Treatment of metastatic TFE3 microphthalmia transcription factor translocation renal cell carcinoma: a case report
Highlight box
Key findings
• This report summarizes the therapeutic strategy for a case of metastatic of transcription factor E3 (TFE3) microphthalmia transcription factor translocation renal cell carcinoma (MiT-RCC), which included immunotherapy (sintilimab) and administration of vascular endothelial growth factor receptor tyrosine kinase inhibitor (VEGFR-TKI) (from pazopanib to sunitinib).
What is known and what is new?
• At present, the main treatment methods for advanced metastatic MiT-RCC include surgery, chemotherapy, immunotherapy, anti-vascular endothelial growth factor or VEGFR inhibitors, mammalian target of rapamycin inhibitors, and targeted therapy against the mesenchymal-epithelial transition factor signaling pathway.
• In this case report, although the patient’s tumor mutational burden and microsatellite instability analysis results suggested limited potential benefit from immune-checkpoint inhibitor monotherapy, sintilimab was nonetheless administered based on the patient’s condition. Due to suggestions gathered at the 4th Congress of Chinese Research Hospital Association-Children’s Oncology Committee-Multidisciplinary Treatment, we changed the treatment of TKIs targeting VEGFR from pazopanib to sunitinib.
What is the implication, and what should change now?
• The current treatment mode for malignant tumors is molecular diagnosis and tailored treatment. For patients with advanced metastatic TFE3 MiT-RCCs, there is an urgent need to develop immunotherapy and identify novel targeted therapeutic agents to improve the survival of these patients.
Introduction
Transcription factor E3 (TFE3) microphthalmia transcription factor translocation renal cell carcinoma (MiT-RCC) is a rare and aggressive renal malignancy, which is significantly more prevalent in children (40%) than in adults (4%) (1). MiT family translocation RCCs mainly include Xp11 translocation renal cell tumors carrying TFE3 gene fusions and t(6;11) RCCs carrying transcription factor EB (TFEB) gene fusions (2). The basic helix-loop-helix leucine zipper transcription factor of the microphthalmia-associated transcription factor/transcription factor E (MiTF/TFE) (MiT) family consists of four closely related members: MiTF, TFE3, TFEB, and TFEC (3). The MiTF/TFE family is now thought to play a key role in organelle biogenesis, nutrient sensing, and energy metabolism (4).
Mutations and/or aberrant expression of MiTF/TFE family members have been associated with different types of cancers in humans, such as renal carcinomas, alveolar sarcomas, and melanomas (4). MiTF/TFE family factors are also involved in the regulation of lysosomal signaling, including mTOR complex 1 (mTORC1) and the Wnt/β-catenin pathway, which are critical for oncogenic signaling (5,6). Most metastatic RCCs are treated with tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor receptor (VEGFR), including pazopanib, sunitinib, sorafenib, bevacizumab, ramucirumab, and cabozantinib (1,7-9). Here, we report a case of advanced metastatic TFE3 MiT-RCC, for which we employed a combination of surgery, chemotherapy, immunotherapy, and targeted therapy. However, the patient eventually developed a significant amount of ascites (including a large number of tumor cells). The family expressed a low willingness for further treatment and decided to discontinue therapy, resulting in the patient’s eventual death. We hope that this single-center case can provide new insights into the treatment of metastatic TFE3 MiT-RCC. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-35/rc).
Case presentation
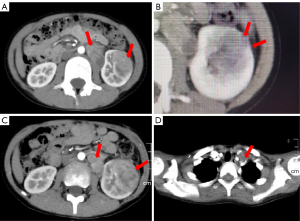
The patient was an 8-year-old boy who attended the local hospital with gross hematuria and intermittent urinary pain for 2 weeks, without frequency or urgency. He was initially examined at a local hospital, where a computed tomography (CT) scan (Figure 1) revealed a malignant tumor in the lower and middle poles of the left kidney. Our CT examination confirmed a mass measuring approximately 35.2 mm × 31.0 mm × 36.8 mm in the lower pole of the left kidney and a mass of about 40.3 mm × 28.0 mm × 65.1 mm in the anterior aspect of the left kidney, suggestive of a renal tumor with retroperitoneal lymph node metastasis. Local invasion of the renal capsule by the tumor was also observed (Figure 1A,1B). Positron emission tomography-CT (PET-CT) demonstrated similar findings and no new distant metastases.
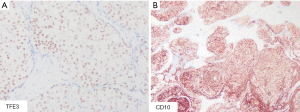
After a multidisciplinary discussion at our center, considering the difficulty of complete resection due to the tumor’s size and the need for a definitive diagnosis, we performed a tumor biopsy surgery. Immunohistochemistry of the postoperative tumor sample (Figure 2A,2B) demonstrated positivity for TFE3. Fluorescence in situ hybridization (FISH) testing for TFE3 on the tumor sample revealed a breakpoint frequency of 44% (44/100, randomly counting 100 tumor cells), surpassing the threshold of 20%. Microscopically, the tumor cells were seen to have a papillary structure, and gravel bodies were scattered within the tumor tissue. Based on these findings, the patient was diagnosed with stage T4N1M1 IV TFE3 MiT-RCC.
Following the surgery, the patient received two cycles of chemotherapy primarily consisting of cyclophosphamide and doxorubicin. Follow-up CT scans revealed an increase in the size of the left renal mass and the retroperitoneal tumor (Figure 1C), and ultrasound indicated lymph node metastasis in the neck. Subsequently, we incorporated sintilimab [a fully human immunoglobulin G4 (IgG4) monoclonal antibody that binds to programmed cell death receptor-1 (PD-1)] into the subsequent chemotherapy regimen. Since the third chemotherapy, the patient has received a total of seven courses of sintilimab.
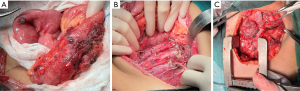
After three cycles of chemotherapy, we performed radical nephrectomy with vascular osteotomy and clearance of multiple tumor implants in the abdominal cavity. Pathological examination confirmed metastases in the perirenal area, small intestine, colon, mesocolon, greater omentum, para-aortic lymph nodes, paracaval lymph nodes, and iliac vascular lymph nodes, consistent with the previous findings (Figure 3A,3B). The patient received sintilimab and additional chemotherapy before and after the surgery, respectively.
The test results for the 831 genes related to the tumor, in combination with the targeted drug matching analysis, indicated that the tumor was microsatellite stable (MSS) with a tumor mutational burden (TMB) of 0.00 muts/Mb. Additionally, a mutation of PALB2-p.Glu650Ter (heterozygous nonsense mutation, 49.3%), which is a member of the DNA damage response (DDR) pathway genes, was detected in the patient. DDR gene mutations lead to an increase in TMB value and infiltration of lymphocytes in the tumor, making patients with DDR gene mutations more likely to benefit from immunotherapy (10). Furthermore, considering the personalized analysis of chemotherapy drug sensitivity, we adjusted the chemotherapy regimen for the patient to achieve precision treatment. During the fifth round of chemotherapy, we incorporated the targeted drug, pazopanib, in addition to the sintilimab-based chemotherapy regimen.
At the 4th Congress of Chinese Research Hospital Association-Children’s Oncology Committee-Multidisciplinary Treatment (CRHA-COC-MDT), which was hosted by our center on August 28, 2022, we shared and discussed this case with all major pediatric solid tumor centers in China. It was suggested that we change the targeted drug from pazopanib to sunitinib. During the sixth round of chemotherapy, a thoracotomy was performed in collaboration with the Thoracic Surgery Department to remove the lymph node metastasis in the supraclavicular region. Surgery was also performed to clear the extensive metastatic lymph nodes in the left superior cervical mediastinum (Figure 3C). During the eighth round of chemotherapy, the treatment regimen was changed to sintilimab + sunitinib + chemotherapeutic drugs. In the tenth round of chemotherapy, the patient developed a large amount of ascites, which was not detected in the previous routine ultrasound examination. Ascitic fluid smear revealed tumor cells, and intraperitoneal chemotherapy was administered. However, the patient responded poorly to the treatment. In the eleventh round of chemotherapy, abdominal ultrasound indicated liver metastasis of the tumor. Due to the family’s low willingness to continue, they ultimately decided to forgo further treatment, after which the patient died.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient’s family consent according to the Institutional Review Board of the Children’s Hospital of Chongqing Medical University.
International multidisciplinary team (iMDT) discussion
Discussion from Department of Pediatric Surgical Oncology, Children’s Hospital of Chongqing Medical University
RCCs are currently thought to account for about 2% to 4% of kidney tumors in children (11,12), and MiT-RCCs, 93.2% of which are TFE3 MiT-RCCs, constitute approximately 41.5% of all RCCs, and the age of patients with MIT-RCCs tends to range from 0 to 20 years (13), and there is no standardized treatment for this disease. The main treatment options include surgery, anti-VEGF/VEGFR or VEGFR therapy, mTOR inhibitors, immunotherapy, MET signaling pathway targeting, and chemotherapy (1).
A 2010 retrospective study from France (14) showed that of 21 cases of metastatic Xp11 translocation RCC, 11 patients were treated with sunitinib and nine with cytokines, with sunitinib appearing to be more effective than cytokine administration. A 2016 study showed that for patients with non-clear cell RCC (nccRCC) including advanced papillary, chromophobe, collecting duct carcinoma (CDC), and Xp11.2 translocation, unclassified RCC, and clear cell RCC (ccRCC) with >20% sarcomatoid features, everolimus is not superior to sunitinib (15). In addition, pembrolizumab monotherapy provides long-lasting antitumor activity for previously untreated patients with nccRCC (16). A 2018 study similarly demonstrated the antitumor activity of PD-1 and programmed death-ligand 1 (PD-L1) inhibitors in nccRCC (17).
Here, we report a case with a primary diagnosis of metastatic TFE3 MiT-RCC. In the patient’s overall treatment regimen, we performed a comprehensive and highly individualized treatment that included biopsy surgery, radical nephrectomy for renal carcinoma, radical tumor resection with vascular osteotomy, cervical and thoracic lymph node dissection, sintilimab administration, TKIs targeting VEGFR (from pazopanib to sunitinib), and chemotherapy (neoadjuvant chemotherapy and postoperative chemotherapy), but unfortunately the patient responded poorly to the aggressive treatments and eventually died.
Although the patient’s TMB and microsatellite instability analysis results suggested limited potential benefit from immune checkpoint inhibitor monotherapy, it has now been shown that translocation RCC contain a considerable density of tumor-infiltrating CD8+ T cells (18), which are susceptible to immune modulation, and the treatment of immune checkpoint inhibition (ICI) makes theoretical sense. However, toward the later stage of treatment, the patient developed a significant amount of bloody ascites. Ultrasound indicated no obvious signs of tumor recurrence at the primary site, but liver metastases were detected. However, it was unclear whether extensive micrometastases were present within the peritoneal cavity, as the liver function did not show significant abnormalities at that time. Unfortunately, the family of the patient had a limited willingness to pursue further treatment, and regrettably, the patient exhibited a poor response to aggressive therapy, ultimately resulting in death.
There are several definitive diagnostic tools available for TFE3 MiT-RCCs, including TFE3 immunohistochemistry, next-generation sequencing (NGS), RNA, and exome sequencing, and TFE3-isolated FISH is the gold standard for diagnosis.
With the advancement of immunotherapy in the first-line treatment of metastatic RCC (19), there has been an increased focus on combination therapies, as evidence by the ongoing NCT03595124 and NCT04704219 trials. Therefore, for the treatment of metastatic TFE3 MiT-RCC, in addition to complete surgical resection at the primary site, early initiation of immunotherapy and the use of precision-targeted drugs are necessary. There is an urgent need for the development of novel immunotherapeutic and targeted therapeutic agents to effectively treat pediatric patients with this condition. Furthermore, additionally research on tumor driver genes and other aspects will be crucial to enhancing the level of precision treatment.
Opinions from the international experts on questions related to diagnosis and treatment of this patient
Q1: What other therapeutic strategies are currently available for the advanced metastatic TFE3 MiT-RCC reported in this study?
Expert opinion 1: Dr. Fumihiko Urabe
Performing nephrectomy prior to chemotherapy might be an option.
Expert opinion 2: Dr. Mauricio Burotto
These translocation RCC looks like to have tumor-infiltrating CD8+ T cells. Which potentially made them hot tumor and susceptible to respond to immuno-modulation. Expert opinion rational and case reports support my suggestion of combine ICI (Ipi-Nivo) or clinical trial with some kind of immune-oncology (IO) in case like this or in general case of TFE3 MiT-RCC.
Expert opinion 3: Dr. Sebastiano Buti & Dr. Giulia Claire Giudice
All the TKIs and the combination treatment options accessible nowadays for the treatment of RCC have been primarily studied in patients with clear-cell RCC, with limited evidence for MiT-RCC. In fact, randomized trials addressing this rare entity are not been held, there is not a defined standard of care treatment and the main evidence comes from retrospective analysis or a few phase II trials.
The International Society of Pediatric Oncology (SIOP) recommends the use of preoperative chemotherapy for the localized RCC, with combination regimes of vincristine and actinomycin-D or epirubicin or doxorubicin (20).
For the advanced disease approach, the European Association of Urology guidelines suggest treatments with temsirolimus, everolimus, sorafenib, sunitinib, cabozantinib and pembrolizumab (21), while the National Comprehensive Cancer Network (NCCN) guidelines (22) underline the importance of enrollment in clinical trials, as the preferred strategy, suggesting TKI (cabozantinib, sunitinib, lenvatinib, axitinib, pazopanib), mTOR inhibitors (everolimus, temsirolimus), VEGFR inhibitors (bevacizumab) or ICI (nivolumab, pembrolizumab), either as monotherapy or combination, in alternative.
Regarding more specifically MiT-RCC, evidence is scarce. According to retrospective analysis by the group of Hirsch et al. on 52 and 17 patients with metastatic MiT-RCC (9), cabozantinib seemed to provide benefit, either in first- or later-lines, with an objective response rate (ORR) of 17.3% and 29%, respectively. Thouvenin and colleagues reported a median progression-free survival (mPFS) of 6.8 months and a median overall survival (mOS) of 18.3 months (23), while Martínez Chanzá et al. collected a median time to treatment failure (mTTF) of 8.3 months and a 1-year OS of 69% (24). Additional evidence comes from the subanalysis of the phase II CaboSun trial, aimed at comparing cabozantinib and sunitinib. Only three patients with MiT-RCC were included and treated with cabozantinib, showing an ORR of 50% and a mPFS of 14 months.
Consequently, cabozantinib could represent a treatment of choice for patients with MiT-RCC, strengthened by the confirmation of MET mutation role in the pathway of MiT (25).
Similarly, pre-clinical study supposed that the secondary resistance to sunitinib may be due to an up-regulation of PD-L1 by TFE3 (26). Consequently, sunitinib may not be the TKI of choice in the treatment of MiT-RCC, with respect to other TKIs.
For instance, the effectiveness of pazopanib was highlighted in a meta-analysis on 15 retrospective or prospective trials on patients with nccRCC, including MiT-RCC. Pazopanib seemed to show good effectiveness in this population, in terms of ORR (range, 27–33%), DCR (range, 81–89%), mPFS (range, 8.1–16.5 months) and mOS (range, 17.3–31.0 months) (27).
Alternatively, axitinib may be a preference, based on the data of a phase II trial on 40 pre-treated patients with metastatic nccRCC receiving axitinib. The subgroup of patients with MiT-RCC (17.5% of the population) seemed to especially benefit from the treatment, with an ORR of 57.1%, a disease control rate (DCR) of 85.7%, a mPFS of 11.1 months and a mOS of 16.9 months (28).
mTOR inhibitors, such as everolimus, may be a good option. Even though the randomized phase II trial, ESPN, failed to show superiority of everolimus over sunitinib, the subgroup of seven patients with MiT-RCC showed mPFS and mOS of 6.1 and 16.2 months with sunitinib and 3 and 8.1 months with everolimus, respectively (15). In addition, data from retrospective analysis may suggest some benefit from a treatment with mTOR inhibitors (14).
Preclinical data also strengthen the importance of the PI3K/AKT/mTOR pathway in MiT-RCC (29).
Regarding ICIs or ICI + TKI combinations, the phase II KEYNOTE-B61 trial evaluated a first-line therapy with lenvatinib + pembrolizumab in patients nccRCC, including six with MiT-RCC, showing an ORR of 67%; similarly, the phase II CaboNivo trial evaluated a first- or second-line therapy with cabozantinib + nivolumab in patients nccRCC, including two with MiT-RCC, showing an ORR of 50% and a mPFS of 14 months. Lastly, a phase II trial of atezolizumab and bevacizumab for patients with metastatic nccRCC enrolled five patients with MiT-RCC, with an ORR of 20% (30). Some additional data may be derived from retrospective analysis. The group of Alhalabi et al. highlighted a scarce response to a dual ICIs treatment on 29 patients affected by MiT-RCC, with an ORR, a mPFS and a mOS of 5%, 2.8 and 17.8 months, respectively; patients seemed to benefit from a combination therapy with ICI and TKI, instead, with an ORR, a mPFS and a mOS of 36%, 5.4 and 30.7 months, respectively (31). In a retrospective analysis of 22 patients with MiT-RCC, an ICI treatment seemed to benefit a higher ORR and longer OS, compared to TKI (ORR 25.0% vs. 0%, OS 62.4 vs. 10.3 months, respectively) (18). Lastly, a real-world data report on patients affected by nccRCC, included nine patients with MiT-RCC receiving systemic treatment; 44.4% of patients received sunitinib while 55.6% ICI + TKI combination. Patients receiving an ICI treatment seemed to show the major benefit with an ORR of 40% and a median PFS of 7.3 months. Sadly, no patient was still alive after 5 years (32).
Considering all the available evidence, a treatment with a combination of ICI and TKI may be of choice in the current treatment of patients with MiT-RCC.
Q2: What should be the timing of the application of targeted agents (e.g., VEGFR-TKI) for cases such as this one?
Expert opinion 1: Dr. Fumihiko Urabe
This timing is the best.
Expert opinion 2: Dr. Mauricio Burotto
I would suggest IO-IO combination in first line, maybe a regime like the COSMIC-313.
IO-IO-TKI could be an option in this subtype of RCC. Would be my first choice in clinical practice.
Expert opinion 3: Dr. Sebastiano Buti & Dr. Giulia Claire Giudice
The nowadays available data are not sufficient enough to define an adequate sequence strategy, although TKIs could be a wise choice in first-line treatment, given the acceptable toxicity for hopefully a long duration of treatment. Particularly, in a retrospective study, cabozantinib seemed to show better benefit when administered in first-line than in later-lines (mPFS of 6.8, 11.7 months for first-line, 6.5 months in later-lines) (23).
Q3: Late in the course of treatment, the patient developed a large amount of ascites in which tumor cells were visible, but imaging suggested no in situ recurrence and the patient’s protein levels were relatively normal: what should be considered the typical cause of this situation?
Expert opinion 1: Dr. Fumihiko Urabe
This mechanism is very difficult.
Expert opinion 2: Dr. Mauricio Burotto
Because of the poor prognostic and aggressive clinical presentation, a peritoneal compromise is not particularly rare. I am treated patient with pleural compromise and lymphangitis and retroperitoneal compromise very aggressive.
Expert opinion 3: Dr. Sebastiano Buti & Dr. Giulia Claire Giudice
In case of ascites onset, every differential cause should be excluded. Taking into account all conditions of ascites, including heart failure, hepatic failure, nephrotic syndrome, pancreatitis, hypothyroidism, venous alterations, amyloidosis, constrictive pericarditis and infections, comprehensive blood and urine tests and heart and abdomen ultrasound evaluations may be performed. Additional instrumental exams may be carried out, such as CT scans, being aware that sensitivity and specificity for detecting peritoneal metastasis seemed to be 68% and 88%, according to a meta-analysis on 37 studies, while magnetic resonance imaging (MRI) may be the exam of choice, with higher sensitivity and specificity (92% and 85% respectively) (33). Lastly an ascitic fluid examination, including biochemical and smear, may clear the diagnosis.
In addition, it is worth remembering that, rarely, TKI may be a cause of nephrotic syndrome, with or without associated proteinuria (34,35).
Lastly, malignant ascites, without visible large mass or other distant metastases, has been reported to be a very rare presentation of RCC (36,37).
Conclusions
Based on this case report of advanced metastatic TFE3 MiT-RCC and other studies, we believe that early molecular diagnosis with genetic testing is necessary for treating advanced, multimetastatic TFE3 MiT-RCC and that the early use of immunotherapy and targeted therapies may be beneficial. Novel targeted drugs and therapeutic strategies are still needed to improve the outcome of patients with metastatic TFE3 MiT-RCC.
Acknowledgments
We wish to thank Dr. Zhu from the Pathology Department of Chongqing Medical University Children’s Hospital for assistance in the successful implementation of this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-35/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-35/coif). M.B. receives consulting fees from ROCHE, BMS, MSD, ASTRAZENECA; and participates in the advisory board of ROCHE, BMS, MSD, ASTRAZENECA. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient’s family consent according to the Institutional Review Board of the Children’s Hospital of Chongqing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Simonaggio A, Ambrosetti D, Verkarre V, et al. MiTF/TFE Translocation Renal Cell Carcinomas: From Clinical Entities to Molecular Insights. Int J Mol Sci 2022;23:7649. [Crossref] [PubMed]
- Caliò A, Segala D, Munari E, et al. MiT Family Translocation Renal Cell Carcinoma: from the Early Descriptions to the Current Knowledge. Cancers (Basel) 2019;11:1110. [Crossref] [PubMed]
- Kuiper RP, Schepens M, Thijssen J, et al. Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res 2004;32:2315-22. [Crossref] [PubMed]
- Martina JA, Diab HI, Li H, et al. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci 2014;71:2483-97. [Crossref] [PubMed]
- Lang M, Schmidt LS, Wilson KM, et al. High-throughput and targeted drug screens identify pharmacological candidates against MiT-translocation renal cell carcinoma. J Exp Clin Cancer Res 2023;42:99. [Crossref] [PubMed]
- Slade L, Pulinilkunnil T. The MiTF/TFE Family of Transcription Factors: Master Regulators of Organelle Signaling, Metabolism, and Stress Adaptation. Mol Cancer Res 2017;15:1637-43. [Crossref] [PubMed]
- Buti S, Bersanelli M, Maines F, et al. First-Line PAzopanib in NOn-clear-cell Renal cArcinoMA: The Italian Retrospective Multicenter PANORAMA Study. Clin Genitourin Cancer 2017;15:e609-14. [Crossref] [PubMed]
- Choueiri TK, Lim ZD, Hirsch MS, et al. Vascular endothelial growth factor-targeted therapy for the treatment of adult metastatic Xp11.2 translocation renal cell carcinoma. Cancer 2010;116:5219-25. [Crossref] [PubMed]
- Hirsch L, Martinez Chanza N, Farah S, et al. Clinical Activity and Safety of Cabozantinib for Brain Metastases in Patients With Renal Cell Carcinoma. JAMA Oncol 2021;7:1815-23. [Crossref] [PubMed]
- Chabanon RM, Rouanne M, Lord CJ, et al. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer 2021;21:701-17. [Crossref] [PubMed]
- Geller JI, Ehrlich PF, Cost NG, et al. Characterization of adolescent and pediatric renal cell carcinoma: A report from the Children's Oncology Group study AREN03B2. Cancer 2015;121:2457-64. [Crossref] [PubMed]
- Pastore G, Znaor A, Spreafico F, et al. Malignant renal tumours incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42:2103-14. [Crossref] [PubMed]
- Cajaiba MM, Dyer LM, Geller JI, et al. The classification of pediatric and young adult renal cell carcinomas registered on the children's oncology group (COG) protocol AREN03B2 after focused genetic testing. Cancer 2018;124:3381-9. [Crossref] [PubMed]
- Malouf GG, Camparo P, Oudard S, et al. Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol 2010;21:1834-8. [Crossref] [PubMed]
- Tannir NM, Jonasch E, Albiges L, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol 2016;69:866-74. [Crossref] [PubMed]
- McDermott DF, Lee JL, Bjarnason GA, et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Clear Cell Renal Cell Carcinoma. J Clin Oncol 2021;39:1020-8. [Crossref] [PubMed]
- McKay RR, Bossé D, Xie W, et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol Res 2018;6:758-65. [Crossref] [PubMed]
- Bakouny Z, Sadagopan A, Ravi P, et al. Integrative clinical and molecular characterization of translocation renal cell carcinoma. Cell Rep 2022;38:110190. [Crossref] [PubMed]
- Powles T, Albiges L, Bex A, et al. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann Oncol 2021;32:1511-9. [Crossref] [PubMed]
- van der Beek JN, Hol JA, Coulomb-l'Hermine A, et al. Characteristics and outcome of pediatric renal cell carcinoma patients registered in the International Society of Pediatric Oncology (SIOP) 93-01, 2001 and UK-IMPORT database: A report of the SIOP-Renal Tumor Study Group. Int J Cancer 2021;148:2724-35. [Crossref] [PubMed]
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022;82:399-410. [Crossref] [PubMed]
- Motzer RJ, Jonasch E, Agarwal N, et al. NCCN Guidelines® Insights: Kidney Cancer, Version 2.2024. J Natl Compr Canc Netw 2024;22:4-16. [Crossref] [PubMed]
- Thouvenin J, Alhalabi O, Carlo M, et al. Efficacy of Cabozantinib in Metastatic MiT Family Translocation Renal Cell Carcinomas. Oncologist 2022;27:1041-7. [Crossref] [PubMed]
- Martínez Chanzá N, Xie W, Asim Bilen M, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol 2019;20:581-90. [Crossref] [PubMed]
- Sun G, Chen J, Liang J, et al. Integrated exome and RNA sequencing of TFE3-translocation renal cell carcinoma. Nat Commun 2021;12:5262. [Crossref] [PubMed]
- Guo X, Li R, Bai Q, et al. TFE3-PD-L1 axis is pivotal for sunitinib resistance in clear cell renal cell carcinoma. J Cell Mol Med 2020;24:14441-52. [Crossref] [PubMed]
- Bersanelli M, Brunelli M, Gnetti L, et al. Pazopanib as a possible option for the treatment of metastatic non-clear cell renal carcinoma patients: a systematic review. Ther Adv Med Oncol 2020;12:1758835920915303. [Crossref] [PubMed]
- Park I, Lee SH, Lee JL. A Multicenter Phase II Trial of Axitinib in Patients With Recurrent or Metastatic Non-clear-cell Renal Cell Carcinoma Who Had Failed Prior Treatment With Temsirolimus. Clin Genitourin Cancer 2018;16:e997-e1002. [Crossref] [PubMed]
- Damayanti NP, Budka JA, Khella HWZ, et al. Therapeutic Targeting of TFE3/IRS-1/PI3K/mTOR Axis in Translocation Renal Cell Carcinoma. Clin Cancer Res 2018;24:5977-89. [Crossref] [PubMed]
- McGregor BA, McKay RR, Braun DA, et al. Results of a Multicenter Phase II Study of Atezolizumab and Bevacizumab for Patients With Metastatic Renal Cell Carcinoma With Variant Histology and/or Sarcomatoid Features. J Clin Oncol 2020;38:63-70. [Crossref] [PubMed]
- Alhalabi O, Thouvenin J, Négrier S, et al. Immune Checkpoint Therapy Combinations in Adult Advanced MiT Family Translocation Renal Cell Carcinomas. Oncologist 2023;28:433-9. [Crossref] [PubMed]
- Izarn F, Allignet B, Gille R, et al. Real World Data of Diagnosis, Survival, and Treatment Outcomes in Patients With Metastatic Non Clear Cell Renal Cell Carcinoma. Clin Genitourin Cancer 2023;21:e35-43. [Crossref] [PubMed]
- van 't Sant I, Engbersen MP, Bhairosing PA, et al. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol 2020;30:3101-12. [Crossref] [PubMed]
- Kust D, Kruljac I, Peternac AŠ, et al. Pleural and pericardial effusions combined with ascites in a patient with severe sunitinib-induced hypothyroidism. Acta Clin Belg 2016;71:175-7. [Crossref] [PubMed]
- Okuno Y, Kume H, Hosoda C, et al. Development of nephrotic syndrome after administration of sorafenib in a case of metastatic renal cell carcinoma. Case Rep Med 2011;2011:710216. [Crossref] [PubMed]
- Boateng AA, Vinson MA, Mutema GK, et al. Malignant ascites and small renal mass: an unusual presentation of advanced renal cell carcinoma. Urology 2013;82:e28-9. [Crossref] [PubMed]
- Gupta R, Mathur SR, Iyer VK, et al. Cytomorphologic consideration in malignant ascites with renal cell carcinoma: A report of two cases. Cytojournal 2010;7:4. [Crossref] [PubMed]