No. 2020JH1/10300001); “Xingliao Talents Program” of Liaoning Province (No. XLYC2008010).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-557/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-557/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-557/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet 2007;369:2106-20. [Crossref] [PubMed]
- Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289-97. [Crossref] [PubMed]
- Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: results of Children's Oncology Group study P9641. J Clin Oncol 2012;30:1842-8. [Crossref] [PubMed]
- Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 2010;363:1313-23. [Crossref] [PubMed]
- Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers 2016;2:16078. [Crossref] [PubMed]
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5--a population-based study. Lancet Oncol 2014;15:35-47. [Crossref] [PubMed]
- Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol 2015;33:3008-17. [Crossref] [PubMed]
- Park JR, Scott JR, Stewart CF, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: a Children's Oncology Group study. J Clin Oncol 2011;29:4351-7. [Crossref] [PubMed]
- Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol 2009;27:1007-13. [Crossref] [PubMed]
- Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer 2009;45:2835-42. [Crossref] [PubMed]
- Rosenquist R, Fröhling S, Stamatopoulos K. Precision medicine in cancer: A paradigm shift. Semin Cancer Biol 2022;84:1-2. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [Crossref] [PubMed]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [Crossref] [PubMed]
- Rosen E, Drilon A, Chakravarty D. Precision Oncology: 2022 in Review. Cancer Discov 2022;12:2747-53. [Crossref] [PubMed]
- Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med 1985;313:1111-6. [Crossref] [PubMed]
- Campbell K, Naranjo A, Hibbitts E, et al. Association of heterogeneous MYCN amplification with clinical features, biological characteristics and outcomes in neuroblastoma: A report from the Children's Oncology Group. Eur J Cancer 2020;133:112-9. [Crossref] [PubMed]
- Katzenstein HM, Bowman LC, Brodeur GM, et al. Prognostic significance of age, MYCN oncogene amplification, tumor cell ploidy, and histology in 110 infants with stage D(S) neuroblastoma: the pediatric oncology group experience--a pediatric oncology group study. J Clin Oncol 1998;16:2007-17. [Crossref] [PubMed]
- Bagatell R, Beck-Popovic M, London WB, et al. Significance of MYCN amplification in international neuroblastoma staging system stage 1 and 2 neuroblastoma: a report from the International Neuroblastoma Risk Group database. J Clin Oncol 2009;27:365-70. [Crossref] [PubMed]
- Otto T, Horn S, Brockmann M, et al. Stabilization of N-Myc is a critical function of Aurora A in human neuroblastoma. Cancer Cell 2009;15:67-78. [Crossref] [PubMed]
- Shang X, Burlingame SM, Okcu MF, et al. Aurora A is a negative prognostic factor and a new therapeutic target in human neuroblastoma. Mol Cancer Ther 2009;8:2461-9. [Crossref] [PubMed]
- Rishfi M, Krols S, Martens F, et al. Targeted AURKA degradation: Towards new therapeutic agents for neuroblastoma. Eur J Med Chem 2023;247:115033. [Crossref] [PubMed]
- Brockmann M, Poon E, Berry T, et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell 2013;24:75-89. [Crossref] [PubMed]
- Mossé YP, Lipsitz E, Fox E, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin Cancer Res 2012;18:6058-64. [Crossref] [PubMed]
- Mossé YP, Fox E, Teachey DT, et al. A Phase II Study of Alisertib in Children with Recurrent/Refractory Solid Tumors or Leukemia: Children's Oncology Group Phase I and Pilot Consortium (ADVL0921). Clin Cancer Res 2019;25:3229-38. [Crossref] [PubMed]
- Michaelis M, Selt F, Rothweiler F, et al. Aurora kinases as targets in drug-resistant neuroblastoma cells. PLoS One 2014;9:e108758. [Crossref] [PubMed]
- DuBois SG, Marachelian A, Fox E, et al. Phase I Study of the Aurora A Kinase Inhibitor Alisertib in Combination With Irinotecan and Temozolomide for Patients With Relapsed or Refractory Neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) Trial. J Clin Oncol 2016;34:1368-75. [Crossref] [PubMed]
- DuBois SG, Mosse YP, Fox E, et al. Phase II Trial of Alisertib in Combination with Irinotecan and Temozolomide for Patients with Relapsed or Refractory Neuroblastoma. Clin Cancer Res 2018;24:6142-9. [Crossref] [PubMed]
- Yang Y, Sheng Y, Sun D, et al. AURKB promotes tumorigenesis and carboplatin resistance by regulating the ERK pathway in neuroblastoma cells. Int J Neurosci 2023;133:1224-32. [Crossref] [PubMed]
- Wood DJ, Endicott JA. Structural insights into the functional diversity of the CDK-cyclin family. Open Biol 2018;8:180112. [Crossref] [PubMed]
- Malumbres M. Cyclin-dependent kinases. Genome Biol 2014;15:122. [Crossref] [PubMed]
- Zhang H, Pandey S, Travers M, et al. Targeting CDK9 Reactivates Epigenetically Silenced Genes in Cancer. Cell 2018;175:1244-1258.e26. [Crossref] [PubMed]
- Turner NC, Slamon DJ, Ro J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 2018;379:1926-36. [Crossref] [PubMed]
- Palbociclib Plus Fulvestrant Maintains Long-Term Overall Survival Benefit in HR+/HER2- Advanced Breast Cancer. Oncologist 2021;26:S5-6. [Crossref] [PubMed]
- Geoerger B, Bourdeaut F, DuBois SG, et al. A Phase I Study of the CDK4/6 Inhibitor Ribociclib (LEE011) in Pediatric Patients with Malignant Rhabdoid Tumors, Neuroblastoma, and Other Solid Tumors. Clin Cancer Res 2017;23:2433-41. [Crossref] [PubMed]
- Pugh TJ, Morozova O, Attiyeh EF, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet 2013;45:279-84. [Crossref] [PubMed]
- Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer 2013;13:397-411. [Crossref] [PubMed]
- Schleiermacher G, Javanmardi N, Bernard V, et al. Emergence of new ALK mutations at relapse of neuroblastoma. J Clin Oncol 2014;32:2727-34. [Crossref] [PubMed]
- Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet 2015;47:864-71. [Crossref] [PubMed]
- Mossé YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 2008;455:930-5. [Crossref] [PubMed]
- Mossé YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 2013;14:472-80. [Crossref] [PubMed]
- Bresler SC, Wood AC, Haglund EA, et al. Differential inhibitor sensitivity of anaplastic lymphoma kinase variants found in neuroblastoma. Sci Transl Med 2011;3:108ra114. [Crossref] [PubMed]
- Foster JH, Voss SD, Hall DC, et al. Activity of Crizotinib in Patients with ALK-Aberrant Relapsed/Refractory Neuroblastoma: A Children's Oncology Group Study (ADVL0912). Clin Cancer Res 2021;27:3543-8. [Crossref] [PubMed]
- Krytska K, Ryles HT, Sano R, et al. Crizotinib Synergizes with Chemotherapy in Preclinical Models of Neuroblastoma. Clin Cancer Res 2016;22:948-60. [Crossref] [PubMed]
- Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:2537-9. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Fischer M, Moreno L, Ziegler DS, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol 2021;22:1764-76. [Crossref] [PubMed]
- Treis D, Umapathy G, Fransson S, et al. Sustained Response to Entrectinib in an Infant With a Germline ALKAL2 Variant and Refractory Metastatic Neuroblastoma With Chromosomal 2p Gain and Anaplastic Lymphoma Kinase and Tropomyosin Receptor Kinase Activation. JCO Precis Oncol 2022;6:e2100271. [Crossref] [PubMed]
- Suk Y, Singh SK. Safety and efficacy of lorlatinib against ALK-driven refractory or relapsed neuroblastoma. Cell Rep Med 2023;4:101071. [Crossref] [PubMed]
- Berko ER, Witek GM, Matkar S, et al. Circulating tumor DNA reveals mechanisms of lorlatinib resistance in patients with relapsed/refractory ALK-driven neuroblastoma. Nat Commun 2023;14:2601. [Crossref] [PubMed]
- Goldsmith KC, Park JR, Kayser K, et al. Lorlatinib with or without chemotherapy in ALK-driven refractory/relapsed neuroblastoma: phase 1 trial results. Nat Med 2023;29:1092-102. [Crossref] [PubMed]
- Stiefel J, Kushner BH, Roberts SS, et al. Anaplastic Lymphoma Kinase Inhibitors for Therapy of Neuroblastoma in Adults. JCO Precis Oncol 2023;7:e2300138. [Crossref] [PubMed]
- Nakagawara A, Arima-Nakagawara M, Scavarda NJ, et al. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 1993;328:847-54. [Crossref] [PubMed]
- Desmet CJ, Peeper DS. The neurotrophic receptor TrkB: a drug target in anti-cancer therapy? Cell Mol Life Sci 2006;63:755-9. [Crossref] [PubMed]
- Minturn JE, Evans AE, Villablanca JG, et al. Phase I trial of lestaurtinib for children with refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. Cancer Chemother Pharmacol 2011;68:1057-65. [Crossref] [PubMed]
- Iyer R, Wehrmann L, Golden RL, et al. Entrectinib is a potent inhibitor of Trk-driven neuroblastomas in a xenograft mouse model. Cancer Lett 2016;372:179-86. [Crossref] [PubMed]
- Doz F, van Tilburg CM, Geoerger B, et al. Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol 2022;24:997-1007. [Crossref] [PubMed]
- Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 2020;21:531-40. [Crossref] [PubMed]
- Hong DS, Bauer TM, Lee JJ, et al. Larotrectinib in adult patients with solid tumours: a multi-centre, open-label, phase I dose-escalation study. Ann Oncol 2019;30:325-31. [Crossref] [PubMed]
- Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 2018;19:705-14. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Ribatti D. Anti-angiogenesis in neuroblastoma. Crit Rev Oncol Hematol 2013;86:212-21. [Crossref] [PubMed]
- Eggert A, Ikegaki N, Kwiatkowski J, et al. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 2000;6:1900-8.
- Peddinti R, Zeine R, Luca D, et al. Prominent microvascular proliferation in clinically aggressive neuroblastoma. Clin Cancer Res 2007;13:3499-506. [Crossref] [PubMed]
- Modak S, Kushner BH, Basu E, et al. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: Results of a phase II study. Pediatr Blood Cancer 2017;
- Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. J Clin Oncol 2008;26:399-405. [Crossref] [PubMed]
- Chuk MK, Widemann BC, Minard CG, et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer 2018;65:e27077. [Crossref] [PubMed]
- Perisa MP, Storey M, Streby KA, et al. Cabozantinib for relapsed neuroblastoma: Single institution case series. Pediatr Blood Cancer 2020;67:e28317. [Crossref] [PubMed]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274-93. [Crossref] [PubMed]
- Yuan R, Kay A, Berg WJ, et al. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2009;2:45. [Crossref] [PubMed]
- Segerström L, Baryawno N, Sveinbjörnsson B, et al. Effects of small molecule inhibitors of PI3K/Akt/mTOR signaling on neuroblastoma growth in vitro and in vivo. Int J Cancer 2011;129:2958-65. [Crossref] [PubMed]
- Gore L, Trippett TM, Katzenstein HM, et al. A multicenter, first-in-pediatrics, phase 1, pharmacokinetic and pharmacodynamic study of ridaforolimus in patients with refractory solid tumors. Clin Cancer Res 2013;19:3649-58. [Crossref] [PubMed]
- Morgenstern DA, Marzouki M, Bartels U, et al. Phase I study of vinblastine and sirolimus in pediatric patients with recurrent or refractory solid tumors. Pediatr Blood Cancer 2014;61:128-33. [Crossref] [PubMed]
- Spunt SL, Grupp SA, Vik TA, et al. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol 2011;29:2933-40. [Crossref] [PubMed]
- Morscher RJ, Brard C, Berlanga P, et al. First-in-child phase I/II study of the dual mTORC1/2 inhibitor vistusertib (AZD2014) as monotherapy and in combination with topotecan-temozolomide in children with advanced malignancies: arms E and F of the AcSé-ESMART trial. Eur J Cancer 2021;157:268-77. [Crossref] [PubMed]
- Phadnis S, Wang X, Daw NC, et al. Everolimus in combination with vandetanib in children, adolescents, and young adults: a phase I study. ESMO Open 2023;8:101609. [Crossref] [PubMed]
- Wienke J, Dierselhuis MP, Tytgat GAM, et al. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur J Cancer 2021;144:123-50. [Crossref] [PubMed]
- Pelosi A, Fiore PF, Di Matteo S, et al. Pediatric Tumors-Mediated Inhibitory Effect on NK Cells: The Case of Neuroblastoma and Wilms' Tumors. Cancers (Basel) 2021;13:2374. [Crossref] [PubMed]
- Nguyen R, Thiele CJ. Immunotherapy approaches targeting neuroblastoma. Curr Opin Pediatr 2021;33:19-25. [Crossref] [PubMed]
- Dyson KA, Stover BD, Grippin A, et al. Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol 2019;12:78. [Crossref] [PubMed]
- Dhillon S. Dinutuximab: first global approval. Drugs 2015;75:923-7. [Crossref] [PubMed]
- Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial. Lancet Oncol 2017;18:946-57.
- Furman WL, Federico SM, McCarville MB, et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin Cancer Res 2019;25:6320-8. [Crossref] [PubMed]
- Furman WL, McCarville B, Shulkin BL, et al. Improved Outcome in Children With Newly Diagnosed High-Risk Neuroblastoma Treated With Chemoimmunotherapy: Updated Results of a Phase II Study Using hu14.18K322A. J Clin Oncol 2022;40:335-44. [Crossref] [PubMed]
- Mody R, Yu AL, Naranjo A, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children's Oncology Group. J Clin Oncol 2020;38:2160-9. [Crossref] [PubMed]
- Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1617-29. [Crossref] [PubMed]
- Yu AL, Gilman AL, Ozkaynak MF, et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin Cancer Res 2021;27:2179-89. [Crossref] [PubMed]
- Mora J, Modak S, Kinsey J, et al. GM-CSF, G-CSF or no cytokine therapy with anti-GD2 immunotherapy for high-risk neuroblastoma. Int J Cancer 2023; Epub ahead of print. [Crossref]
- Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14:1264-70. [Crossref] [PubMed]
- Cherkassky L, Hou Z, Amador-Molina A, et al. Regional CAR T cell therapy: An ignition key for systemic immunity in solid tumors. Cancer Cell 2022;40:569-74. [Crossref] [PubMed]
- Straathof K, Flutter B, Wallace R, et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci Transl Med 2020;12:eabd6169. [Crossref] [PubMed]
- Del Bufalo F, De Angelis B, Caruana I, et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N Engl J Med 2023;388:1284-95. [Crossref] [PubMed]
- Trochet D, Bourdeaut F, Janoueix-Lerosey I, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. Am J Hum Genet 2004;74:761-4. [Crossref] [PubMed]
- Warren M, Matsuno R, Tran H, et al. Utility of Phox2b immunohistochemical stain in neural crest tumours and non-neural crest tumours in paediatric patients. Histopathology 2018;72:685-96. [Crossref] [PubMed]
- Ma Y, Feng J, Zhao J, et al. PHOX2B as a Reliable Marker for Neuroblastoma in Tissue and Cytology Specimens. J Neuropathol Exp Neurol 2021;80:1108-16. [Crossref] [PubMed]
- Melaiu O, Mina M, Chierici M, et al. PD-L1 Is a Therapeutic Target of the Bromodomain Inhibitor JQ1 and, Combined with HLA Class I, a Promising Prognostic Biomarker in Neuroblastoma. Clin Cancer Res 2017;23:4462-72. [Crossref] [PubMed]
- Zeng L, Xu H, Li SH, et al. Cross-cohort analysis identified an immune checkpoint-based signature to predict the clinical outcomes of neuroblastoma. J Immunother Cancer 2023;11:e005980. [Crossref] [PubMed]
- Nawijn MC, Alendar A, Berns A. For better or for worse: the role of Pim oncogenes in tumorigenesis. Nat Rev Cancer 2011;11:23-34. [Crossref] [PubMed]
- Brunen D, de Vries RC, Lieftink C, et al. PIM Kinases Are a Potential Prognostic Biomarker and Therapeutic Target in Neuroblastoma. Mol Cancer Ther 2018;17:849-57. [Crossref] [PubMed]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol 2004;36:189-204. [Crossref] [PubMed]
- Zhu P, Zhou K, Lu S, et al. Modulation of aryl hydrocarbon receptor inhibits esophageal squamous cell carcinoma progression by repressing COX2/PGE2/STAT3 axis. J Cell Commun Signal 2020;14:175-92. [Crossref] [PubMed]
- Wu PY, Liao YF, Juan HF, et al. Aryl hydrocarbon receptor downregulates MYCN expression and promotes cell differentiation of neuroblastoma. PLoS One 2014;9:e88795. [Crossref] [PubMed]
- Wu PY, Yu IS, Lin YC, et al. Activation of Aryl Hydrocarbon Receptor by Kynurenine Impairs Progression and Metastasis of Neuroblastoma. Cancer Res 2019;79:5550-62. [Crossref] [PubMed]
- Xu T, Luo Y, Xie HQ, et al. Systematic identification of molecular mechanisms for aryl hydrocarbon receptor mediated neuroblastoma cell migration. Environ Int 2022;168:107461. [Crossref] [PubMed]
- Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry 1996;35:6735-44. [Crossref] [PubMed]
- Corrias MV, Faulkner LB, Pistorio A, et al. Detection of neuroblastoma cells in bone marrow and peripheral blood by different techniques: accuracy and relationship with clinical features of patients. Clin Cancer Res 2004;10:7978-85. [Crossref] [PubMed]
- Gu Y, Lv F, Xue M, et al. The deubiquitinating enzyme UCHL1 is a favorable prognostic marker in neuroblastoma as it promotes neuronal differentiation. J Exp Clin Cancer Res 2018;37:258. [Crossref] [PubMed]
- Yamashiro S, Gokhin DS, Kimura S, et al. Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton (Hoboken) 2012;69:337-70. [Crossref] [PubMed]
- Bettinsoli P, Ferrari-Toninelli G, Bonini SA, et al. Favorable prognostic role of tropomodulins in neuroblastoma. Oncotarget 2018;9:27092-103. [Crossref] [PubMed]
- Altun Z, Yuan H, Baran B, et al. Whole-exome sequencing reveals genetic variants in low-risk and high-risk neuroblastoma. Gene 2023;860:147233. [Crossref] [PubMed]
- Swanson KD, Winter JM, Reis M, et al. SOS1 mutations are rare in human malignancies: implications for Noonan Syndrome patients. Genes Chromosomes Cancer 2008;47:253-9. [Crossref] [PubMed]
- Zhang X, Dong Z, Zhang C, et al. Critical Role for GAB2 in Neuroblastoma Pathogenesis through the Promotion of SHP2/MYCN Cooperation. Cell Rep 2017;18:2932-42. [Crossref] [PubMed]
- Valencia-Sama I, Ladumor Y, Kee L, et al. NRAS Status Determines Sensitivity to SHP2 Inhibitor Combination Therapies Targeting the RAS-MAPK Pathway in Neuroblastoma. Cancer Res 2020;80:3413-23. [Crossref] [PubMed]
- Chantrain CF, Jijon P, De Raedt T, et al. Therapy-related acute myeloid leukemia in a child with Noonan syndrome and clonal duplication of the germline PTPN11 mutation. Pediatr Blood Cancer 2007;48:101-4. [Crossref] [PubMed]
- Mutesa L, Pierquin G, Janin N, et al. Germline PTPN11 missense mutation in a case of Noonan syndrome associated with mediastinal and retroperitoneal neuroblastic tumors. Cancer Genet Cytogenet 2008;182:40-2. [Crossref] [PubMed]
- Pirone D, Montella A, Sirico DG, et al. Label-free liquid biopsy through the identification of tumor cells by machine learning-powered tomographic phase imaging flow cytometry. Sci Rep 2023;13:6042. [Crossref] [PubMed]
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531-48. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 2019;16:409-24. [Crossref] [PubMed]
- Pinzani P, D'Argenio V, Del Re M, et al. Updates on liquid biopsy: current trends and future perspectives for clinical application in solid tumors. Clin Chem Lab Med 2021;59:1181-200. [Crossref] [PubMed]
- Chicard M, Colmet-Daage L, Clement N, et al. Whole-Exome Sequencing of Cell-Free DNA Reveals Temporo-spatial Heterogeneity and Identifies Treatment-Resistant Clones in Neuroblastoma. Clin Cancer Res 2018;24:939-49. [Crossref] [PubMed]
- Bosse KR, Giudice AM, Lane MV, et al. Serial Profiling of Circulating Tumor DNA Identifies Dynamic Evolution of Clinically Actionable Genomic Alterations in High-Risk Neuroblastoma. Cancer Discov 2022;12:2800-19. [Crossref] [PubMed]
- Trinidad EM, Juan-Ribelles A, Pisano G, et al. Evaluation of circulating tumor DNA by electropherogram analysis and methylome profiling in high-risk neuroblastomas. Front Oncol 2023;13:1037342. [Crossref] [PubMed]
- Ruas JS, Silva FLT, Euzébio MF, et al. Somatic Copy Number Alteration in Circulating Tumor DNA for Monitoring of Pediatric Patients with Cancer. Biomedicines 2023;11:1082. [Crossref] [PubMed]
- Lodrini M, Wünschel J, Thole-Kliesch TM, et al. Circulating Cell-Free DNA Assessment in Biofluids from Children with Neuroblastoma Demonstrates Feasibility and Potential for Minimally Invasive Molecular Diagnostics. Cancers (Basel) 2022;14:2080. [Crossref] [PubMed]
- Combaret V, Bréjon S, Iacono I, et al. Determination of 17q gain in patients with neuroblastoma by analysis of circulating DNA. Pediatr Blood Cancer 2011;56:757-61. [Crossref] [PubMed]
- Lak NSM, Seijger A, van Zogchel LMJ, et al. Cell-Free RNA from Plasma in Patients with Neuroblastoma: Exploring the Technical and Clinical Potential. Cancers (Basel) 2023;15:2108. [Crossref] [PubMed]
- Moss TJ, Sanders DG. Detection of neuroblastoma cells in blood. J Clin Oncol 1990;8:736-40. [Crossref] [PubMed]
- Träger C, Kogner P, Lindskog M, et al. Quantitative analysis of tyrosine hydroxylase mRNA for sensitive detection of neuroblastoma cells in blood and bone marrow. Clin Chem 2003;49:104-12. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]
- Morgenstern DA, London WB, Stephens D, et al. Metastatic neuroblastoma confined to distant lymph nodes (stage 4N) predicts outcome in patients with stage 4 disease: A study from the International Neuroblastoma Risk Group Database. J Clin Oncol 2014;32:1228-35. [Crossref] [PubMed]
- Moroz V, Machin D, Hero B, et al. The prognostic strength of serum LDH and serum ferritin in children with neuroblastoma: A report from the International Neuroblastoma Risk Group (INRG) project. Pediatr Blood Cancer 2020;67:e28359. [Crossref] [PubMed]