
The blood routine test holds screening values for influenza A in 2023: a retrospective study
Highlight box
Key findings
• Children with influenza A always demonstrate decreased lymphocyte multiplied by platelet. It exhibits the best predictive value of influenza infection.
What is known and what is new?
• Previous studies have found that routine blood parameters are helpful for early identification of influenza. However, these studies are grouped according to the results of nucleic acid test and focus on adults.
• In the present study, grouped patient subjects by the results of rapid antigen tests (positive and negative), we have found that the blood routine test still holds screening values for influenza A.
What is the implication, and what should change now?
• Blood routine tests combined with rapid antigen tests would lead to early diagnosis of influenza A in the children. It could decrease the morbidity, hospitalization time, and mortality of influenza A.
Introduction
Influenza is an acute and sometimes very severe respiratory infectious disease worldwide, accounting for up to one billion infections annually, with 3–5 million severe patients and approximately 400,000 to 500,000 deaths. The major symptoms are fever, cough, sore throat, runny nose, muscle pain, headache, and fatigue (1). Influenza A is the most common type virus isolated from clinic visitors during the flu seasons. Despite its essence of self-healing, the disease in some patients rapidly develops into severe pneumonia and meningitis. Therefore, early diagnosis is essential for decreasing morbidity, hospitalization time, and mortality.
Diagnosis of influenza largely relies on symptoms, clinical signs, epidemiological knowledge and laboratory tests. Laboratory methods mainly are antigen detection, antibody detection, nucleic acid tests and virus isolation. Although nucleic acid tests and virus isolation are the gold standard (2), they are time-consuming, relatively expensive and technically difficult, which limit their wide employment in outpatients and emergency department. Rapid antigen testing is specific, but negative results are common in early phase of mild influenza A infection. On the other hand, blood test is commonly performed in most outpatients, and is an important guide for diagnosing the presence, type, and severity of infection. It is commonly applied for differing viral infections from bacterial infections in practice (3). In addition to complete blood panel (4,5), related hematological parameters, such as neutrophil-to-lymphocyte ratio (NLR) (6,7), platelet-to-lymphocyte ratio (PLR) (8), and lymphocyte (LYM) multiplied by platelet (LYM*PLT) (9), are reported to be important screening indicators in infectious diseases. A number of previous studies have confirmed that routine blood parameters are helpful for early identification of influenza (8,10,11). However, their predictive value in influenza A has not been well studied in pediatric patients. Moreover, these studies were grouping according to the results of nucleic acid test, which is inconsistent with the practical clinical situation (6,8,12). It is necessary to combine blood routine tests with rapid antigen testing for influenza A screening. We present this article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-435/rc).
Methods
Study design
The detailed study design was demonstrated in Figure 1. The minimal sample size in this study were estimated by PASS (version: 21.0.3) in analysis module of one-way ANOVA.
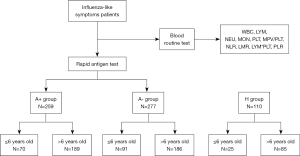
Patients
In this study, we included patients less than sixteen years old with influenza-like symptoms who presented at the outpatient pediatric clinic of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China, from February to April 2023. All of the patients underwent routine blood tests and rapid antigen testing. Influenza-like symptoms included: fever, sore throat, headache, cough or muscle pain. Inclusion criteria included fever, sore throat, headache, cough, muscle pain or respiratory tract infection (1,13). The exclusion criteria were: (I) systemic chronic diseases, for example, cardiovascular diseases, liver disease, nephropathy, anemia, hematological disease, and cancer; (II) immunodeficiency or immunosuppression; (III) patients who declined blood routine examination or rapid antigen testing; (IV) long-term use of any medications that may affect the result of the blood routine; (V) patients who had undergone antiviral therapy before the initial clinic visit.
Routine blood parameters
Capillary blood samples of blood routine tests were obtained by finger prick (12,14) and detected by a routine analyzer (BC-6800, Mindray, Shenzhen, China). The complete blood count results included white blood cell (WBC), neutrophil (NEU), LYM, monocyte (MON), platelet (PLT), and mean PLT volume (MPV). In addition, other hematological parameters were calculated, including NLR, lymphocyte-to-monocyte ratio (LMR), LYM*PLT, PLR and MPV divided by PLT (MPV/PLT).
Influenza A detection by rapid antigen testing
The type of samples submitted for testing was nasal swab of both nasal mucosae obtained by trained physicians. Colloidal gold immunochromatography was applied in the influenza A virus antigen detection kit (Guangzhou Wondfo Biotech Co., Ltd, Guangzhou, China). Double antibody sandwich method was used to detect influenza A antigen. 80 mL of the treated sample extract were added dropwise to the sample well of the test card and the displayed results were recorded within 15–20 minutes.
Statistical analyses
GraphPad Prism version 8.0 was applied to statistical analysis and P value <0.05 was considered as statistically significant. Continuous variables were expressed as mean ± standard deviation when conforming to a normal distribution. Categorical variables were expressed as frequencies. t-test was used for between two-groups comparisons. Chi-squared test or Fisher’s exact test were applied to the comparison of frequencies. Kruskal-Wallis H test or one-way ANOVA were applied to continuous variables between multiple groups. Using influenza A negative-result group (A− group) or healthy control group (H group) as a reference, the sensitivity, specificity and the area under the curve (AUC) of LYM, MON, PLT, NLR, LMR, LYM*PLT, PLR and MPV/PLT were calculated with the receiver operating characteristic (ROC) curve model. Logistic regression model was used to explore the optimal combinations of different blood routine parameters for predicting influenza A. this logistic model was developed by glm function in R software (version: 4.1.1). Then, ROC analysis was performed to examine the accuracy of this model in GraphPad Prism version 8.0.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Wuxi Branch of Shanghai Ruijin Hospital Ethics Committee (No. 001). Informed consent was taken from all the patients’ legal guardians.
Results
Patient characteristics
In brief, the minimal sample size calculated in PASS using one-way ANOVA module is 27. To improve the accuracy and reliability of our study, we collected more samples than this estimated sample size. Totally, 537 patients were enrolled in the study and were divided into influenza A-positive-result patients (A+ group) (n=259) and influenza A-negative-result patients (A− group) (n=277) according to rapid antigen tests. Simultaneously, 110 children without infectious diseases were selected as the healthy control group (H group). Meanwhile, children were divided into two age groups: the ≤6 years old and the >6 years old group. No statistically significant difference was found in gender nor age among the three groups (A+ group/A− group/H group) in each age group (P>0.05) (Table 1).
Table 1
| Variables | ≤6 years old | >6 years old | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A+ group (n=259) | A− group (n=277) | H group (n=110) | P | A+ group (n=259) | A− group (n=277) | H group (n=110) | P | ||
| Male/female | 32/38 | 46/45 | 15/10 | 0.466 | 109/80 | 101/85 | 37/48 | 0.092 | |
| Age, years (mean ± SD) | 4.70±1.52 | 4.37±1.66 | 5.04±0.92 | 0.060 | 10.10±2.04 | 9.78±2.00 | 10.02±1.87 | 0.293 | |
| Fever | 70 | 89 | 0.505 | 187 | 164 | 9.679e–06 | |||
| Cough | 58 | 73 | 0.690 | 162 | 116 | 2.823e–07 | |||
| Sore throat | 12 | 16 | 1 | 81 | 72 | 0.462 | |||
| Abdominal pain | 5 | 3 | 0.296 | 5 | 6 | 0.770 | |||
| Diarrhea | 4 | 1 | 0.168 | 6 | 3 | 0.503 | |||
| Vomit | 12 | 11 | 0.374 | 20 | 19 | 1 | |||
| Dizzy | 2 | 5 | 0.451 | 27 | 32 | 0.480 | |||
| Headache | 6 | 6 | 0.765 | 30 | 32 | 0.781 | |||
| Fatigue | 11 | 9 | 0.337 | 21 | 27 | 0.356 | |||
| Muscle pain | 4 | 5 | 1 | 21 | 15 | 0.381 | |||
A+ group, influenza A-positive-result group; A− group, influenza A-negative-result group; H group, healthy control group.
Results of routine blood parameters
Tables 2,3 list the parameters in the routine blood tests of the influenza A children. In both the ≤6 years old group and >6 years old group, there were significant differences in WBC, NEU, LYM, MON, PLT, NLR, LMR, LYM*PLT, PLR and MPV/PLT between A+ group, A− group and H group. The WBC, NEU, LYM, MON, PLT, NLR, LMR, LYM*PLT, PLR and MPV/PLT level in the A+ group, A− group and H group were significantly different in the subgroups of patients ≤6 years old (Table 2) and >6 years old (Table 3). Compared to the H group, patients in the A+ group had significantly decreased number in WBC, LYM, PLT, LMR and LYM*PLT values, while NEU, MON, NLR, PLR and MPV/PLT values were significantly increased (Tables 2,3) in both age subgroups. In the ≤6 years old group, WBC, LYM, PLT, NLR, LMR, and LYM*PLT were significantly different between the A+ group and the A− group (Figure 2, Figure S1 and Table 2). In the >6 years old group, WBC, NEU, LYM, PLT, LMR, LYM*PLT and MPV/PLT were significantly different between the A+ group and the A− group (Figure 2, Figure S1 and Table 3).
Table 2
| Parameters | A+ group | A− group | H group | P | Pa | Pb |
|---|---|---|---|---|---|---|
| WBC (109/L) | 6.28±2.30 | 7.49±3.78 | 6.92±1.72 | 0.0430 | 0.0136 | 0.2110 |
| NEU (%) | 65.4±17.1 | 61.4±17.4 | 41.0±14.5 | <0.0001 | 0.0600 | <0.0001 |
| LYM (%) | 23.3±15.3 | 27.4±15.6 | 49.0±13.7 | <0.0001 | 0.0147 | <0.0001 |
| MON (%) | 9.8±3.5 | 9.2±3.2 | 6.2±1.7 | <0.0001 | 0.2651 | <0.0001 |
| NEU (109/L) | 4.27±2.17 | 4.85±3.25 | 2.78±0.98 | 0.0001 | 0.1824 | <0.0001 |
| LYM (109/L) | 1.34±0.99 | 1.86±1.09 | 3.46±1.40 | <0.0001 | 0.0022 | <0.0001 |
| MON (109/L) | 0.54±0.24 | 0.63±0.28 | 0.42±0.13 | 0.0001 | 0.2903 | <0.0001 |
| PLT (109/L) | 206.90±50.31 | 230.10±67.06 | 312.80±70.48 | <0.0001 | 0.0132 | <0.0001 |
| MPV (fL) | 9.53±1.08 | 9.58±0.93 | 9.38±1.39 | 0.6690 | 0.6970 | 0.5853 |
| LMR | 9.53±1.96 | 3.26±2.16 | 8.40±2.91 | <0.0001 | 0.0308 | <0.0001 |
| NLR | 4.64±3.70 | 3.69±3.91 | 1.20±1.53 | <0.0001 | 0.0158 | <0.0001 |
| MPV/PLT | 0.049±0.015 | 0.046+0.016 | 0.032±0.009 | <0.0001 | 0.2197 | <0.0001 |
| LYM*PLT | 271.7±189.3 | 436.2±295.2 | 1,118.0±534.7 | <0.0001 | <0.0001 | <0.0001 |
| PLR | 212.7±130.0 | 177.8±156.2 | 111.5±66.4 | <0.0001 | 0.1236 | <0.0001 |
Data are presented as mean ± SD. a, compared to the A− group, significance P<0.05; b, compared to the H group, significance P<0.05. A+ group, influenza A-positive-result group; A− group, influenza A-negative-result group; H group, healthy control group. WBC, white blood cell count; LYM, lymphocyte; NEU, neutrophil; MON, monocyte; PLT, platelet; MPV, mean platelet volume; MPV/PLT, MPV divided by PLT; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LYM*PLT, LYM multiplied by PLT; PLR, platelet-to-lymphocyte ratio.
Table 3
| Parameters | A+ group | A− group | H group | P | Pa | Pb |
|---|---|---|---|---|---|---|
| WBC (109/L) | 5.93±2.40 | 7.12±3.88 | 6.53±1.44 | <0.0001 | 0.0004 | 0.0111 |
| NEU (%) | 68.3±14.3 | 66.2±16.2 | 43.5±9.3 | <0.0001 | 0.3271 | <0.0001 |
| LYM (%) | 20.5±13.0 | 23.0±15.1 | 45.0±9.8 | <0.0001 | 0.0839 | <0.0001 |
| MON (%) | 10.0±3.7 | 9.2±3.5 | 6.8±1.3 | <0.0001 | 0.0369 | <0.0001 |
| NEU (109/L) | 4.22±2.23 | 5.02±3.54 | 2.88±1.06 | <0.0001 | 0.0097 | <0.0001 |
| LYM (109/L) | 1.08±0.58 | 1.41±0.80 | 2.93±0.75 | <0.0001 | <0.0001 | <0.0001 |
| MON (109/L) | 0.56±0.24 | 0.60±0.27 | 0.44±0.11 | <0.0001 | 0.1739 | <0.0001 |
| PLT (109 /L) | 213.20±59.19 | 235.60±60.07 | 288.00±65.17 | <0.0001 | 0.0003 | <0.0001 |
| MPV (fL) | 9.52±1.15 | 9.58±1.16 | 9.81±1.15 | 0.1527 | 0.6012 | 0.0895 |
| LMR | 2.27±1.19 | 2.83±2.34 | 6.89±2.14 | <0.0001 | 0.0352 | <0.0001 |
| NLR | 5.23±4.24 | 4.96±4.56 | 1.07±0.62 | <0.0001 | 0.2004 | <0.0001 |
| MPV/PLT | 0.049±0.019 | 0.045±0.018 | 0.036±0.012 | <0.0001 | 0.0191 | <0.0001 |
| LYM*PLT | 233.1±153.2 | 340.6±249.9 | 857.2±336.4 | <0.0001 | <0.0001 | <0.0001 |
| PLR | 252.1±147.4 | 230.4±159.0 | 103.9±34.51 | <0.0001 | 0.1712 | <0.0001 |
Data are presented as mean ± SD. a, compared to the A− group, significance P<0.05; b, compared to the H group, significance P<0.05. A+ group, influenza A-positive-result group; A− group, influenza A-negative-result group; H group, healthy control group. WBC, white blood cell count; LYM, lymphocyte; NEU, neutrophil; MON, monocyte; PLT, platelet; MPV, mean platelet volume; MPV/PLT, MPV divided by PLT; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LYM*PLT, LYM multiplied by PLT; PLR, platelet-to-lymphocyte ratio.

Screening values of blood parameters in influenza A
In patients ≤6 years old A+ group, when referred to the A− group, based on the AUC, the best parameter that predicted influenza A was the LYM*PLT. The optimal cutoff value of LYM*PLT was 221.6, the AUC, the sensitivity and specificity were 0.6830, 55.71% and 76.92%, respectively. The cutoff value of LMR was 1.917, the AUC, the sensitivity and specificity were 0.6489, 47.14% and 75.82%, respectively. When referred to the H group, the maximum AUC was obtained for the LMR; the cutoff value of the LMR was 4.712, the AUC, the sensitivity and specificity were 0.9377, 90% and 92%, respectively (Figure 3). The cutoff value of LYM*PLT was 598.4, the AUC, the sensitivity and specificity were 0.9211, 94.29% and 84%, respectively.
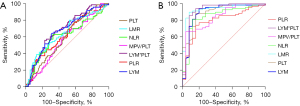
In patients >6 years old group, the best performance was identified by LYM*PLT regardless of A− group or H group as a reference. When referred to the A− group, the cutoff value was 196.7, the AUC, the sensitivity and specificity were 0.6448, 53.97% and 70.81%, respectively. When referred to the H group was used as a reference, the cutoff value was 461.9, the AUC, the sensitivity and specificity were 0.9713, 91.53% and 92.94%, respectively (Figure 4).

Predictive model for children with influenza A based on multivariate logistic regression
To further improve the screening accuracy of influenza A, we used multivariate logistic regression to find the optimal combinations of different blood routine parameters and developed a screening model based on our results. For children in ≤6 years old, the method that included all independent variables using the ENTER method achieved the highest Akaike information criterion (AIC) value and AUC. The results of logistic regression were displayed in Table 4. This predictively model was described as: Influenza A = 6.40 + WBC×−0.95 + NEU×0.81 + LYM×2.69 + MON×1.55 + PLT×−0.005 + MPV×0.037 + LMR×−0.071 + NLR×0.167 + (MPV/PLT) ×−79.58 + (LYM*PLT) ×−0.012 + PLR×−0.005. Interestingly, LYM*PLT was still the most significant of all parameters, which was consistent with our above analysis. Notably, using 0.399 as the optimal cutoff value, the sensitivity, specificity and AUC have reached 0.857, 0.5111, 0.7202 in this screening model, respectively and obviously achieved better performance than single blood routine parameter (Figure 5A). For children in >6 years old, the method that included all independent variables using the ENTER method also achieved the highest AIC value and AUC. The results of logistic regression were displayed in Table 5. This predictively model was described as: Influenza A = 4.32 + WBC×−0.57 + NEU×0.47 + LYM×−0.35 + MON×1.28 + PLT×−0.003 + MPV×−0.24 + LMR×−0.06 + NLR×−0.057 + (MPV/PLT)×3.40 + (LYM*PLT)×−0.00004 + PLR×−0.003. Interestingly, although LYM*PLT was not significant in this model, using 0.386 as the optimal cutoff value, the sensitivity, specificity and AUC have reached 0.56, 0.70, 0.6760 in this screening model, respectively and obtained better performance than single blood routine parameter (Figure 5B).
Table 4
| Parameters | Estimate | Std. error | z value | Crude OR (95% CI) | Crude P value | Adj. OR (95% CI) | P (Wald’s test) |
|---|---|---|---|---|---|---|---|
| (Intercept) | 6.40 | 3.22 | 1.99 | 0.0469* | |||
| WBC | −0.95 | 1.12 | −0.85 | 0.9 (0.8, 1) | 0.03 | 0.4 (0, 3.4) | 0.39 |
| NEU | 0.81 | 1.13 | 0.72 | 0.9 (0.8, 1) | 0.21 | 2.3 (0.2, 20.5) | 0.47 |
| LYM | 2.69 | 1.57 | 1.71 | 0.6 (0.4, 0.8) | 0.00 | 14.7 (0.7, 319.9) | 0.09 |
| MON | 1.55 | 1.88 | 0.83 | 0.5 (0.1, 1.6) | 0.22 | 4.7 (0.1, 187.7) | 0.41 |
| PLT | −0.01 | 0.01 | −0.40 | 0.99 (0.99, 1.0) | 0.03 | 0.994 (0.969, 1.020) | 0.69 |
| MPV | 0.04 | 0.30 | 0.12 | 0.9 (0.7, 1.3) | 0.60 | 1 (0.6, 1.9) | 0.90 |
| LMR | −0.07 | 0.23 | −0.31 | 0.8 (0.7, 1) | 0.05 | 0.9 (0.6, 1.5) | 0.76 |
| NLR | 0.17 | 0.18 | 0.91 | 1.1 (1, 1.2) | 0.14 | 1.2 (0.8, 1.7) | 0.36 |
| MPV/PLT | −79.58 | 43.68 | −1.82 | 2.30E+05 (0, 1.58E+14) | 0.23 | 0 (0, 412.5) | 0.07 |
| LYM*PLT | −0.01 | 0.01 | −2.14 | 0.997 (0.99, 0.999) | <0.001 | 1 (1, 1) | 0.03* |
| PLR | 0.00 | 0.00 | −1.02 | 1.00 (0.999, 1.004) | 0.15 | 0.995 (0.986, 1.004) | 0.31 |
Significance: *, P<0.05. AIC: 213.79. OR, odds risk; CI, confidence interval; WBC, white blood cell count; LYM, lymphocyte; NEU, neutrophil; MON, monocyte; PLT, platelet; MPV, mean platelet volume; MPV/PLT, MPV divided by PLT; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LYM*PLT, LYM multiplied by PLT; PLR, platelet-to-lymphocyte ratio; AIC, Akaike information criterion.
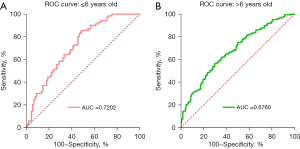
Table 5
| Parameters | Estimate | Std. error | z value | Crude OR (95% CI) | Crude P value | Adj. OR (95% CI) | P (Wald’s test) |
|---|---|---|---|---|---|---|---|
| (Intercept) | 4.32 | 1.80 | 2.40 | 0.0164* | |||
| WBC | −0.57 | 1.00 | −0.57 | 0.9 (0.8, 1) | <0.001 | 0.6 (0.1, 4) | 0.57 |
| NEU | 0.47 | 1.01 | 0.46 | 0.9 (0.8, 1) | 0.01 | 1.6 (0.2, 11.4) | 0.64 |
| LYM | −0.35 | 1.30 | −0.27 | 0.5 (0.4, 0.7) | <0.001 | 0.7 (0.1, 9) | 0.79 |
| MON | 1.28 | 1.24 | 1.03 | 0.6 (0.3, 1.4) | 0.22 | 3.6 (0.3, 40.5) | 0.30 |
| PLT | 0.00 | 0.01 | −0.41 | 0.993 (0.99, 0.997) | <0.001 | 0.9974 (0.985, 1.01) | 0.68 |
| MPV | −0.24 | 0.15 | −1.62 | 1 (0.8, 1.1) | 0.66 | 0.8 (0.6, 1.1) | 0.11 |
| LMR | 0.06 | 0.13 | 0.46 | 0.9 (0.8, 1) | 0.01 | 1.1 (0.8, 1.4) | 0.65 |
| NLR | 0.06 | 0.09 | 0.66 | 1 (1, 1.1) | 0.54 | 1.1 (0.9, 1.3) | 0.51 |
| MPV/PLT | 3.40 | 15.82 | 0.22 | 1,061,983.3 (10.5, 1.08E+11) |
0.02 | 29.9 (0, 8.69E+14) | 0.83 |
| LYM*PLT | 0.00 | 0.00 | −0.15 | 0.997 (0.996, 0.999) | <0.001 | 0.9996 (0.99, 1.01) | 0.88 |
| PLR | 0.00 | 0.00 | −1.09 | 1.001 (0.9996, 1.002) | 0.17 | 0.997(0.99, 1.002) | 0.28 |
Significance: *, P<0.05. AIC: 503.09. OR, odds risk; CI, confidence interval; WBC, white blood cell count; LYM, lymphocyte; NEU, neutrophil; MON, monocyte; PLT, platelet; MPV, mean platelet volume; MPV/PLT, MPV divided by PLT; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; LYM*PLT, LYM multiplied by PLT; PLR, platelet-to-lymphocyte ratio; AIC, Akaike information criterion.
Discussion
Clinically, the common manifestations of influenza A infection are fever, cough and sore throat. Based on these typical symptoms, physicians further employ routine blood tests and rapid antigen tests to confirm the infection (Table 1). Although rapid influenza test is routinely performed, delayed diagnosis is common. Influenza A mostly causes mild symptoms and is self-limited, while critical cases cause severe economic and social burden. Routine blood tests in combination with rapid antigen test are commonly used and have proved to be effective for early diagnosis of influenza A (15). In this study, we analyzed and compared the parameters of the routine blood tests in influenza patients in order to find a better way for early diagnosis. We found that LYM, PLT, LMR and LYM*PLT had significant differences between A+ group and A− group or healthy group regardless of age. Furthermore, the LYM*PLT and LMR exhibited the best predictive value of influenza infection according to the AUC.
Although children less than 16 years were chosen in our study, subgroups of ≤6 years old and >6 years old were still set. Physiologically, the count of LYM and NEU have their second crossover between 4 and 6 years old, then LYM decreases and NEU increases to the levels commonly observed in adults (16). Moreover, children generally start primary school at the age of six and the odd of cross-infections substantially elevates at the same time. This is why our study applied 6 years as the age cut-off. Either in ≤6 years old and >6 years old group, patients were divided into A+ group and A− group based on the results of rapid antigen testing.
A significant decrease in LYM was found in A+ group in children of both age subgroups. This is consistent with previous studies showing decreased LYM counts in children with influenza A by inducing the apoptosis of LYMs (12,17,18). Meanwhile, LYMs redistribution and migration in respiratory system further aggravate the decrease in circulating LYMs (19). Lymphopenia is helpful for distinguishing influenza A infection from other virus infection that causes increased LYM count including respiratory syncytial virus, rhinovirus, adenovirus, Epstein-Barr virus, cytomegalovirus (11,20-24). Our study achieved the sensitivity, specificity and AUC of 64.29%, 68.13%, 0.669 respectively compared to A− group in the ≤6 years old group. Similarly, in the >6 years old group, sensitivity 68.25%, specificity 52.97%, AUC 0.622 were observed versus A− group. Severe lymphopenia is useful to recognize patients at risk of severe complications and poor outcomes (25).
Another finding was decreased PLT and increased MPV/PLT in the A+ group compared to those in the A− and H group in both age subgroups, which is consistent with previous study (12). Our study achieved the sensitivity, specificity and AUC of 68.57%, 49.45%, 0.5962, respectively compared to A− group in the ≤6 years old group. Similarly, in the >6 years old group, sensitivity, specificity, and AUC were 61.38%, 60.75%, and 0.6162, respectively versus A− group. PLT consumption is caused by inflammation-induced coagulation and phagocytosing influenza virus particles that would be cleared from circulation (26). Other possible reasons include activation of PLT by virus antibodies (27) and influenza-induced immune thrombocytopenia (28). Dysregulated PLTs or thrombocytopenia may result in systemic inflammation and organ damage (29). Decreased PLT and increased WBC counts are often found in non-survivor influenza-infected patients (30). These findings suggest that PLT count can be recognized as a key characteristics of influenza and helps identify high-risk cases and predict prognosis.
A significant increase in MON was found in the A+ group compared to H group in both age subgroups, consistent with previous studies (31,32). Increased MONs coincide with the role of MONs in host defense function against influenza infection (33). MON populations peak on at second and fourth day of symptomatic illness (32), which explains decreased LYMs but increased MONs in our study. In fact, most outpatients receive routine blood tests in the first 3 days since the onset of symptoms, when MONs are yet to reach its peak. Because we found decreased LYMs in A+ group, we assumed that a combination of LYM and MON may be a practical biomarker for the prediction of influenza A infection. According to our results, the application of LMR to predictive diagnosis is more meaningful for children at six years old and younger with healthy children as reference.
Besides LMR, we also selected LYM*PLT as a predictive parameter of influenza A. AUC was 0.683 in ≤6 years old group compared to A− group, which is in line with an AUC in the previous studies (9,12). According to our results, LYM*PLT is more efficient for influenza A diagnosis in children, and has higher predictive value and screening value. A retrospective study found that LYM*PLT had the largest AUC and the best screening value, with the sensitivity and specificity of 57.59% and 72.60%, respectively (9). Moreover, a logistic regression model based on all blood routine parameters achieved better predictive performance than single parameters both in ≤6 years old group and >6 years old group, which demonstrates that combinations of different blood routine parameters could improve the improve the screening accuracy. Collectively, our study suggests that it is feasible to use routine blood tests in combination with rapid antigen test to assist in the early diagnosis of influenza A infection.
The innovative point of the present study is that we grouped patient subjects by the results of rapid antigen tests (positive and negative), which is different from previous studies grouping according to the results of nucleic acid test. The benefit of this is its being close to the real clinical setting because blood routine test combined with antigen is commonly used for influenza A screening. The limitation of this study is that there was a difference in the time from symptom onset to initial physician visit, thus some subjects only showed little changes in their routine blood testing and with the negative result of the rapid antigen. Finally, the sample size of the current study is relatively small, which can be improved by further investigation.
Conclusions
Blood routine tests are convenient, inexpensive, rapid and easy to follow up. Blood routine tests coupled with rapid antigen testing are useful for screening of influenza A patients at early stage. A significantly lower LMR*PLT was seen in influenza A children, regardless of age groups. The LYM*PLT demonstrated the best performance either using A− group or H group as a reference. Logistic regression model based on all blood routine parameters significantly improves the screening accuracy.
Acknowledgments
We thank Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine for the help and all the children enrolled in this study. We would like to thank all the healthcare workers who worked on the frontline of pediatric emergency.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-435/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-435/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-435/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-435/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Wuxi Branch of Shanghai Ruijin Hospital Ethics Committee (No. 001). Informed consent was taken from all the patients’ legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Han SB, Rhim JW, Kang JH, et al. Clinical features and outcomes of influenza by virus type/subtype/lineage in pediatric patients. Transl Pediatr 2021;10:54-63. [Crossref] [PubMed]
- Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers 2018;4:3. [Crossref] [PubMed]
- Wang W, Li SH. Use of common blood parameters for the differential diagnosis of childhood infections. PLoS One 2022;17:e0273236. [Crossref] [PubMed]
- Russell CD, Parajuli A, Gale HJ, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J Infect 2019;78:339-48. [Crossref] [PubMed]
- Oh GH, Chung SP, Park YS, et al. Mean Platelet Volume to Platelet Count Ratio as a Promising Predictor of Early Mortality in Severe Sepsis. Shock 2017;47:323-30. [Crossref] [PubMed]
- Temel H, Gündüz M, Tosun AI, et al. The Importance of Neutrophil/Lymphocyte and Lymphocyte/Monocyte Ratios in The Diagnosis of Influenza in Children. Clin Lab 2021;67: [Crossref] [PubMed]
- Wang G, Lv C, Liu C, et al. Neutrophil-to-lymphocyte ratio as a potential biomarker in predicting influenza susceptibility. Front Microbiol 2022;13:1003380. [Crossref] [PubMed]
- Liao Y, Liu C, He W, et al. Study on the Value of Blood Biomarkers NLR and PLR in the Clinical Diagnosis of Influenza a Virus Infection in Children. Clin Lab 2021;67: [Crossref] [PubMed]
- Fei Y, Zhang H, Zhang C. The application of lymphocyte*platelet and mean platelet volume/platelet ratio in influenza A infection in children. J Clin Lab Anal 2019;33:e22995. [Crossref] [PubMed]
- Shen C, Tan M, Song X, et al. Comparative Analysis of Early-Stage Clinical Features Between COVID-19 and Influenza A H1N1 Virus Pneumonia. Front Public Health 2020;8:206. [Crossref] [PubMed]
- Cunha BA, Pherez FM, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis 2009;49:1454-6. [Crossref] [PubMed]
- Zhu R, Chen C, Wang Q, et al. Routine blood parameters are helpful for early identification of influenza infection in children. BMC Infect Dis 2020;20:864. [Crossref] [PubMed]
- Jing J, Wang L, Wang G, et al. A human infection case with avian-origin H10N3 influenza virus. Quant Imaging Med Surg 2021;11:4508-10. [Crossref] [PubMed]
- He J, Zhang G, Wang Y, et al. The possibility of automatic capillary blood testing in routine blood tests: an evaluation of the automatic mode of the Mindray BC-7500 CRP Auto Hematology Analyzer for capillary blood testing. Cardiovasc Diagn Ther 2023;13:465-73. [Crossref] [PubMed]
- Nolte FS, Gauld L, Barrett SB. Direct Comparison of Alere i and cobas Liat Influenza A and B Tests for Rapid Detection of Influenza Virus Infection. J Clin Microbiol 2016;54:2763-6. [Crossref] [PubMed]
- Zhang X, Ding Y, Zhang Y, et al. Age- and sex-specific reference intervals for hematologic analytes in Chinese children. Int J Lab Hematol 2019;41:331-7. [Crossref] [PubMed]
- Xie D, Bai H, Liu L, et al. Apoptosis of lymphocytes and monocytes infected with influenza virus might be the mechanism of combating virus and causing secondary infection by influenza. Int Immunol 2009;21:1251-62. [Crossref] [PubMed]
- Fleming EH, Ochoa EE, Nichols JE, et al. Reduced activation and proliferation of human lymphocytes exposed to respiratory syncytial virus compared to cells exposed to influenza virus. J Med Virol 2018;90:26-33. [Crossref] [PubMed]
- Cheng Y, Zhao H, Song P, et al. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J Infect Public Health 2019;12:878-83. [Crossref] [PubMed]
- Coşkun O, Avci IY, Sener K, et al. Relative lymphopenia and monocytosis may be considered as a surrogate marker of pandemic influenza a (H1N1). J Clin Virol 2010;47:388-9. [Crossref] [PubMed]
- McClain MT, Park LP, Nicholson B, et al. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol 2013;58:689-95. [Crossref] [PubMed]
- Biserni GB, Scarpini S, Dondi A, et al. Potential Diagnostic and Prognostic Biomarkers for Adenovirus Respiratory Infection in Children and Young Adults. Viruses 2021;13:1885. [Crossref] [PubMed]
- Zangger N, Oxenius A. T cell immunity to cytomegalovirus infection. Curr Opin Immunol 2022;77:102185. [Crossref] [PubMed]
- Kerr JR. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol 2019;72:651-8. [Crossref] [PubMed]
- Lalueza A, Folgueira D, Díaz-Pedroche C, et al. Severe lymphopenia in hospitalized patients with influenza virus infection as a marker of a poor outcome. Infect Dis (Lond) 2019;51:543-6. [Crossref] [PubMed]
- Raadsen M, Du Toit J, Langerak T, et al. Thrombocytopenia in Virus Infections. J Clin Med 2021;10:877. [Crossref] [PubMed]
- Boilard E, Paré G, Rousseau M, et al. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood 2014;123:2854-63. [Crossref] [PubMed]
- Hamiel U, Kventsel I, Youngster I. Recurrent Immune Thrombocytopenia After Influenza Vaccination A Case Report. Pediatrics 2016;138:e20160124. [Crossref] [PubMed]
- Koupenova M, Corkrey HA, Vitseva O, et al. The role of platelets in mediating a response to human influenza infection. Nat Commun 2019;10:1780. [Crossref] [PubMed]
- Rondina MT, Tatsumi K, Bastarache JA, et al. Microvesicle Tissue Factor Activity and Interleukin-8 Levels are Associated with Mortality in Patients with Influenza A/H1N1 Infection. Crit Care Med 2016;44:e574-8. [Crossref] [PubMed]
- Chen J, Pan Y, Li G, et al. Distinguishing between COVID-19 and influenza during the early stages by measurement of peripheral blood parameters. J Med Virol 2021;93:1029-37. [Crossref] [PubMed]
- Turner JS, Lei T, Schmitz AJ, et al. Impaired Cellular Immune Responses During the First Week of Severe Acute Influenza Infection. J Infect Dis 2020;222:1235-44. [Crossref] [PubMed]
- Vangeti S, Yu M, Smed-Sörensen A. Respiratory Mononuclear Phagocytes in Human Influenza A Virus Infection: Their Role in Immune Protection and As Targets of the Virus. Front Immunol 2018;9:1521. [Crossref] [PubMed]


