
Analysis of clinical features of heart failure in children with cardiomyopathy and improved ejection fraction
Highlight box
Key findings
• Virus infection, low expression of suppression of tumorigenicity 2 (ST2; cutoff value =1.89 ng/mL), and treatment with intravenous immunoglobulin (IVIG) were predictors of improvement in left ventricular ejection fraction (LVEF) in patients with heart failure after treatment.
What is known and what is new?
• The prognosis of children with heart failure is highly variable. After treatment, LVEF can be improved in some children.
• Virus infection, low ST2 expression, and treatment with IVIG were predictors of improvement in LVEF.
What is the implication, and what should change now?
• ST2 and viral etiology examination should be included in the management of the children with heart failure with recovered ejection fraction (defined as LVEF ≤40% on the first day of hospitalization and >40% at 1 year after treatment, with an increase of ≥10% from baseline).
• IVIG can be used to treat heart failure in children.
Introduction
Pediatric heart failure is a common critical disease caused by a variety of causes, most commonly congenital heart diseases, coronary artery diseases, valve diseases, and cardiomyopathy, with the latter accounting for about 19.4% of heart failure in children (1). Moreover, the prognosis of heart failure varies greatly according to the cause. In recent years, evidence-based medical findings have shown that in some patients, left ventricular ejection fraction (LVEF) gradually increases or even recovers. Therefore, the concept of heart failure with recovered ejection fraction (HFrecEF) has been proposed (2). Suppression of tumorigenicity 2 (ST2) is a member of the interleukin (IL)-1 receptor family, which is divided into ST2 ligand (ST2L) and soluble ST2 (sST2). ST2L is a membrane-bound receptor, and its ligand is IL-33. Combining ST2L with IL-33 can exert antihypertrophic, antifibrotic, and antiapoptotic effects. In contrast, sST2, as a soluble decoy receptor, can effectively reduce the concentration of IL-33 that can bind to ST2L, thereby weakening the cardioprotective effect of IL-33. The predictive value of ST2 is not affected by age, renal function damage, or body mass index, making it clearly preferable to B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), which are widely used at present (3).
However, there is a lack of relevant clinical research data on children in this context. Therefore, this study analyzed the clinical characteristics of children with HFrecEF from a single center to provide a reference for subsequent clinical work. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-447/rc).
Methods
General information
In this retrospective, case-control study, children admitted to the Pediatric Heart Center of Beijing Anzhen Hospital, Capital Medical University, from January 2018 to December 2021 due to heart failure were included for analysis. History, clinical manifestations, laboratory results, and echocardiography were recorded for all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing Anzhen Hospital (No. KS2023027), and individual consent for this retrospective analysis was waived. Children were consecutively enrolled in this study, and those missing significant data or lost to follow-up were excluded. There was no obvious selection bias in the data. The flowchart of patient inclusion is shown in Figure 1.
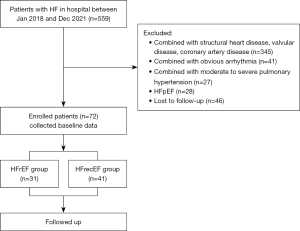
Inclusion criteria and exclusion criteria
The inclusion criteria for patients were as follows: (I) aged between 0–18 years old; (II) meeting the diagnostic criteria of cardiomyopathy (4); (III) a left ventricular end-diastolic diameter (LVEDD) increase exceeding the normal mean by two standard deviations (SDs) (according to age) as assessed by echocardiography and LVEF <40%; and (IV) increase in NT-proBNP level to >450 pg/mL or in BNP level to >100 pg/mL).
Meanwhile, the exclusion criteria were the following: (I) diagnosis of pediatric myocarditis in children according to the 2021 American Heart Association (AHA) scientific statement (5); (II) complication with congenital heart disease, ischemic coronary artery disease, hypertension, rheumatic valvular heart disease, Kawasaki disease, Marfan syndrome, or other cardiovascular diseases that can cause cardiomyopathy; (III) diagnosis of arrhythmogenic cardiomyopathy; (IV) diagnosis of arrhythmogenic right ventricular cardiomyopathy or moderate-to-severe pulmonary hypertension or other diseases that can affect right heart function in children; (V) presence of neuromuscular diseases, chronic obstructive pulmonary disease, connective tissue disease, metabolic disease, or other diseases that may cause heart disease; and (VI) previous use of drugs (such as anthracyclines) that are known to cause cardiomyopathy.
Clinical data collection
Age, sex, height, weight, underlying diseases, past medical history, oral medication, and other information of the enrolled children were recorded. For electrocardiographic data collection, the enrolled children were placed in the decubitus or lateral decubitus position, and their heart conditions were examined in the state of calm or medicated sleep. In this study, baseline and follow-up data were measured with the iE33 ultrasonic diagnostic instrument with S5-1 (frequency 1–5 MHz) and S8-3 probes (frequency 3.0–8.0 MHz) (Philips, iE33, the Netherlands). The electrocardiographic records were synchronously connected, and ≥3 cardiac cycles were measured and averaged. The height and weight of the children were recorded, and a z value was calculated (http://hdb.nbscn.org/zscore). Blood sampling and echocardiography was completed on the first hospital day.
Grouping
Grouping was completed according to the 2022 AHA guidelines (6) for heart failure as follows: (I) patients with LVEF ≤40% on the first day of hospitalization and LVEF <40% or an LVEF increase by less than 10% at 1 year after treatment were placed in the heart failure with reduced ejection fraction (HFrEF) group; (II) patients with LVEF ≤40% on the first day of hospitalization and LVEF >40% with an increase of ≥10% at 1 year after treatment were placed in the HFrecEF group.
Statistical analysis
SPSS 23.0 software (IBM Corp., Armonk, New York, USA) was used for data analysis. Normally distributed continuous variables are expressed as the mean ± SD, and the t-test was used for comparison between groups. Measurement of data with a nonnormal distribution are represented as the media and interquartile range, and the rank-sum test was used for comparison between groups. Statistical data are expressed as numbers and percentages, and the χ2 test was used for comparison between groups. Spearman correlation analysis was used to further analyze the correlation between the improvement of ejection fraction and each index. Predictors of ejection fraction improvement in patients with heart failure were analyzed using univariate logistic regression analysis. A P value <0.05 was considered to be statistically significant.
Results
General clinical data of the children.
A total of 72 children with an average age of 59.35±63.27 months were included in this study, including 41 females (56.9%) and 31 males (43.1%). Of these children, 31 (43.1%) were in the HFrEF group and 41 (56.9%) in the HFrecEF group. Compared with the HFrEF group, the HFrecEF group showed no statistically significant differences in sex, New York Heart Association (NYHA) cardiac function grade, history of preinfection, or history of viral infection (all P values >0.05); however, patients in the HFrecEF group were significantly younger (P<0.001), and patients in the HFrecEF group were more likely to be treated with intravenous immunoglobulin (IVIG) (P=0.008), as shown in Table 1. Follow-up of all children was continued until December 2022, with an average follow-up of 35.87 months. Finally, 26 children recovered to more than 60% ejection fraction, 2 children in the HFrEF group died from cardiac-related issues, and 4 children in the HFrEF group received heart transplantation. No cardiac death or heart transplantation occurred in the HFrecEF group.
Table 1
| Characteristics | Group | F/χ² value | P value | |
|---|---|---|---|---|
| HFrEF group (n=31) | HFrecEF group (n=41) | |||
| Clinical characteristics | ||||
| Sex, male/female | 16/15 | 15/26 | 1.617 | 0.208 |
| Age (months) | 93.15±68.59 | 33.80±44.98 | 14.398 | 0.000** |
| NYHA heart function grade III–IV | 23 (74.19) | 33 (80.49) | 0.803 | 0.373 |
| Heart rate (bpm) | 115.00±17.56 | 128.33±26.73 | 1.120 | 0.036* |
| History of bacterial infection | 14 (45.16) | 16 (39.02) | 0.186 | 0.668 |
| History of viral infection | 11 (35.48) | 17 (41.46) | 0.677 | 0.414 |
| Cardiac medication | ||||
| Use of cardiotonic drugs | 31 (100.00) | 41 (100.00) | – | – |
| Use of ACEIs | 23 (74.19) | 32 (78.05) | 0.557 | 0.708 |
| Use of beta-blockers | 10 (32.26) | 13 (31.71) | 0.010 | 0.961 |
| Use of diuretic agents | 31 (100.00) | 41 (100.00) | – | – |
| Use of glucocorticoids | 11 (35.48) | 20 (48.78) | 3.581 | 0.266 |
| Use of IVIG | 15 (48.39) | 32 (78.05) | 13.699 | 0.008* |
| Outcome | ||||
| LVEF recovery >60% | 1 (3.26) | 25 (60.98) | – | – |
| End-point event | 6 (19.35) | 0 (0.00) | – | – |
Data are presented as n, n (%) or mean ± standard deviation. *, P<0.05; **, P<0.01. HFrEF, heart failure with reduced ejection fraction (defined as LVEF ≤40% on the first day of hospitalization and less than 40% or increased by less than 10% at 1 year after treatment); HFrecEF, heart failure with recovered ejection group (defined as LVEF ≤40% on the first day of hospitalization and >40% at 1 year after treatment and increased by ≥10% from baseline); NYHA, New York Heart Association; ACEI, angiotensin-converting enzyme inhibitor; IVIG, intravenous immunoglobulin; LVEF, left ventricular ejection fraction.
Test results of children
Compared with the HFrEF group, the HFrecEF group had a lower creatinine level (P<0.001), lower ST2 expression (P=0.019), and lower platelet-to-lymphocyte (PLT:LYM) ratio (P=0.043). There were no significant differences in the levels of BNP, high-sensitivity troponin I (hs-TnI), creatine kinase-MB (CK-MB), high-sensitivity C-reactive protein (hs-CRP), IL-4, IL-6, or IL-10 (all P values >0.05), as shown in Table 2.
Table 2
| Characteristics | Group | F/χ² value | P value | |
|---|---|---|---|---|
| HFrEF group (n=31) | HFrecEF group (n=41) | |||
| Laboratory results | ||||
| BNP (ng/mL) | 670.00 (142.00, 2,126.00) | 329.50 (93.00, 826.50) | 0.629 | 0.230 |
| CK-MB (ng/mL) | 2.10 (1.35, 3.70) | 2.95 (1.78, 4.23) | 0.308 | 0.256 |
| hs-TnI (pg/mL) | 4.45 (1.65, 7.93) | 6.10 (4.90, 13.00) | 0.521 | 0.288 |
| NE:LYM | 1.20 (0.58, 1.85) | 0.76 (0.45, 1.33) | 0.168 | 0.152 |
| PLT:LYM | 77.54 (62.33, 110.83) | 58.91 (42.27, 77.98) | 0.401 | 0.043 |
| Creatinine (ng/mL) | 45.40 (26.70, 54.88) | 23.30 (19.00, 33.25) | 8.782 | <0.001* |
| hs-CRP (ng/mL) | 0.45 (0.29, 1.32) | 0.37 (0.19, 1.74) | 2.20 | 0.435 |
| ST2 (ng/mL) | 32.58 (22.38, 52.98) | 2.62 (1.90, 7.97) | 11.178 | 0.019* |
| IL-4 (ng/mL) | 3.75 (0.00, 11.55) | 8.15 (1.17, 17.90) | 0.277 | 0.322 |
| IL-6 (ng/mL) | 2.70 (0.00, 3.48) | 1.17 (0.00, 4.53) | 2.513 | 0.373 |
| IL-10 (ng/mL) | 0.00 (0.00, 3.48) | 0.00 (0.00, 2.23) | 2.983 | 0.383 |
| Echocardiogram—baseline | ||||
| LAD z value | 5.75±4.12 | 3.17±2.50 | 7.333 | 0.002* |
| MR (moderate to severe) | 8 (25.81) | 10 (24.39) | 1.378 | 0.057 |
| LVEDD z value | 6.08±1.64 | 5.73±1.87 | 0.154 | 0.409 |
| LVEF (%) | 32.15±8.44 | 34.23±7.45 | 1.689 | 0.286 |
| E peak (cm/s) | 101.67±32.03 | 102.51±28.52 | 0.822 | 0.909 |
| A peak (cm/s) | 69.58±25.29 | 65.65±23.88 | 0.187 | 0.557 |
| Echocardiogram—1-year follow-up | ||||
| LAD z value | 5.47±4.33 | 2.02±1.89 | 11.300 | <0.001* |
| MR (moderate to severe) | 8 (25.81) | 3 (7.31) | 0.122 | 0.203 |
| LVEDD z value | 5.54±2.11 | 2.93±1.84 | 0.463 | <0.001* |
| LVEF (%) | 37.75±9.85 | 57.54±10.10 | 0.001 | <0.001* |
Data are presented as median (interquartile range), n (%) or mean ± standard deviation. *, P<0.05. HFrEF, heart failure with reduced ejection fraction (defined as LVEF ≤40% on the first day of hospitalization and less than 40% or increased by less than 10% at 1 year after treatment); HFrecEF, heart failure with recovered ejection group (defined as LVEF ≤40% on the first day of hospitalization and >40% at 1 year after treatment and increased by ≥10% from baseline); BNP, B-type natriuretic peptide; CK-MB, creatine kinase-MB; hs-TnI, high-sensitivity troponin I; NE:LYM, neutrophil-to-lymphocyte ratio; PLT:LYM, platelet-to-lymphocyte ratio; hs-CRP, high-sensitivity C-reactive protein; ST2, suppression of tumorigenicity 2; IL, interleukin; LAD, left atrial diameter; MR, mitral regurgitation; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
Ultrasonic results of children
The LVEDD z value, LVEF, mitral regurgitation (MR), E peak, and A peak did not significantly differ between the two groups at baseline, but the left atrial diameter (LAD) of the HFrEF group was significantly larger than that in the HFrecEF group (P<0.001); however, at 1 year, the LVEDD z value and LVEF of the two groups were significantly different (P<0.001), as shown in Table 2.
Predictive factors of LVEF improvement
Spearman correlation analysis showed that age, heart rate, PLT:LYM, creatinine level, ST2 expression, LAD, and IVIG were correlated with ejection fraction improvement (P<0.05), as shown in Table 3. Logistic analysis of all the above indicators showed that the combination of virus infection [odds ratio (OR) =1.279; 95% confidence interval (CI): 0.374–4.379; P=0.007], low ST2 expression (OR =1.042; 95% CI: 1.007–1.082; P=0.032), and treatment with IVIG (OR =5.077; 95% CI: 1.458–17.684; P=0.011) were predictors of improvement in LVEF in patients with heart failure after treatment.
Table 3
| Characteristics | LVEF recovery at 1 year | Normal LVEF in 2022 | |||
|---|---|---|---|---|---|
| Correlation coefficient | P value | Correlation coefficient | P value | ||
| Clinical characteristics | |||||
| Sex | 0.150 | 0.208 | 0.238 | 0.049* | |
| Age | −0.390 | 0.001* | −0.349 | 0.003* | |
| NYHA heart function grade | −0.108 | 0.365 | 0.107 | 0.383 | |
| Heart rate | 0.274 | 0.034* | 0.456 | <0.001* | |
| History of bacterial infection | −0.052 | 0.668 | 0.042 | 0.735 | |
| History of viral infection | 0.107 | 0.414 | −0.056 | 0.759 | |
| Laboratory results | |||||
| BNP (ng/mL) | −0.196 | 0.117 | −0.09 | 0.484 | |
| CK-MB (ng/mL) | 0.199 | 0.115 | 0.046 | 0.719 | |
| hs-TnI (pg/mL) | 0.312 | 0.194 | 0.155 | 0.54 | |
| NE:LYM | −0.239 | 0.063 | −0.276 | 0.036* | |
| PLT:LYM | −0.345 | 0.007* | −0.204 | 0.125 | |
| Creatinine (ng/mL) | −0.448 | <0.001* | −0.311 | 0.014* | |
| hs-CRP (ng/mL) | −0.088 | 0.509 | 0.091 | 0.503 | |
| ST2 (ng/mL) | −0.088 | 0.509 | −0.114 | 0.624 | |
| IL-4 (ng/mL) | 0.242 | 0.183 | 0.078 | 0.678 | |
| IL-6 (ng/mL) | −0.042 | 0.818 | −0.068 | 0.716 | |
| IL-10 (ng/mL) | −0.039 | 0.833 | −0.097 | 0.605 | |
| Echocardiogram—baseline | |||||
| LVEDD z value | −0.115 | 0.347 | −0.075 | 0.547 | |
| LVEF (%) | 0.137 | 0.253 | 0.091 | 0.461 | |
| E peak (cm/s) | 0.026 | 0.832 | 0.032 | 0.801 | |
| A peak (cm/s) | −0.068 | 0.621 | 0.150 | 0.290 | |
| LAD z value | −0.595 | <0.001* | −0.525 | <0.001* | |
| Cardiac medication | |||||
| Use of glucocorticoids | 0.133 | 0.266 | 0.019 | 0.876 | |
| Use of IVIG | 0.308 | 0.008* | 0.147 | 0.228 | |
*, P<0.05. LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; BNP, B-type natriuretic peptide; CK-MB, creatine kinase-MB; hs-TnI, high-sensitivity troponin I; NE:LYM, neutrophil-to-lymphocyte ratio; PLT:LYM, platelet-to-lymphocyte ratio; hs-CRP, high-sensitivity C-reactive protein; ST2, suppression of tumorigenicity 2; IL, interleukin; LVEDD, left ventricular end-diastolic diameter; LAD, left atrial diameter; IVIG, intravenous immunoglobulin.
Discussion
Heart failure has a variable prognosis and is also a common clinical disease, having received extensive attention. In previous studies, left ventricular reverse remodeling (LVRR) was often used to evaluate the improvement of heart failure, which was defined as a decrease in left ventricular diameter or volume accompanied by significant improvement in left ventricular systolic function (7). In 2011, Punnoose et al. proposed the concept of HFrecEF for the first time (2), which was adopted by the Expert Consensus on Recovery of Heart Failure by Ejection Fraction as published in the Journal of the American College of Cardiology (JACC) in 2020 (8). In this study, HFrecEF was selected as the primary endpoint due to the young average age of the included children, low rate of heart transplantation and cardiogenic mortality, and the proportion of the children who gradually recovered to normal ejection fraction after treatment. In our study, we found that the combination of viral infection, low ST2 expression, and the application of IVIG therapy were independent predictors of the improvement of LVEF in patients with heart failure after treatment.
In our study, ST2 was found to be a predictor of LVEF improvement in patients with heart failure after treatment. As a member of the IL-1 receptor/toll-like receptor superfamily, ST2 is mainly secreted by vascular endothelial cells, cardiac fibroblasts, and cardiomyocytes and can be divided into sST2 and transmembrane ST2 (ST2L) isomers (9). sST2 may influence the progression of heart failure by promoting mitochondrial fusion in human cardiac fibroblasts (HCFs), increasing oxidative stress, and secreting inflammatory markers of nuclear factor-κB (NF-κB) (10,11). Previous studies have found there to be no significant difference between ST2 in different types of heart failure, namely heart failure with preserved ejection fraction (HFpEF), heart failure with midrange ejection fraction (HFmrEF), and HFrEF. In 2018 (12), JACC published a large cohort study involving six chronic heart failure cohorts and a total of 4,268 adult patients, which found that sST2 had a strong independent predictive value for patient prognosis, and its predictive ability was superior to that of NT-proBNP and high-sensitivity troponin T (hs-TnT) (13,14). The results of our study are roughly similar to those of previous studies on adults. However, as there is no unified diagnostic threshold for children, the predicted cutoff value needs to be further studied with a large sample size to facilitate further clinical application.
In addition, we found that viral infection was a predictor of improvement in LVEF. The possible reason is that inflammation and immune response play an important role in the pathogenesis of cardiomyopathy (15). Echocardiographic review with a minimum interval of 12 months from baseline excluded changes in LVEF due to short-term changes in heart rate or hemodynamic load in favor of the prognosis gap due to intrinsic pathogenesis. Differential analysis of cardiomyopathy microarray data from Gene Expression Omnibus (GEO) has been previously conducted, and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment has revealed that differentially expressed genes are significantly enriched in cytokine-cytokine receptor interactions, natural killer cell-mediated cytotoxicity (16,17), cell adhesion molecules, and Th17 cell differentiation signaling pathways. Viral infection may cause the above-mentioned immune response and affect the prognosis of children.
We further found that IVIG therapy was also a predictor of improvement in LVEF. In terms of therapeutic mechanism, immunoglobin G (IgG) in IVIG is involved in both specific and nonspecific immune responses. IVIG can bind to macrophages and regulate the release of related inflammatory cytokines, thereby regulating antibody levels, promoting myocardial cell remodeling, and improving cardiac function (18). It has been gradually established as a form of treatment for cardiomyopathy (19,20). Our findings are consistent with those of previous studies that reported left ventricular function improving significantly after IVIG treatment as compared with control treatment (21,22). In our study, all parents were informed about the current status of the use of IVIG therapy, and the final decision on whether to use IVIG therapy was made by the parents. However, due to its cost, related blood products, and other reasons, IVIG has a low application rate and should be further promoted.
In this study, the mean follow-up time was 35.87 months. By 2022, the LVEF of 36.1% of the children had returned to normal, which was roughly the same as the 39.8% rate reported in previous single-center studies. In Ciuca et al.’s study, the average follow-up time was 7.8±4.9 years, and the 1-year and 5-year survival rates without heart transplantation were 83.6% and 69.8%, respectively. However, in our study, the average age of the children was younger, and only 8.3% of the children had cardiac death or heart transplantation. Therefore, it is necessary to extend the follow-up time to obtain long-term results (23).
Some limitations to our study should be addressed. First, we employed a single-center, retrospective design with consecutive inclusion, but some children were lost to follow-up, so the results may be biased. Second, the LVEF values of all patients were obtained from different echocardiographic reports rather than by the same doctor. Third, the standard boundary value of ST2 has not yet been determined in this field for children, and due to the relatively small sample size in this study, the cutoff value was not calculated (21). Finally, the follow-up time of this study was short, and a large number of children still did not return to normal LVEF or had endpoint events; therefore, the follow-up time should be further extended to improve the study.
Conclusions
The clinical prognosis of patients with HFrecEF is fair, and in a certain proportion of children, the LVEF eventually returns to normal. The combination with a history of viral infection, low ST2 expression, and the application of IVIG therapy were found to be independent predictors for the improvement of LVEF in patients who experience heart failure after treatment.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-447/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-447/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-447/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-447/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Beijing Anzhen Hospital (No. KS2023027) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Massin MM, Astadicko I, Dessy H. Epidemiology of heart failure in a tertiary pediatric center. Clin Cardiol 2008;31:388-91. [Crossref] [PubMed]
- Punnoose LR, Givertz MM, Lewis EF, et al. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail 2011;17:527-32. [Crossref] [PubMed]
- Demyanets S, Kaun C, Pentz R, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol 2013;60:16-26. [Crossref] [PubMed]
- Lipshultz SE, Law YM, Asante-Korang A, et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement From the American Heart Association. Circulation 2019;140:e9-e68. [Crossref] [PubMed]
- Law YM, Lal AK, Chen S, et al. Diagnosis and Management of Myocarditis in Children: A Scientific Statement From the American Heart Association. Circulation 2021;144:e123-35. Erratum in: Circulation 2021;144:e149. [Crossref] [PubMed]
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:1757-80. [Crossref] [PubMed]
- Kass DA, Baughman KL, Pak PH, et al. Reverse remodeling from cardiomyoplasty in human heart failure. External constraint versus active assist. Circulation 1995;91:2314-8. [Crossref] [PubMed]
- Wilcox JE, Fang JC, Margulies KB, et al. Heart Failure With Recovered Left Ventricular Ejection Fraction: JACC Scientific Expert Panel. J Am Coll Cardiol 2020;76:719-34. [Crossref] [PubMed]
- Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol 2015;115:3B-7B. [Crossref] [PubMed]
- Song Y, Li F, Xu Y, et al. Prognostic value of sST2 in patients with heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol 2020;304:95-100. [Crossref] [PubMed]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 2008;7:827-40. [Crossref] [PubMed]
- Huang A, Qi X, Hou W, et al. Prognostic value of sST2 and NT-proBNP at admission in heart failure with preserved, mid-ranged and reduced ejection fraction. Acta Cardiol 2018;73:41-8. [Crossref] [PubMed]
- Emdin M, Aimo A, Vergaro G, et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J Am Coll Cardiol 2018;72:2309-20. [Crossref] [PubMed]
- Liu BH, Li YG, Liu JX, et al. Assessing inflammation in Chinese subjects with subtypes of heart failure: an observational study of the Chinese PLA Hospital Heart Failure Registry. J Geriatr Cardiol 2019;16:313-9. [Crossref] [PubMed]
- Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021;18:169-93. [Crossref] [PubMed]
- You H, Zhao Q, Ren S, et al. Pathogenesis of dilated cardiomyopathy based on GEO data mining. Journal of Shanxi Medical University 2022;53:556-65.
- Chen T, Xuan X, Ni J, et al. Selection of key genes for dilated cardiomyopathy based on machine learning algorithms and assessment of diagnostic accuracy. J Thorac Dis 2023;15:4445-55. [Crossref] [PubMed]
- Guidelli GM, Tenti S, Pascarelli NA, et al. Granulomatosis with polyangiitis and intravenous immunoglobulins: a case series and review of the literature. Autoimmun Rev 2015;14:659-64. [Crossref] [PubMed]
- Maisch B, Alter P. Treatment options in myocarditis and inflammatory cardiomyopathy : Focus on i. v. immunoglobulins. Herz 2018;43:423-30. [Crossref] [PubMed]
- Maisch B. Cardio-Immunology of Myocarditis: Focus on Immune Mechanisms and Treatment Options. Front Cardiovasc Med 2019;6:48. [Crossref] [PubMed]
- Prasad AN, Chaudhary S. Intravenous immunoglobulin in children with acute myocarditis and/or early dilated cardiomyopathy. Indian Pediatr 2014;51:583-4. [Crossref] [PubMed]
- Heidendael JF, Den Boer SL, Wildenbeest JG, et al. Intravenous immunoglobulins in children with new onset dilated cardiomyopathy. Cardiol Young 2018;28:46-54. [Crossref] [PubMed]
- Ciuca C, Ragni L, Hasan T, et al. Dilated cardiomyopathy in a pediatric population: etiology and outcome predictors - a single-center experience. Future Cardiol 2019;15:95-107. [Crossref] [PubMed]


