
RYR2 receptor gene mutation associated with catecholaminergic polymorphic ventricular tachycardia in children: a case report & literature review
Highlight box
Key findings
• In this systemic review of 14 articles, all reported patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) presented with syncopal attack, seizure, or sudden death. Ryanodine receptor 2 (RYR2) gene mutation detected in all cases with few articles reported association with family history of RYR2 gene mutation.
What is known and what is new?
• RYR2 gene mutation is a rare gene mutation commonly associated with CPVT.
• With regards to our patient, she has RYR2 gene mutation with cardiac tumour which can be another triggering factor for her CPVT.
What is the implication and what should change now?
• In recent years, RYR2 gene mutation identification associated with CPVT has been well recognised. It helps in terms of choice of treatment in the event where patient with RYR2 gene mutation develops cardiac arrest. Nevertheless, it will reduce the rate of mortality and morbidity of patient with RYR2 gene mutations.
Introduction
Ryanodine receptor 2 (RYR2) is primarily found in cardiac muscles to facilitate calcium release from sarcoplasmic reticulum, leading to muscle contractions. RYR2 gene mutation can cause uncontrolled muscle contraction and causing arrythmias and lead to cardiac arrest. Catecholaminergic polymorphic ventricular tachycardia (CPVT) is one of the most identified causes of cardiac arrest in paediatric populations and manifested as syncopal attack induced by exercise. CPVT is diagnosed by unexplained exercise-induced ventricular tachycardia (VT) or ventricular fibrillation (VF) in a normal heart structure and electrocardiogram (ECG). Eighty percent of cases showed positive family history with 10 index cases and each family at least experienced a case of either sudden infant death syndrome (SIDS) or drowning at time of presentation (1). CPVT was reported to contribute to about 30% of cardiac arrest by causing a spontaneous efflux of calcium ions, activating the sympatho-adrenergic system giving rise to ventricular arrythmias (2). Approximately 50–65% of CPVT-type 1 cases are associated with RYR2 gene mutation. Petrungaro et al. found that CPVT can also give cardiac rhythm disturbances and be associated with overlap syndromes with non-compact myocardium in which patient presented with atrioventricular (AV) block (3). Although, intravenous adrenaline is the first choice of pharmaceutical therapy in cardiac resuscitation, the use of adrenaline in patient with RYR2 gene receptor mutation will lead to CPVT and death. In CPVT, beta-blocker could be beneficial though treatment failure was reported mainly due to poor adherence (4).
Another known complication of RYR2 gene mutation is epilepsy which may be due to either the nature of the disease or as a complication of recurrent episodes of cardiac arrest leading to hypoxic ischaemic encephalopathy (HIE). In 2021, a study in China demonstrated a Benign Epilepsy of childhood of Centrotemporal Spike (BECTS) associated with RYR2 gene missense mutation. Five of the subjects had onset of childhood-onset focal seizures with two probands with family history of arrythmias (5). On the other hand, recent studies showed association of RYR gene mutation with tumours, particularly in adults. There is no case reported in children so far. Tumour mutational burden (TMB) was found higher in those with RYR gene mutations, mainly in RYR2 gene mutations (6). Till date, only two types of cancer have been reported which are oesophageal cancer and lung cancer (7,8). RYR2 gene mutation is known to be mostly expressed in the epithelial cells, which can cause either adenocarcinoma or squamous cell carcinoma (9).
In this article, we report a case of RYR2 gene mutation presenting with cardiac arrest, eventually had multiple episodes of syncopal attack and seizure, with an interesting accidental finding of cardiac tumour. Subsequently, we proceed to systematically review the literature for cases of CPVT with documented RYR2 mutations among paediatric population, focusing on the main clinical manifestation and their genetic mutation profile. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-255/rc).
Case presentation
A 1-year-old girl with no previous medical illness was found unconscious at home by her father after playing with her sister. There was no choking episode or seizure witnessed prior to the event. This was her first cardiac arrest episode and no history of syncopal attack before. Her father initiated the cardio-pulmonary resuscitation (CPR) with rescue breath at home and brought her immediately to the hospital. It took 25 minutes to reach the hospital and the child did not regain consciousness in between. Upon her arrival in resus zone, her Glasgow Coma Scale (GCS) was 3/10 (E1V1M1). She was immediately intubated, and VF was noted in the cardiac monitoring. Direct current (DC) shock of 50 Joules with six times intravenous Adrenaline 0.1 mg/kg and total bolus of intravenous Normal Saline 40 mL/kg were given. CPR was commenced for 20 minutes with return of spontaneous circulation (ROSC), and was haemodynamically unsupported. During the acute period, she developed multiple episodes of focal seizure. Neurological examination revealed power of at least 3/5 bilateral upper and lower limbs with brisk knee jerk reflexes. Subsequent assessment with computed tomography (CT) brain showed generalised cerebral sulci effacement and loss of grey-white matter differentiation, although electroencephalography (EEG) showed no epileptiform changes. Cerebral spinal fluid examination was not suggestive of infection and other blood investigations were normal. Chest radiography showed clear lungs field bilaterally. As she continued to had seizures, she was started on syrup levetiracetam 20 mg BD.
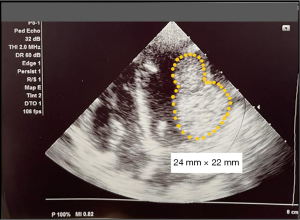
Her echocardiogram assessment revealed presence of two large tumours at the posterior wall of left ventricle with the largest size of 24 mm × 22 mm (Figure 1) otherwise with good ventricular contractility. Brain magnetic resonance arteriogram/venogram (MRA/MRV) and cardiac magnetic resonance imaging (MRI) showed profound hypotensive type of HIE and two intramyocardial masses largest 37 mm × 30 mm along lateral wall of left ventricle suggestive of rhabdomyomas or fibromas. She was discharged home after 23 days of admission with both the parents were educated with basic life support (BLS) in the event of cardiac arrest. Since discharged, she was admitted several times with multiple episodes of syncopal attack at home precipitated by crying and was documented of VT upon presentation to the emergency department. She was then initiated on oral amiodarone of 5 mg/kg/dose twice daily to control the arrythmias. Whole exome sequencing (WES) was performed, and RYR2 gene mutation was found in the patient and her father. She is currently on Gross Motor Function Classification (GMFCS) level 5 with swallowing incoordination requiring nasogastric Ryle’s tube feeding assistance. Despite on the medication, she still had two episodes of VT per week precipitated by triggers such as crying, anger, or stress. The decision for conservative management for the index case was made after a discussion in a multidisciplinary meeting, taking into consideration of risk outweigh the benefit in this age group.
We report a patient based on retrospective review of information via electronic medical record, digital laboratory system, and digital radiological, image and reports in Hospital Pakar Kanak-Kanak (HPKK), National University of Malaysia. For systemic review, four large databases: PubMed, Ovid, Scopus and Google Scholar were used to find articles with keywords “RYR2 gene mutations” and “CPVT” up to December 2022. Selected articles based on title and abstract included are: (I) case reports and articles on children 18 years old and below with (II) treatment response on CPVT and (III) written in English only. We excluded reports of asymptomatic patients with RYR2 gene mutations and articles written in other languages.
We have summarised the results of the literature search using PRISMA flow diagram (Figure 2). The data of clinical presentation, clinical findings, radiology modalities and findings, laboratory results, confirmatory tests, and treatments were collected and tabulated.
Following inclusion and exclusion criteria, 14 articles were selected, summarised in Table 1. A total of 90 patients were included in the review, including our patients. The patients’ age ranged from 4 weeks old to 18 years old, with the mean age at presentation 11±4 years old. Only 3 patients (3.3%) presented below the age of 1 year old.
Table 1
| Case No. | Mutation | Gender | Exercise-induced | Syncope | Seizure | Age* | Reference |
|---|---|---|---|---|---|---|---|
| 1 | A2254V | Female | Yes | Yes | Not documented | 8 | Postma et al. (10) |
| 2 | A2387T | Female | Yes | Yes | Not documented | 18 | Tester et al. (1) |
| 3 | A2394G | Female | Yes | Yes | Yes | 9 | Postma et al. (10) |
| 4 | A2403T | Female | Yes | Yes | Not documented | 14 | Choi et al. (11) |
| 5 | A2403T | Male | Yes | Yes | Not documented | 7 | Choi et al. (11) |
| 6 | A2403T | Female | Yes | Yes | Not documented | 16 | Tester et al. (1) |
| 7 | A4510T | Male | Yes | Yes | Not documented | 15 | Choi et al. (11) |
| 8 | A4510T | Male | Yes | Yes | Not documented | 11 | Tester et al. (1) |
| 9 | A4860G | Female | Yes | Yes | Not documented | 7 | Priori et al. (12) |
| 10 | A169G | Male | Yes | Yes | Not documented | 18 | Hsueh et al. (13) |
| 11 | c. 6800G>A | NS | Yes | SIDS | Not documented | 6 months | Tester et al. (1) |
| 12 | c. 6800G>A | NS | Yes | SIDS | Not documented | 4 weeks | Tester et al. (1) |
| 13 | c.7580T > G | Male | Yes | Yes | Yes | 9 | Duan et al. (14) |
| 14 | c.7580T > G | Male | Yes | Yes | Yes | 3 months | Duan et al. (14) |
| 15 | c.12244G>C | Male | Yes | Yes | Not documented | 12 | Nathani et al. (15) |
| 16 | c.12470G>A | Female | Yes | Yes | Not documented | 15 | Del Franco et al. (16) |
| 17 | c.12520T>A | Male | Yes | Yes | Not documented | 17 | Seildmayer et al. (2) |
| 18 | c.12670G > T | Male | Yes | Yes | Yes | 3 | Hu et al. (17) |
| 19 | c.1458A>C | Male | Yes | Sudden death | Not documented | 17 | Larsen (18) |
| 20 | c.6497G>A | Female | Yes | Yes | Not documented | 13 | Mahlke et al. (19) |
| 21 | c.6800G>A | Male | No | No | Not documented | 15 | Kohli et al. (20) |
| 22 | c.7169c > t | Male | Yes | Yes | Yes | 11 | Watanabe et al. (21) |
| 23 | c.7210C>A | Female | Yes | Sudden death | Not documented | 13 | Beckmann et al. (22) |
| 24 | c.9872A>T= | Female | Yes | Yes | Not documented | 9 | Blancard et al. (23) |
| 25 | D3291V | Female | Yes | Yes | Not documented | 10 | Blancard et al. (23) |
| 26 | E1724K | Female | Yes | Yes | Not documented | 9 | Postma et al. (10) |
| 27 | E2311D | Male | Yes | Yes | Not documented | 8 | Priori et al. (12) |
| 28 | E243K | Male | Yes | Yes | Not documented | 13 | Roston et al. (4) |
| 29 | E4076K | Male | Yes | Yes | Not documented | 10 | Postma et al. (10) |
| 30 | E4950K | Male | Yes | Yes | Not documented | 10 | Priori et al. (12) |
| 31 | F4020L | Male | Yes | Yes | Yes | 4 | Postma et al. (10) |
| 32 | G14876A | Male | Yes | Yes | Not documented | 10 | Allouis et al. (24) |
| 33 | G14876A | Male | Yes | Yes | Not documented | 6 | Allouis et al. (24) |
| 34 | G14876A | Female | Yes | Yes | Not documented | 11 | Allouis et al. (24) |
| 35 | G14876A | Male | No | Yes | Not documented | 12 | Allouis et al. (24) |
| 36 | G14876A | Female | Yes | Yes | Not documented | 15 | Allouis et al. (24) |
| 37 | G14876A | Female | Yes | Yes | Not documented | 13 | Allouis et al. (24) |
| 38 | G375S | Male | Yes | Yes | Not documented | 14 | Heiner et al. (25) |
| 39 | G3946A | Male | Yes | Yes | Not documented | 6 | Pizzale et al. (26) |
| 40 | G3946S | Male | Yes | Yes | Not documented | 14 | Priori et al. (12) |
| 41 | G3946S | Male | Yes | Yes | Not documented | 9 | Priori et al. (12) |
| 42 | G3946S | Male | Yes | Yes | Not documented | 11 | Wilde et al. (27) |
| 43 | G4076L | Female | Yes | Yes | Not documented | 10 | Wilde et al. (27) |
| 44 | G4671R | Male | Yes | Yes | Not documented | 11 | Choi et al. (11) |
| 45 | G4671R | Male | Yes | Yes | Not documented | 10 | Tester et al. (1) |
| 46 | G4936L | Male | Yes | Yes | Not documented | 17 | Itoh et al. (28) |
| 47 | H4108N | Female | Yes | Yes | Yes | 4 | Postma et al. (10) |
| 48 | H4108Q | Female | Yes | Yes | Not documented | 6.5 | Postma et al. (10) |
| 49 | H4762P | Female | Yes | Yes | Yes | 13 | Postma et al. (10) |
| 50 | I4756S | Female | Yes | Yes | Not documented | 16 | Letsas et al. (29) |
| 51 | I4848V | Female | Yes | Yes | Not documented | 14 | Choi et al. (11) |
| 52 | I4848V1 | Female | Yes | Yes | Not documented | 16 | Choi et al. (11) |
| 53 | I4848V | Female | Yes | Yes | Not documented | 14 | Tester et al. (1) |
| 54 | I4855M | Female | No | No | Not documented | 10 | Roston et al. (4) |
| 55 | I4867M | Male | Yes | Yes | Not documented | 9 | Priori et al. (12) |
| 56 | L2534V | Male | Yes | Yes | Not documented | 13 | Hasdemir et al. (30) |
| 57 | L3778F | Male | Yes | Yes | Not documented | 10 | Priori et al. (12) |
| 58 | M4109R | Male | Yes | Yes | Not documented | 15 | Nof et al. (31) |
| 59 | M4109R | Female | No | Yes | Not documented | 12 | Nof et al. (31) |
| 60 | n.A12476C | Female | No | No | Not documented | 2 | Di Pino et al. (32) |
| 61 | N4104I | Male | Yes | Yes | Yes | 7 | Postma et al. (10) |
| 62 | N4104K | Male | Yes | Yes | Not documented | 9 | Priori et al. (12) |
| 63 | N4895D | Male | Yes | Yes | Not documented | 9 | Priori et al. (12) |
| 64 | NS | Female | Yes | Yes | Not documented | 13 | Bhuiyan et al. (33) |
| 65 | NS | Female | Yes | Yes | Not documented | 12 | Roston et al. (34) |
| 66 | NS | Female | Yes | Yes | Not documented | 9 | Saito et al. (35) |
| 67 | NS | Male | Yes | Yes | Not documented | 16 | Saito et al. (35) |
| 68 | P164S | Male | Yes | Yes | Not documented | 17 | Choi et al. (11) |
| 69 | P4511L | Male | Yes | Yes | Not documented | 17 | Wilde et al. (27) |
| 70 | P466A | Male | Yes | Yes | Not documented | 9 | Tester et al. (1) |
| 71 | P4902S | Female | Yes | Yes | Not documented | 13 | Postma et al. (10) |
| 72 | R169Q | Female | Yes | Yes | Not documented | 6 | Nozaki et al. (36) |
| 73 | R169Q | Female | Yes | Yes | Not documented | 5 | Nozaki et al. (36) |
| 74 | R169Q | Female | Yes | Yes | Yes | 7 | Nozaki et al. (36) |
| 75 | R176Q | Male | Yes | Yes | Not documented | 12 | Tester et al. (1) |
| 76 | R2474S | Male | Yes | Yes | Not documented | 8 | Priori et al. (12) |
| 77 | R414L | Male | Yes | Yes | Not documented | 11 | Choi et al. (11) |
| 78 | R414L | Male | Yes | Yes | Not documented | 17 | Tester et al. (1) |
| 79 | R4959Q | Female | Yes | Yes | Not documented | 12 | Tester et al. (1) |
| 80 | R4959Q | Female | Yes | Yes | Yes | 11 | Roston et al. (4) |
| 81 | S2246L | Male | Yes | Yes | Not documented | 2 | Priori et al. (12) |
| 82 | S2246L | Female | Yes | Yes | Not documented | 9 | Priori et al. (12) |
| 83 | S2246L | Female | Yes | Yes | Not documented | 11 | Aizawa et al. (37) |
| 84 | S4124T | Female | Yes | Yes | Not documented | 14 | Tester et al. (1) |
| 85 | V4471I | Female | Yes | Yes | Not documented | 8 | Roston et al. (4) |
| 86 | V4471I | Female | Yes | Yes | Yes | 18 | Roston et al. (4) |
| 87 | V4771I | Male | Yes | Yes | Not documented | 6 | Priori et al. (12) |
| 88 | V4771I | Female | Yes | Yes | Yes | 12 | Postma et al. (10) |
| 89 | 230 C>T | Male | Yes | Sudden death | Not documented | 17 | d’Amati et al. (38) |
| 90 | c.294+A>G | Female | Yes | Yes | Yes | 1 | Abdullah and Ali |
*, the unit of Age is “year” unless otherwise specified. SIDS, sudden infant death syndrome.
Forty-six of the reviewed cases were males, 42 were females and 2 were unspecified. 13 of the patients (14.4%) presented with both syncopal attack and seizure during the initial presentation; 72 patients (80%), including our patient presented with typical syncopal attack. Only 4 (4.4%) patients presented with CPVT without exercise or stress-induced event. Three of them (3.3%) presented with sudden cardiac arrest and were diagnosed with RYR2 gene mutation post-mortem.
Thirteen genetic variants or coding effects were detected to be associated with CPVT and considered as pathogenic. Figure 3 showed the top 15 variants/coding effect that represents nearly 45% of the total cases reviewed. To date, none of the literature review showed similar RYR2 gene mutation with c.294+3A>G variant as in our patient. The nucleotide mutation results are shown in Figure 4.


All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the legal guardians for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
Discussion
RYR2 receptor gene mutation leading to CPVT has been documented in several cases reported in children and adult, and their manifestations may range from benign to severe life-threatening conditions. Nonetheless, the choice of pharmacological treatment may need adjustment in patients with RYR2 gene mutation. In this review, we will focus on characteristics of pathogenic RYR2 gene mutation and its variants among paediatric population.
Ohno et al. has described in 2015, several gene receptors which can cause cardiac arrest in a young patient namely, RYR2, CASQ2, KCNJ2, TRDN and CALM1 with more than 60% of CPVT patients carry mutations in RYR2 (9). RYR2 gene mutation can be autosomal dominant (AD) or autosomal recessive (AR) in which the receptor functions primarily in controlling the calcium release from sarcoplasmic reticulum in each cardiac cycle (39). In our patient, she has heterozygotes condition of RYR2 gene mutation with c.294+3A>G variant. According to the latest International Guidelines on Sudden Cardiac Death, CPVT can be diagnosed with either: (I) exercise or emotion-induced bidirectional or polymorphic VT with normal heart structure and normal baseline ECG at rest, or (II) based on heterozygous state that causes pathogenic (or likely pathogenic) variants in RYR2, CALM1, CALM2, CALM3, CASQ2, or KCNJ2 or biallelic pathogenic (or likely pathogenic) variants in CASQ2, TECRL, or TRD (40). Our patient fulfilled both diagnostic criteria of CPVT.
Phenotypes of RYR2 gene mutation has been described by Leung et al. in 2022, which the group reported as mainly occurring in female gender with the median age of presentation of eight years old, clinically presenting with syncopal attack (41). Koponen et al. showed similar results with predominantly CPVT occurring in female gender. This study focused on RYR2 p2328S gene mutation which resulted on cardiac arrest in 5% of patients and 25% of patients had syncopal attack due to exercise or stress (42). Based on the cardiac abnormality reported, presence of VT or VF was one of the commonest cardiac manifestations in patients with RYR2 gene mutation (4). Furthermore, the same study also reported in nine patients who had positive respond to adrenaline or epinephrine challenge test after inducing VT. Similarly in a case series reported by Bellamy et al., they also demonstrated in three different patients (age range, 4–10 years old), a positive response to epinephrine challenge test by inducing arrythmias in patients with RYR2 gene mutation, which successfully reverted by nadolol and flecainide (43).
In comparison to our patient, she had two precipitating factors that can provoke her CPVT episode which are the underlying RYR2 gene mutation and the incidental finding of the cardiac tumour. Generally, in paediatrics population, rhabdomyoma is the commonest cardiac tumour in children, commonly associated with tuberous sclerosis, with other possible differential diagnoses of cardiac tumour being fibroma and myxoma. In our study and Miyake et al. study in 2011 showed out of 173 patients in Children’s Boston Hospital, rhabdomyoma contributed the highest numbers of patients with cardiac tumour. On the other hand, fibroma demonstrated highest total number of patients presented with VT (16 patients) or cardiac arrest (2 patients) (44). Hypothetically, the cardiac tumour can further complicate the underlying condition caused by RYR2 gene mutation and resulted in uncontrolled CPVT. In cases with the size of the cardiac tumour is significant and/or in haemodynamically unstable patient, surgical resection will be the best choice to control the symptoms (44). Till date, our patient is the only reported case of RYR2 gene mutation manifesting with concomitant CPVT and cardiac tumour, which may lead for her recalcitrant arrythmias.
Choices of pharmacological treatment depend on individual response. Most of the literature review reported beta blocker as the treatment of choice in CPVT. According to American College of Cardiology nadolol, a non-selective beta-1-receptor blocker in heart and vascular smooth muscle is suggested as the first line treatment in CPVT, combined with flecainide (45), though this treatment it is not easily accessible in most part of the world. Other choices of beta-blocker include propranolol (non-selective beta blocker), atenolol, bisoprolol and metoprolol (selective beta-1 receptor blocker). The main reason of using beta-blocker as the main choice in CPVT due to the longer half-life with once daily dose and less potential central nervous side effects (46). The next step to consider after pharmacological therapy is the implantable cardiac defibrillator (ICD). ICD can be implanted either via epicardial or transvenous approach. Epicardial is the best approach in our patient due to her young age and small size. Primary indication in our patient is channelopathy disease with high risk factors; (I) onset at young age; (II) previous history of cardiac arrest; and (III) genetic variants (47).
Conclusions
The patients reviewed in this article showed similar clinical presentations and complications with the previously reported patients with RYR2 gene mutation. RYR2 gene mutation with c.294+3A>G variant may be another novel mutation which uniquely associated the formation of cardiac tumour. In our case, it could be speculated that her CPVT may also be provoked by underlying cardiac tumour with early onset at the age of 1 year old. The mean age for the onset usually at 2 years old.
Acknowledgments
We would like to express our gratitude towards the Faculty of Medicine, National University of Malaysia and Hospital Pakar Kanak-Kanak (HPKK) Research Centre for supporting our publication.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-255/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-255/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-255/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the legal guardians for publication of this case report and accompanying images. A copy of written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tester DJ, Kopplin LJ, Will ML, et al. Spectrum and prevalence of cardiac ryanodine receptor (RyR2) mutations in a cohort of unrelated patients referred explicitly for long QT syndrome genetic testing. Heart Rhythm 2005;2:1099-105. [Crossref] [PubMed]
- Seidlmayer LK, Riediger F, Pagonas N, et al. Description of a novel RyR2 mutation in a juvenile patient with symptomatic catecholaminergic polymorphic ventricular tachycardia in sleep and during exercise: a case report. J Med Case Rep 2018;12:298. [Crossref] [PubMed]
- Petrungaro M, Scarà A, Borrelli A, et al. CPVT and Complete Atrio-Ventricular Block: The Flipside of the Same Coin. J Cardiovasc Dev Dis 2023;10:97. [Crossref] [PubMed]
- Roston TM, Vinocur JM, Maginot KR, et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633-42. [Crossref] [PubMed]
- Ma MG, Liu XR, Wu Y, et al. RYR2 Mutations Are Associated With Benign Epilepsy of Childhood With Centrotemporal Spikes With or Without Arrhythmia. Front Neurosci 2021;15:629610. [Crossref] [PubMed]
- Wang F, Yu J, Lin P, et al. The ryanodine receptor mutational characteristics and its indication for cancer prognosis. Sci Rep 2022;12:16113. [Crossref] [PubMed]
- Liu Z, Liu L, Jiao D, et al. Association of RYR2 Mutation With Tumor Mutation Burden, Prognosis, and Antitumor Immunity in Patients With Esophageal Adenocarcinoma. Front Genet 2021;12:669694. [Crossref] [PubMed]
- Ren W, Li Y, Chen X, et al. RYR2 mutation in non-small cell lung cancer prolongs survival via down-regulation of DKK1 and up-regulation of GS1-115G20.1: A weighted gene Co-expression network analysis and risk prognostic models. IET Syst Biol 2022;16:43-58. [Crossref] [PubMed]
- Ohno S, Hasegawa K, Horie M. Gender Differences in the Inheritance Mode of RYR2 Mutations in Catecholaminergic Polymorphic Ventricular Tachycardia Patients. PLoS One 2015;10:e0131517. [Crossref] [PubMed]
- Postma AV, Denjoy I, Kamblock J, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 2005;42:863-70. [Crossref] [PubMed]
- Choi G, Kopplin LJ, Tester DJ, et al. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation 2004;110:2119-24. [Crossref] [PubMed]
- Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69-74. [Crossref] [PubMed]
- Hsueh CH, Weng YC, Chen CY, et al. A novel mutation (Arg169Gln) of the cardiac ryanodine receptor gene causing exercise-induced bidirectional ventricular tachycardia. Int J Cardiol 2006;108:276-8. [Crossref] [PubMed]
- Duan H, Lu Y, Yan S, et al. A delayed diagnosis of catecholaminergic polymorphic ventricular tachycardia with a mutant of RYR2 at c.7580T>G for 6 years in a 9-year-old child. Medicine (Baltimore) 2018;97:e0368. [Crossref] [PubMed]
- Nathani Y, Berent R, Shah A. 262: CPVT Triggered by influenza b in a child due to previously described point mutation in RYR2 gene. Critical Care Medicine 2019;47:112.
- Del Franco A, Gualandi F, Malagù M, et al. A Clinical Case of Catecholaminergic Polymorphic Ventricular Tachycardia: The Clinical Suspicious and the Need of Genetics. Cardiology 2017;138:69-72. [Crossref] [PubMed]
- Hu J, Gao X, Chen L, et al. A novel mutation in ryanodine receptor 2 (RYR2) genes at c.12670G>T associated with focal epilepsy in a 3-year-old child. Front Pediatr 2022;10:1022268. [Crossref] [PubMed]
- Larsen MK. Sudden unexpected death and genetic heart disease: a molecular autopsy study. Aarhus University: Denmark; 2012.
- Mahlke N, Dittmann S, Schulze-Bahr E, et al. Sudden unexpected cardiac death and postmortem identification of a novel RYR2 gene mutation. Int J Legal Med 2019;133:1835-8. [Crossref] [PubMed]
- Kohli U, Nayak HM. SIDS associated RYR2 p.Arg2267His variant may lack pathogenicity. J Electrocardiol 2020;60:23-6. [Crossref] [PubMed]
- Watanabe T, Ohno S, Shirai M, et al. Inherited catecholaminergic polymorphic ventricular tachycardia due to RYR2 mutation. Pediatr Int 2016;58:512-5. [Crossref] [PubMed]
- Beckmann BM, Wilde AA, Kääb S. Dual inheritance of sudden death from cardiovascular causes. N Engl J Med 2008;358:2077-8. [Crossref] [PubMed]
- Blancard M, Touat-Hamici Z, Aguilar-Sanchez Y, et al. A Type 2 Ryanodine Receptor Variant in the Helical Domain 2 Associated with an Impairment of the Adrenergic Response. J Pers Med 2021;11:579. [Crossref] [PubMed]
- Allouis M, Probst V, Jaafar P, et al. Unusual clinical presentation in a family with catecholaminergic polymorphic ventricular tachycardia due to a G14876A ryanodine receptor gene mutation. Am J Cardiol 2005;95:700-2. [Crossref] [PubMed]
- Heiner JD, Bullard-Berent JH, Inbar S. Deadly proposal: a case of catecholaminergic polymorphic ventricular tachycardia. Pediatr Emerg Care 2011;27:1065-8. [Crossref] [PubMed]
- Pizzale S, Gollob MH, Gow R, et al. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:1319-21. [Crossref] [PubMed]
- Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 2008;358:2024-9. [Crossref] [PubMed]
- Itoh H, Murayama T, Kurebayashi N, et al. Sudden death after inappropriate shocks of implantable cardioverter defibrillator in a catecholaminergic polymorphic ventricular tachycardia case with a novel RyR2 mutation. J Electrocardiol 2021;69:111-8. [Crossref] [PubMed]
- Letsas KP, Prappa E, Bazoukis G, et al. A novel variant of RyR2 gene in a family misdiagnosed as congenital long QT syndrome: The importance of genetic testing. J Electrocardiol 2020;60:8-11. [Crossref] [PubMed]
- Hasdemir C, Payzin S, Kocabas U, et al. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm 2015;12:1584-94. [Crossref] [PubMed]
- Nof E, Belhassen B, Arad M, et al. Postpacing abnormal repolarization in catecholaminergic polymorphic ventricular tachycardia associated with a mutation in the cardiac ryanodine receptor gene. Heart Rhythm 2011;8:1546-52. [Crossref] [PubMed]
- Di Pino A, Caruso E, Costanzo L, et al. A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm 2014;11:1480-3. [Crossref] [PubMed]
- Bhuiyan ZA, van den Berg MP, van Tintelen JP, et al. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 2007;116:1569-76. [Crossref] [PubMed]
- Roston TM, Yuchi Z, Kannankeril PJ, et al. The clinical and genetic spectrum of catecholaminergic polymorphic ventricular tachycardia: findings from an international multicentre registry. Europace 2018;20:541-7. [Crossref] [PubMed]
- Saito A, Ohno S, Nuruki N, et al. Three cases of catecholaminergic polymorphic ventricular tachycardia with prolonged QT intervals including two cases of compound mutations. J Arrhythm 2018;34:291-3. [Crossref] [PubMed]
- Nozaki Y, Kato Y, Uike K, et al. Co-Phenotype of Left Ventricular Non-Compaction Cardiomyopathy and Atypical Catecholaminergic Polymorphic Ventricular Tachycardia in Association With R169Q, a Ryanodine Receptor Type 2 Missense Mutation. Circ J 2020;84:226-34. [Crossref] [PubMed]
- Aizawa Y, Mitsuma W, Ikrar T, et al. Human cardiac ryanodine receptor mutations in ion channel disorders in Japan. Int J Cardiol 2007;116:263-5. [Crossref] [PubMed]
- d'Amati G, Bagattin A, Bauce B, et al. Juvenile sudden death in a family with polymorphic ventricular arrhythmias caused by a novel RyR2 gene mutation: evidence of specific morphological substrates. Hum Pathol 2005;36:761-7. [Crossref] [PubMed]
- Fowler ED, Zissimopoulos S. Molecular, Subcellular, and Arrhythmogenic Mechanisms in Genetic RyR2 Disease. Biomolecules 2022;12:1030. [Crossref] [PubMed]
- Lodola F, Morone D, Denegri M, et al. Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis 2016;7:e2393. [Crossref] [PubMed]
- Leung J, Lee S, Zhou J, et al. Clinical Characteristics, Genetic Findings and Arrhythmic Outcomes of Patients with Catecholaminergic Polymorphic Ventricular Tachycardia from China: A Systematic Review. Life (Basel) 2022;12:1104. [Crossref] [PubMed]
- Koponen M, Marjamaa A, Tuiskula AM, et al. Genealogy and clinical course of catecholaminergic polymorphic ventricular tachycardia caused by the ryanodine receptor type 2 P2328S mutation. PLoS One 2020;15:e0243649. [Crossref] [PubMed]
- Bellamy D, Nuthall G, Dalziel S, et al. Catecholaminergic Polymorphic Ventricular Tachycardia: The Cardiac Arrest Where Epinephrine Is Contraindicated. Pediatr Crit Care Med 2019;20:262-8. [Crossref] [PubMed]
- Miyake CY, Del Nido PJ, Alexander ME, et al. Cardiac tumors and associated arrhythmias in pediatric patients, with observations on surgical therapy for ventricular tachycardia. J Am Coll Cardiol 2011;58:1903-9. [Crossref] [PubMed]
- Peltenburg PJ, Kallas D, Bos JM, et al. An International Multicenter Cohort Study on β-Blockers for the Treatment of Symptomatic Children With Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation 2022;145:333-44. [Crossref] [PubMed]
- Van Herendael H, Dorian P. Amiodarone for the treatment and prevention of ventricular fibrillation and ventricular tachycardia. Vasc Health Risk Manag 2010;6:465-72. [Crossref] [PubMed]
- Writing Committee Members. 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients: Executive Summary. Ann Pediatr Cardiol 2022;15:323-46. [Crossref] [PubMed]



