
Recurrent Serratia marcescens osteomyelitis eight years after a contaminated open fracture: a case report and review of the literature
Highlight box
Key findings
• Serratia marcescens (S. marcescens) may remain indolent within a healthy host for years before disease recurrence, and a thorough investigation of distant exposures and trauma is important.
What is known and what is new?
• S. marcescens osteoarticular infections may occur after open trauma with environmental contamination in an otherwise healthy host.
• The organism may remain indolent for years after initial infection before recurrence of disease. This has been rarely reported in adults, but not in children.
What is the implication, and what should change now?
• Infection following open trauma should be treated aggressively with antibiotics and consideration of deeper-seated infection is imperative. In the case of hardware-associated infections after open trauma, empiric antibiotic therapy should take into consideration environmental bacteria that may complicate open fractures.
Introduction
Serratia marcescens (S. marcescens) is a facultative, anaerobic, Gram-negative rod with an interesting history owing to its characteristic red pigment that was mistaken for blood for millennia (1). It is ubiquitous in the environment in water, soil, and plumbing (1). The first human infection by this organism was reported in 1913, but it was largely considered non-pathogenic until the mid-1960s (1,2). It is now known to cause a wide variety of disease manifestations and has been implicated in numerous healthcare and community-acquired outbreaks (1-5). The presence of an underlying immunodeficiency or invasive device are risk factors for infection, and the ability of S. marcescens to form a biofilm may make it difficult to eradicate an infection. Osteoarticular infections are extremely rare (4,6-9). Infections occurring outside of trauma or specific risk factors should lead to an evaluation for an immunodeficiency, especially chronic granulomatous disease (CGD) (10). The bacterium has few virulence factors but may remain indolent for years within a host before disease recurrence (11-13). We detail the case of an adolescent male with a two-month history of progressive lower extremity pain and swelling years after an open fracture to the same leg, who was found to have extensive tibial osteomyelitis secondary to S. marcescens. We present this case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-492/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A previously healthy 16-year-old Caucasian male presented with a two-month history of progressive left lower extremity pain and swelling that temporally coincided with increased weight-lifting exercises. He had no overlying skin redness or drainage on his initial evaluation. An ultrasound to assess for a deep venous thrombosis was negative. A radiograph of the leg demonstrated an increased density in the mid-tibial shaft associated with lateral periosteal thickening which was interpreted as a possible stress injury related to his recent increase in physical activity. He then developed overlying skin erythema with progressive swelling concerning for infection and he was started on empiric trimethoprim/sulfamethoxazole (TMP/SMX) twice daily. An outpatient lower extremity magnetic resonance imaging (MRI) demonstrated a 1.8 cm × 1.2 cm × 1.7 cm intraosseous abscess, sinus tract to the overlying skin, and osteomyelitis spanning the length of the diaphysis with extension to the proximal metaphysis and epiphysis (Figure 1). The MRI findings led to admission for further diagnostic workup and management.

On admission, he was well-appearing, and his vital signs were normal. The left leg was diffusely swollen from the knee to the ankle, with erythema, pain, and a fluctuant abscess overlying the proximal tibia (Figure 1). The remainder of his exam was normal. Laboratory results revealed a white blood cell (WBC) count of 11.0×103/mL, absolute neutrophil count of 5.0×103/mL, erythrocyte sedimentation rate of 15 mm/hr, and C-reactive protein of 2.3 mg/dL (normal ≤1.0 mg/dL). In the operating room, an incision and drainage with corticotomy was performed by orthopedic surgery. No foreign bodies were identified. He was started on empiric vancomycin and cefazolin post-operatively to cover the most likely etiologies of hematogenous osteomyelitis, including Staphylococcus aureus and Streptococcus pyogenes. He required a second debridement two days later with primary closure of the wound. The wound and bone cultures grew grayish round colonies identified as S. marcescens by matrix-assisted laser desorption/ionization time-of-flight, and his antibiotics were changed to cefepime. Bone histopathology demonstrated marked acute inflammation and necrosis. Flow cytometric neutrophil oxidative burst assay by measuring dihydrorhodamine fluorescence was normal, ruling out CGD.
The unexpected culture results in an apparently immunocompetent host led to a thorough evaluation of his past medical history. Eight years and nine months earlier the patient had suffered a Gustilo/Anderson type 3 B open left tibial and fibular fracture following an all-terrain vehicle accident with significant wound contamination (Figure 2). He was started on preemptive penicillin, gentamicin, and cefazolin and underwent initial debridement and irrigation within 12 hours of injury. There was no damage to the dorsalis pedis or posterior tibialis blood vessels. He required external fixation with a Delta frame type construct with two pins in the proximal tibia and one in the calcaneus (Figure 2). On day six after injury, he underwent an autologous latissimus dorsi muscle free flap with end-to-end anastomosis of the posterior tibial artery and the thoracodorsal artery and split-thickness skin graft. On day eight, he required debridement of necrotic tissue from his skin flap with significant discharge noted. On day 11, the wound culture was positive for 4+ S. marcescens, 1+ Stenotrophomonas maltophilia, and 1+ Enterococcus species. Additional debridement of the necrotic skin flap was performed, and a new split-thickness skin graft was constructed. His antibiotic regimen was changed to vancomycin, piperacillin/tazobactam, and gentamicin. This regimen did not provide adequate coverage for S. maltophilia. The S. marcescens isolate was reported susceptible to ceftriaxone [minimal inhibitory concentration (MIC) ≤1 µg/mL] which was used as a surrogate for piperacillin/tazobactam MIC, but this did not take into consideration inducible Amp-C production. The isolate was susceptible to gentamicin. The antibiotics were discontinued after five days of therapy, with resolution of fever and improved appearance of the wound, and the patient was discharged. There was no consideration for a deeper focus of infection that may have led to the modification of the type and duration of antibiotic therapy.
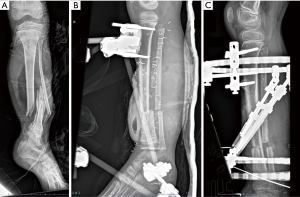
One month later, secondary fixation was accomplished with a Taylor spatial frame utilizing the two half pins from his original fixation (Figure 2). Two months following this procedure, he developed a proximal tibia pin site infection with a surrounding lucency on plain films (Figure 3), that did not improve with clindamycin therapy and required pin removal. No cultures were obtained, and he received no additional antibiotics. He required multiple corrective surgeries, with eventual removal of all hardware about five months after his initial injury (Figure 3). He reported intermittent pain over the left tibia during the ensuing eight years that did not limit activity, with full functional use of the lower extremity. He had periodic radiographs to monitor bone healing and leg-length discrepancy during this time, none of which raised the concern for osteomyelitis. He had no history of immunodeficiency, and specifically no history of pneumonia, recurrent skin and soft tissue infections, or bacteremia and no recent activities suspected to have increased his exposure to S. marcescens. He had a history of attention deficit hyperactivity disorder for which he was no longer on medication, and he denied alcohol, nicotine, or other drug use. There was no family history of immunodeficiency.

In consideration of his prior trauma history and subacute presentation for the new infection, he received six weeks of intravenous (IV) cefepime followed by oral antibiotics for chronic osteomyelitis. He was initially transitioned to oral sulfamethoxazole-trimethoprim (two double-strength tablets, 800 mg/160 mg, three times daily), but experienced a significant rise in creatinine of greater than 20% above baseline which resulted in a change to oral ciprofloxacin (750 mg twice daily) for the remainder of his course. He required one additional surgery for wound dehiscence near the end of his IV antibiotic course, but repeat cultures were negative and inflammatory biomarkers were normal. Antibiotics were discontinued after 19 weeks of therapy, which was about three months following his last surgery. He had normal repeat inflammatory biomarkers, symptom resolution, and radiographic improvement. A timeline of his clinical course is depicted in Figure 4.

Discussion
S. marcescens is an increasingly recognized source of healthcare-associated and community-acquired infections (2-5), but isolated osteoarticular infection in a healthy host without hardware is uncommon (14). The mechanism by which osteomyelitis develops in a patient is primarily determined by the isolated organism and the individual’s history and physical exam. Most cases of osteoarticular cases in children are hematogenous in origin and caused by S. aureus, and in younger children, Kingella kingae, and less commonly S. pyogenes (7,15). Hematogenous osteomyelitis secondary to S. marcescens would be highly unusual without specific risk factors. A history of trauma, surgery, or a contiguous focus of infection may help identify non-hematogenous sources of infection. In the absence of such risk factors, the isolation of a rare organism may suggest an underlying immunodeficiency. In North America, S. marcescens is one of the five most common infections in patients with CGD (10), which should be considered when this organism is implicated in a severe infection without another clear predisposing risk factor. Our patient demonstrated a normal neutrophil oxidative burst capacity and lacked other historical findings to suggest an immunodeficiency disorder. Rather, the patient’s prior open fracture and S. marcescens infection was suspected to be the risk factor for his recurrent infection years later.
Indolent osteoarticular infections by S. marcescens have rarely been reported (Table 1). Each of the patients presented with S. marcescens infection years after the initial surgery or trauma, but in only one other case was S. marcescens confirmed as causing infection at both time periods. Three of the patients had documented retained foreign bodies, which likely served as a nidus for biofilm formation and persistence. Our patient is the first pediatric patient to present with recurrent infection years after trauma, and we were able to confirm the isolation of S. marcescens with both the initial injury and recurrent infection. Our patient did have retained staples from his skin flap, but no other foreign objects were identified at the site of his infection. Various mechanisms have been proposed that might explain the persistence of S. marcescens for lengthy periods of time within a host, including biofilm formation, persister cells, small-colony variants (SCV) and quorum-sensing (17), and it is likely that multiple mechanisms occur together. SCVs have been demonstrated in S. aureus and have been implicated in disease recurrence years after apparent cure of the initial infection (18).
Table 1
| Ref | Age/sex | Comorbidities | Mechanism of injury or surgery | Location | Primary infection | Relapse interval | Secondary infection | Treatment† | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| (11) | 43/M | Healthy | Metal spike penetrating injury through rubber soled shoes | Right foot | Abscess requiring drainage, antibiotics. No culture data reported | 11 years | S. marcescens right cuboid osteomyelitis with retained rubber fragments | IV tobramycin and ceftazidime: 6 weeks | Recovered |
| (12) | 74/M | None stated | Gunshot wound to left thigh | Femur | Recurrent SSTI for two years after injury secondary to retained swab. No culture data reported | 8 years | S. marcescens left femur osteomyelitis | IV ceftazidime and gentamicin: 1 mo | Recovered |
| PO ciprofloxacin: 6 mo | |||||||||
| (13) | 59/M | Healthy | Suspected metallic debris from power tools | Femur | None reported | – | S. marcescens femur osteomyelitis with retained metallic fragment± | PO ciprofloxacin: 6 mo | Recovered |
| (16) | 71/M | Diabetes mellitus, coronary artery disease, prostate cancer | CABG | Sternum | Post-operative superficial sternal infections, no culture data available | 13 years | S. marcescens sternal osteomyelitis with absorbable suture and sternal wire in the wound bed | PO sulfamethoxazid-trimethoprim: 3 mo | Developed an abscess cavity and sinus tract on initial PO therapy requiring repeat I&D |
| IV piperacillin/tazobactam for 6 weeks | Recovered following second surgery and IV piperacillin/tazobactam | ||||||||
| (17) | 61/M | Dilated cardiomyopathy; cardiac transplant at 46 years | Cardiac transplant | Sternum | Folliculitis (S. marcescens), pericardial effusion (S. marcescens) | 15 years | S. marcescens sternal osteomyelitis with abscess§ | IV ceftriaxone and gentamicin: 19 days¶ | Died from multivisceral organ failure |
| Present case | 16/M | Healthy | Open fracture with gross contamination | Left tibia | Skin flap infection due to S. marcescens, S. maltophilia and E. faecalis | 8 years | S. marcescens tibial osteomyelitis | IV cefepime: 6 weeks | Recovered |
| PO TMP/SMX → PO ciprofloxacin: 13 weeks |
†, treatment refers to antibiotics administered for the recurrent infection after S. marcescens was confirmed as the etiology of the infection. ±, the authors favored a hematogenous route with damaged bone from a prior foreign body serving as a nidus for infection. The patient was a plumber and by occupation would have exposure to environmental sources of S. marcescens, though biofilm formation on a retained foreign body is a plausible explanation as demonstrated in other cases. As the timing of the foreign body injury was not definitively known the relapse interval was uncertain but it appears to have been many years. §, genotypic analysis performed, closely related by Tenover criteria. ¶, the article does not clearly state how long the patient received IV antibiotic therapy. He had initial clinical improvement on these antibiotics and was discharged after 10 days of treatment, apparently with continued parenteral therapy. He returned nine days later with a systemic illness and hypotensive shock, ultimately succumbing to multivisceral organ failure reported to be secondary to metabolic disorders and acute renal failure. Blood cultures on readmission, while on antibiotics, were negative. M, male; IV, intravenous; SSTI, skin or soft tissue infection; PO, oral; mo, month(s); CABG, coronary artery bypass grafting; I&D, incision and drainage; TMP/SMX, trimethoprim/sulfamethoxazole.
The optimal approach to therapy of S. marcescens osteomyelitis is not established. Current clinical practice guidelines for pediatric osteomyelitis focus on acute hematogenous routes of infection and may not be directly applicable to guide the optimal class or duration of antibiotics in our patient’s scenario (15). A wide variety of antimicrobial approaches have been reported for S. marcescens, as can be seen in Table 1. Serratia species may harbor chromosomally encoded AmpC, an Ambler Class C beta-lactamase, whose expression may be induced by beta-lactam therapy, particularly second and third generation cephalosporins. This may result in a rise in mean inhibitory concentration during therapy, and potential resistance (19). The clinical significance of this has been questioned, however, as overexpression of AmpC occurs in <5% of isolates (20) and varies by antibiotic (19). Nonetheless, mutants with derepressed AmpC production, who have constitutive rather than inducible production, may emerge during treatment. This may be particularly relevant in high inoculum or sequestered infections and when prolonged therapy is indicated (19), as may occur in chronic osteomyelitis. The duration of IV therapy will need to be individualized based on the severity of the infection and the host. Fluoroquinolones and TMP/SMX are oral agents that can be considered for completion of therapy for osteomyelitis, dependent upon antibiotic susceptibility testing. Fluoroquinolones have been studied extensively in osteoarticular infections in adults and have the advantages of excellent oral bioavailability, good bone penetration, and less frequent dosing intervals. However, the potential adverse effect profile of these agents, including tendinopathy and Clostridium difficile infections, are of concern with prolonged therapy in children (15). TMP/SMX is an alternative option that may be preferable in children, though hypersensitivity reactions and myelosuppression are notable concerns, and there is very limited data on the effectiveness of this agent in osteomyelitis (15,21). Further studies are needed to guide the optimal approach to therapy.
Our patient is suspected to have developed osteomyelitis following his initial injury despite preemptive antibiotics with early irrigation and debridement, as recommended in current practice guidelines (22). Unfortunately, when he developed a skin flap infection, a deeper focus of infection was not considered, and he received a suboptimal duration of therapy. He subsequently developed a pin-site infection, but empiric therapy was not based on prior culture results and antibiotics were not continued after pin removal. While the infection was subsequently contained by his immune system, a chronic, latent infection likely developed.
A limitation of this case report is the inability to compare isolates from both time points. While the isolates had identical susceptibility patterns, the initial isolate was not available for paired molecular characterization or genotype sequencing, preventing us from definitively excluding a new infection. However, in the absence of recent trauma or other risk factors, the ability of S. marcescens to remain quiescent within a host, and the isolation of an unexpected organism with similar susceptibility profiles as the former isolate, a recurrence of the initial infection is suspected.
Conclusions
This case stresses the importance of considering a deeper-seated infection when an open fracture is complicated by a wound infection to ensure an appropriate regimen and duration of antibiotics is prescribed. When an uncommon organism is isolated from a sterile site, obtaining a thorough history of past trauma, even years before, is imperative. The case highlights the potential for S. marcescens to remain quiescent for years within a host, through biofilm formation, persister cells, or SCV, but further research is needed to better understand the mechanisms by which immune evasion can occur.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-492/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-492/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-492/coif). J.A.M. received funding from the Indiana University Immunology and Infectious Disease Training Program T32 (No. NIH AI060519) and the Pediatric Infectious Diseases Society Fellowship Award funded by Stanley and Susan Plotkin and Sanofi, which supported his salary during fellowship training. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu VL. Serratia marcescens: historical perspective and clinical review. N Engl J Med 1979;300:887-93. [Crossref] [PubMed]
- Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev 2011;24:755-91. [Crossref] [PubMed]
- Laupland KB, Parkins MD, Gregson DB, et al. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis 2008;27:89-95. [Crossref] [PubMed]
- Martins HF, Raposo A, Baptista I, et al. Serratia marcescens osteomyelitis in Cushing's disease. BMJ Case Rep 2015;2015:bcr2015212872. [Crossref] [PubMed]
- Engel HJ, Collignon PJ, Whiting PT, et al. Serratia sp. bacteremia in Canberra, Australia: a population-based study over 10 years. Eur J Clin Microbiol Infect Dis 2009;28:821-4. [Crossref] [PubMed]
- Lin CS, Horng JT, Yang CH, et al. RssAB-FlhDC-ShlBA as a major pathogenesis pathway in Serratia marcescens. Infect Immun 2010;78:4870-81. [Crossref] [PubMed]
- Dartnell J, Ramachandran M, Katchburian M. Haematogenous acute and subacute paediatric osteomyelitis: a systematic review of the literature. J Bone Joint Surg Br 2012;94:584-95. [Crossref] [PubMed]
- Hadid H, Usman M, Thapa S. Severe Osteomyelitis and Septic Arthritis due to Serratia marcescens in an Immunocompetent Patient. Case Rep Infect Dis 2015;2015:347652. [Crossref] [PubMed]
- Marin L, Rowan R, Mantilla A, et al. Lower-Extremity Infections Caused by Serratia marcescens A Report of Three Cases and a Literature Review. J Am Podiatr Med Assoc 2017;107:231-9. [Crossref] [PubMed]
- Marciano BE, Spalding C, Fitzgerald A, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015;60:1176-83. [Crossref] [PubMed]
- Greene WB. Unrecognized foreign body as a focus for delayed Serratia marcescens osteomyelitis and septic arthritis. Two case reports. J Bone Joint Surg Am 1989;71:754-7.
- Warren NP, Coombs RR. Delayed Serratia marcescens osteomyelitis following a gunshot injury. Injury 1991;22:493-4. [Crossref] [PubMed]
- Hannah S, McConnell J. Serratia marcescens: A case history to illustrate the value of radiographer history taking in the face of poor health professional communication. Radiography 2009;15:e34-e43.
- Thomas JM, Lowes JA, Tabaqchali S. Serratia marcescens in mixed aerobic infections of bone. A report of two patients. J Bone Joint Surg Br 1980;62:389-90. [Crossref] [PubMed]
- Woods CR, Bradley JS, Chatterjee A, et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J Pediatric Infect Dis Soc 2021;10:801-44. [Crossref] [PubMed]
- Chinn A, Knabel M, Sanger JR, et al. Chronic Serratia marcescens sternal infection presenting 13 years after coronary artery surgery. Int J Surg Case Rep 2019;62:50-3. [Crossref] [PubMed]
- Paquin A, Lepelletier D, Leprince C, et al. Relapse of Serratia marcescens sternal osteitis 15 years after the first episode. J Clin Microbiol 2012;50:184-6. [Crossref] [PubMed]
- Plata K, Rosato AE, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol 2009;56:597-612.
- Harris PN, Ferguson JK. Antibiotic therapy for inducible AmpC β-lactamase-producing Gram-negative bacilli: what are the alternatives to carbapenems, quinolones and aminoglycosides? Int J Antimicrob Agents 2012;40:297-305. [Crossref] [PubMed]
- Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis 2023;ciad428. [Crossref] [PubMed]
- McDaniel LM, Fiawoo S, Tamma PD, et al. Trimethoprim-Sulfamethoxazole for Pediatric Osteoarticular Infections. J Pediatric Infect Dis Soc 2023;12:534-9. [Crossref] [PubMed]
- Goldman AH, Tetsworth K. AAOS Clinical Practice Guideline Summary: Prevention of Surgical Site Infection After Major Extremity Trauma. J Am Acad Orthop Surg 2023;31:e1-8. [Crossref] [PubMed]


