
Efficacy and tolerability of perampanel in pediatric patients with Dravet syndrome
Highlight box
Key findings
• We provided evidence of promising therapeutic potentials for perampanel (PER) among some patients with Dravet syndrome (DS), with clinical data supporting the value of this treatment.
What is known and what is new?
• PER was recently approved as an adjunctive anti-epileptic medication for patients as young as 4 years old for primary generalized tonic-clonic seizures.
• Patients who received the medication at the age of 8 years and younger had a significantly higher efficacy rate in comparison to older patients.
What is the implication, and what should change now?
• PER should be included in the treatment modality of patients presenting with epilepsy and genetically diagnosed with DS.
Introduction
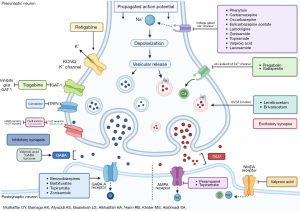
Epilepsy is a chronic brain disorder characterized by the tendency to develop recurrent unprovoked seizures at least 24 hours apart. In 2017, the International League Against Epilepsy (ILAE) established a classification of seizures and epilepsies (1), which aided clinicians in different diagnostic and therapeutic approaches depending on the form of epilepsy. In 1978, Charlotte Dravet first described a form of seizure termed Dravet syndrome (DS), previously known as severe myoclonic epilepsy of infancy (SMEI) (2). It is a form of genetic epilepsy with early-onset and rare prevalence. It manifests as intractable epilepsy with multiple seizure patterns and neurodevelopmental delays (3). Remarkably, the vast majority of patients with DS carry a de novo mutation in the SCN1A gene (4,5). This gene encodes for the alpha-one subunit of the voltage-gated sodium channel (6). The sequence variants in the mutated gene result in a broad spectrum of clinical features ranging from asymptomatic carriers to severe epilepsy phenotypes (7). Additionally, 20–30% of phenotypical DS patients could have other mutations (8,9). In the pediatric population, DS can lead to refractory seizures that are resistant to therapy and occasionally present in severe forms that are associated with regression of the normal development in the child’s first few years of life (especially during the first 4 to 6 years). Other features like cognitive decline, intellectual disability like hyperactivity and attention deficit, and oppositional defiant behavior could also be present in pediatric patients with DS (10). In 1990, DS was reported to have an incidence of 1:40,000 live births (11). However, in another study conducted in 1992, the figures were reported to be between 1:20,000 and 1:30,000, with a male-to-female ratio of 2:1 (12). Many individuals with DS fail multiple anti-seizure medications (ASMs). As such, some studies investigated the role of ketogenic diet and presented data of its potential safe application, however, these studies were weakened with the level of evidence (13) and prominent compliance issues (14). As for the use of ASMs, a recent meta-analysis that assessed eight placebo-controlled trials on the efficacy of stiripentol, pharmaceutical-grade cannabidiol, fenfluramine hydrochloride, and soticlestat for patients with DS, the study presented first-class evidence that their use may support in the treatment paradigm to control seizure among patients with DS (15). The study proposed a superiority for fenfluramine hydrochloride and stiripentol in comparison to the other options, however, a higher risk of adverse events was reported among some of these medications which promotes the investigation of other pharmacological options that could potentially present with lower risks of adverse events and higher efficacy, especially given that such study limited with low number of evidence with some results being based on a single observation. Therefore, we discuss a new option, that is, perampanel (PER) which is a selective, noncompetitive antagonist of the αamino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) glutamate receptor (16) (Figure 1). PER was recently approved as an ASM for patients as young as 4 years old after it was indicated as an adjunctive treatment for partial-onset seizures in patients older than 12 years old and as an adjunctive treatment for primary generalized tonic-clonic seizure (GTCS) in patients with epilepsy at the same age group (17,18) and has been favored over other ASMs due to the ease of use of the titration scheme (19). Furthermore, PER showed efficacy and appropriate tolerability among other epilepsy syndromes that are known to be refractory to many ASMs including Lennox-Gastaut syndrome (20), nonetheless, firm conclusions are still not established on its use as a first-line treatment and more studies are needed to assess its long-term effects. It also presented potential in treating patients with refractory seizures compared to other ASMs (16), however, several possible adverse events for its administration have been reported such as dizziness, somnolence, headache, and fatigue which was frequently reported (21-23) as well as other psychiatric side effects (17,19,24). Moreover, only a few studies investigated and assessed the use of PER in patients with DS (24-30). In addition, studies conducted had a limited number of cases. Therefore, our study aims to assess the efficacy and tolerability of PER among pediatric patients with DS. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-581/rc).
Methods
Study design and setting
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Unit of Biomedical Ethics at the Faculty of Medicine at King Abdulaziz University approved this study (reference number: 244-23) on May 2, 2023. Informed consent was obtained from the parents or legal guardians of all patients. Informed consent was also obtained for off-label use of PER on the patients. Following the approval, we retrospectively reviewed the medical records of all pediatric patients (14 years and younger during the first admission) diagnosed with DS. The study extracted and included the data of all patients with a history of using PER or are currently on the medication. The search time frame was set from the date of PER approval that is October 2012 to August 2023. Patient records that did not indicate any PER prescription throughout their life were excluded. The diagnosis of DS was based on the following criteria: (I) refractory epilepsy with multiple seizure types including prolonged febrile convulsions, myoclonic jerks, atypical absences, GTCS and complex focal seizures; (II) seizure before 1 year of age in a previously normal infant; (III) developmental delay; (IV) electroencephalogram (EEG) with generalized spike and polyspike waves; (V) genetic diagnosis of SCN1A mutation or other reported genetic variants that can present with DS like: PCDH19, SCN1B, GABRA1, STXBP1, CHD2 and SCN2A. The criteria were based on the ILAE 2022 definition. We evaluated seizure frequency before and after administering PER and the adverse events that occurred following its administration. Patients were followed for at least 3 months to determine the efficacy of PER. PER treatment was considered effective when seizure frequency had been reduced by more than 50%. We continued observation until the dose of concomitant ASMs had increased or until patients started taking another ASM. Adverse events were determined via physical examination, laboratory testing, or based on reports from patients and their families. The collected data included age, gender, seizure onset, seizure type and semiology, genetic mutations, age of PER initiation, duration of PER usage, PER maximum dose, the number of concomitant ASMs, past failed ASMs, PER efficacy, EEG, and magnetic resonance imaging findings.
Statistical analysis
Data were entered into Microsoft Excel version 20. A descriptive statistical analysis was conducted using the Statistical Package for the Social Science (SPSS) version 25 (IBM© Corp., Armonk, NY, USA). Measures of central tendency were calculated to describe quantitative variables, while frequencies and percentages were used for categorical variables. Person correlation was used to assess the relationship between the age and the dose with the drug efficacy. While independent t-test was used to assess the relationship between the age groups (≤8 and >8 years) with the drug efficacy. The drug retention probability curves were calculated by the Kaplan-Meier method. Confidence interval was set at 95% and P value were considered statistically significant at >0.05. Charts were created using GraphPad Prism version 5.01 for Windows (GraphPad Software Inc., San Diego, CA, USA; www.graphpad.com).
Results
Clinical characteristics
A total of 18 pediatric patients were included in this study. Gender distribution was as follows: nine boys and nine girls. All had been diagnosed with DS according to the diagnosis criteria established in the methodology section. The youngest patient was 4 years old, while the oldest patient was 15 years old and the median age of the participating patients was 10 years [interquartile range (IQR), 6.00–13.25 years]. The youngest age at which PER was initiated was at 1 year old for two patients. Moreover, three patients were started on PER at 13 years old, the latest among the study participants. The median age of PER initiation was 8 years (IQR, 4.75–10.75 years). Further individualized details on the patients, including their weight and seizure onset, are presented in Table 1. Most of the patients had two types of seizures (61.1%), followed by three types (22.2%). Among different seizure types, GTCS was the most frequently reported form and manifested in all participating patients. The youngest age of seizure onset was 4 months, while the oldest was at 3 years old. Surgical procedures were performed on six patients; and only one had diet modification (ketogenic diet). Patients’ characteristics are displayed in Table 2. Regarding the patients’ genetic background, whole exome sequencing (WES) testing revealed a mutation in the SCN1A gene in 94.4% with the remaining having a mutation in the PCDH19 gene. Heterozygosity was confirmed among the majority of the mutation carriers (61.1%). Detailed data on the pathogenic variant were presented in Table 3.
Table 1
| Case No. | Age (years)/sex | Weight (kg) | Age of seizure onset | Age at initiation of PER | Seizure types at PER introduction |
|---|---|---|---|---|---|
| 1 | 10/F | 28 | 3 years | 8 years | Focal febrile seizure, GTCS, recurrent status epilepticus |
| 2 | 13/M | 15 | 6 months | 9 years | Focal febrile seizure, GTCS |
| 3 | 14/F | 77 | 6 months | 1 year | Focal febrile seizure, GTCS |
| 4 | 6/F | 19 | 10 months | 5 years | Focal febrile seizure, GTCS |
| 5 | 12/M | 23 | 1 year | 10 years | Focal febrile seizure, GTCS, myoclonic seizure |
| 6 | 6/M | 23 | 4 months | 5 years | Focal febrile seizure, GTCS |
| 7 | 10/F | 39 | 8 months | 9 years | Focal febrile seizure, GTCS |
| 8 | 9/M | 25 | 6 months | 7 years | Complex febrile partial seizure, GTCS, myoclonic seizure, head drops |
| 9 | 11/M | 13 | 4 months | 9 years | GTCS, tonic seizure |
| 10 | 10/F | 28 | 1 year | 2 years | Focal febrile seizure, GTCS |
| 11 | 4/M | 13 | 4 months | 3 years | Focal febrile seizure, GTCS, myoclonic seizure |
| 12 | 15/M | 47 | 1 year | 13 years | Focal seizure, GTCS |
| 13 | 5/M | 18 | 6 months | 1 year | Drop attack, GTCS, myoclonic seizure |
| 14 | 5/F | 17 | 6 months | 4 years | GTCS |
| 15 | 8/F | 25 | 1 year | 6 years | Focal febrile seizure, GTCS |
| 16 | 15/F | 37 | 1 year | 13 years | GTCS |
| 17 | 15/F | 57 | 1 year | 13 years | Focal seizure, GTCS |
| 18 | 11/M | 27 | 6 months | 8 years | Focal febrile seizure, GTCS |
M, male; F, female; PER, perampanel; GTCS, generalized tonic-clonic seizure.
Table 2
| Variables | Mean | SD | Median | IQR |
|---|---|---|---|---|
| Age (years) | 9.94 | 3.670 | 10.00 | 6.00–13.25 |
| Age at initiation of PER (years) | 7.67 | 3.865 | 8.00 | 4.75–10.75 |
| Maximum dose of PER taken (mg) | 6.67 | 1.680 | 8.00 | 5.50–8.00 |
| % of drug efficacy | 29.17 | 29.368 | 25.00 | 0.00–50.00 |
PER, perampanel; SD, standard deviation; IQR, interquartile range.
Table 3
| Case No. | Gender | Age of onset | Pathogenic genetic mutation | Zygosity |
|---|---|---|---|---|
| 1 | Female | 3 years | SCN1A: NM001165963:exon16:c.3135delA:p.L1045fs | Heterozygous |
| 2 | Male | 6 months | SCN1A: C.680T>G p.lle227Ser Chr2 166909376 Exon 5 | N/A |
| 3 | Female | 6 months | SCN1A: NM_001165963.2:exon3–8:chr2:166903258-166913051del9793bp | Heterozygous |
| 4 | Female | 10 months | SCN1A | N/A |
| 5 | Male | 1 year | SCN1A: NM_001165963:exon16:c.2985T>G:p.F995L | Heterozygous |
| 6 | Male | 4 months | SCN1A: NM_001165963:exon24:c.4497delT:p.F1499fs | Heterozygous |
| 7 | Female | 8 months | SCN1A: NM_001165963:exon16:c.3225T>A:p.Y1075X | Heterozygous |
| 8 | Male | 6 months | SCN1A: NM_0011659631:exon :c.3867_3869del:p.F1289del chr2:166868628 | Heterozygous |
| 9 | Male | 4 months | SCN1A | N/A |
| 10 | Female | 1 years | SCN1A | N/A |
| 11 | Male | 4 months | SCN1A: NM_001165963.4:exon14:c.1852C>T:p.Arg618Cys | Heterozygous |
| 12 | Male | 1 year | SCN1A: NM_001165963.4:exon11:c.1177C>T:p.Arg393Cys | Heterozygous |
| 13 | Male | 6 months | SCN1A | N/A |
| 14 | Female | 6 months | SCN1A: NM_001165963:exon16:c.3091T>C:p.Y1031H | Heterozygous |
| 15 | Female | 1 year | SCN1A: NM_001165963:exon26:c.5010_5013del:p.L1670fs | Heterozygous |
| 16 | Female | 1 year | SCN1A | N/A |
| 17 | Female | 1 year | PCDH19: NM_001184880.1:exon1:c.464A>G:p.Asp155Gly | N/A |
| 18 | Male | 6 months | SCN1A: NM_0011659631:exon :c.5010_5013del:p.F1671Tfs | Heterozygous |
N/A, not available.
PER efficacy and concomitant ASMs
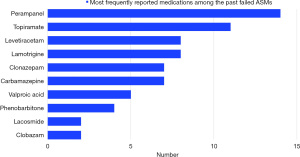
The mean efficacy of PER was 29.17%±29.368%, with only one patient with 100% efficacy to PER. The mean maximum dose of PER in milligrams per day (mg/day) was 6.67±1.680 mg. The used doses ranged from 4 to 8 mg. The mean duration in which the patients took PER in weeks was 37.22±48.35, with a patient (case No. 13) being the only one exceeding more than 52 weeks on PER (208 weeks) (Table 4). Five patients were currently on two or fewer ASMs. A single patient had the highest number of concomitant ASMs with a total of five medications, including lamotrigine, topiramate, clobazam, valproic acid, and stiripentol. The most commonly used concomitant ASM was valproic acid and clobazam (n=12) (Figure 2). Our patients took different ASMs in the past, but many of them failed to manage their symptoms. Six patients had two or fewer failed ASMs, while others had more than two failed medications. PER was the most frequently reported medication among the past failed ASMs (n=14).
Table 4
| Case No. | Age at initiation of PER (years) | Maximum dose of PER (mg) | Adverse effects of PER | Current ASMs used by the patient | Number of past failed ASMs used by the patient | Non-pharmacological intervention | PER efficacy (%) |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 4 | Sleepiness | Lamotrigine, topiramate, clobazam, valproic acid, stiripentol | Lacosamide, perampanel | No | 0 |
| 2 | 9 | 6 | Sleepiness | Valproic acid, zonisamide | Topiramate, levetiracetam, steroid, clonazepam, clobazam, rufinamide, lamotrigine, cannabinoid, perampanel | No | 50 |
| 3 | 1 | 8 | None | Levetiracetam, perampanel, clobazam, valproic acid | Topiramate, carbamazepine, lamotrigine, rufinamide | No | 50 |
| 4 | 5 | 4 | None | Valproic acid, phenobarbitone, clobazam | Perampanel, levetiracetam, topiramate | Surgery: vagal nerve stimulation | 0 |
| 5 | 10 | 6 | Drowsiness | Levetiracetam, valproic acid, topiramate | lamotrigine, perampanel | No | 0 |
| 6 | 5 | 8 | None | Perampanel, clobazam, levetiracetam, topiramate | Valproic acid | No | 100 |
| 7 | 9 | 6 | None | Valproic acid, clobazam | Perampanel, levetiracetam, Topiramate, lamotrigine, carbamazepine, phenobarbitone, clonazepam | No | 0 |
| 8 | 7 | 4 | Drowsiness Sleepiness | Clobazam, stiripentol | Perampanel, topiramate, carbamazepine, clonazepam, oxcarbazepine, valproic acid, levetiracetam, lamotrigine, ethosuximide, cannabinoid | Surgery: vagal nerve stimulation + corpus callosotomy | 0 |
| 9 | 9 | 6 | Drowsiness | Lacosamide | Perampanel, levetiracetam, valproic acid, clonazepam, clobazam, phenobarbitone | No | 30 |
| 10 | 2 | 8 | None | Levetiracetam, clobazam, topiramate | Valproic acid, carbamazepine, lamotrigine, phenobarbitone, perampanel | No | 50 |
| 11 | 3 | 8 | None | Perampanel, levetiracetam, valproic acid, lamotrigine | Lacosamide, phenobarbitone, topiramate, carbamazepine, prednisone, clonazepam | Diet modification: keto diet | 60 |
| 12 | 13 | 8 | None | Lacosamide, lamotrigine, clobazam | Perampanel, levetiracetam | Surgery: focal epilepsy surgery (focal cortical dysplasia) | 0 |
| 13 | 1 | 8 | None | Perampanel, valproic acid, phenobarbitone | Levetiracetam, clonazepam, Carbamazepine, topiramate | No | 50 |
| 14 | 4 | 4 | Severe drowsiness | Clobazam | Perampanel, valproic acid, phenytoin, topiramate | No | 60 |
| 15 | 6 | 8 | None | Clobazam, levetiracetam, valproic acid | Perampanel, topiramate | No | 25 |
| 16 | 13 | 8 | Drowsiness | Clobazam, levetiracetam, valproic acid | Perampanel, topiramate, lamotrigine | Surgery: vagal nerve stimulation | 25 |
| 17 | 13 | 8 | None | Topiramate, valproic acid, levetiracetam | Perampanel, carbamazepine | Surgery: temporal lobectomy (gliosis) | 25 |
| 18 | 8 | 8 | None | Clobazam, valproic acid, stiripentol | Perampanel, topiramate, levetiracetam, clonazepam, lamotrigine | Surgery: temporal lobectomy (gliosis): vagal nerve stimulation | 0 |
PER, perampanel; ASM, anti-seizure medication.
Adverse effects of PER
Among the patients, seven patients reported side effects after the administration of PER. These adverse effects included sleepiness (n=3) and drowsiness (n=5). The severity of those adverse effects were variables as demonstrated in Table 4. A single patient reported a severe form of drowsiness. Other patients reported no adverse effects after the administration of PER. Detailed data on each patient were presented in Table 4.
Bivariate analysis
When assessing the factors affecting the efficacy of PER, patient age had an inverse correlation, as patients younger in age had a higher efficacy rate (r=−0.383, P=0.11). Furthermore, when dividing the patients into two groups (≤8 years old; 6 patients, and >8 years old; 12 patients), the first group had a significantly higher efficacy rate when using PER than the second (49.17%±34.120% vs. 19.17%±21.829%, P=0.03). There was a positive correlation between the max dose and the efficacy of PER, but it was not significant (r=0.358, P=0.14). However, the only patient with 100% efficacy was one with the maximum drug dose (8 mg). The number of drug side effects was positively correlated with the drug efficacy. However, the relationship was not significant (r=−0.235, P=0.34). Nevertheless, the only patient with more than one side effect had an efficacy rate of 0% (Figure 3).

Discussion
In this study, we investigated the efficacy and tolerability of PER in patients with DS. Despite its rare prevalence, it manifests with interactable epilepsy that requires timely intervention and an accurate diagnosis (3). Next-generation sequencing (NGS), especially WES testing, aids personalized management strategies for patients and families. These advances in NGS are key in diagnosing and guiding treatment in current clinical practice (31). In the case of patients with DS, early and accurate diagnosis can lead to withholding specific ASMs that proven less effectivity among those patients, namely, carbamazepine, lamotrigine, phenytoin due to their inhibitory role on sodium channels (32). However, there are several more effective substitutes that includes levetiracetam, valproic acid, topiramate, clobazam, zonisamide, and stiripentol (33). However, some DS patients still have interactable seizures, and the causes remain unknown for some. Medication availability can also be an issue, with certain countries having limitations on drugs like clobazam and stiripentol. Previous studies found that around 80% of patients with DS have a mutation in the SCN1A gene, which is a subunit gene of the voltage-gated sodium channel (4) (Table 3). Speculating into the molecular level, evidence has been demonstrated that most of these mutations are paternally derived due to higher rates of mitoses during spermatogenesis than oogenesis (34). The mean age of our patients was 9.94±3.670 years (range, 4–15 years) (Table 2), and the average age in the studies by Yoshitomi et al. [2019] and Lin et al. [2018] was 11.5±2.2 years (range, 7–15 years) and 14.4±2.3 years (range, 12–17 years), respectively (28,30). Although this study comes to fill the gap of prior studies and to assess the efficacy among younger populations, further studies are required among younger age groups and toddlers that frequently exhibit refractory epilepsy (35-37). Seven of our patients (38.9%) presented with an efficacy of ≥50%. In other literature, percentages of favorable seizure reduction (≥50%) were observed among 80% of DS patients (28), While other studies included a single patient with DS (26,27), and two patients (25). Collectively, the efficacy rate was estimated at 66.7% of patients with DS. Other studies with a slightly higher number of participants showed that the efficacy rate was moderately reduced (62.5%) (30). Moreover, our results found younger patients showed a significantly higher PER efficacy rate compared to older ones. There have been varying views in the literature about the correlation, as Fernandes et al. [2021] noted a similar trend but encouraged further studies to confirm such findings (38), while Swiderska et al. [2017] and Hwang et al. [2020] noted no significant relationship between age and efficacy (27,39). A study conducted by Rohracher et al. [2018] found the contrary, in which the prevalence of patients who became seizure-free was observed among higher age groups (40). A summary of other studies was presented in Table 5. The most common concomitant ASMs are represented in Figure 2. A study by Goa et al. [2022] showed that early add-ons (defined as previously using two or fewer ASMs) had a greater responder rate than a late add-on. However, there was no statistical significance (41). Notable side effects including irritability with aggressiveness, loss of appetite and diplopia were reported (26). In a different study, a couple of patients developed suicidal thoughts after commencing PER, where these suicidal thoughts subsequently resolved after the withdrawal of PER in the two patients (27). Furthermore, some observation suggested an action pattern of “all-or-nothing” for PER. This description for such observation was set after noticing that if PER is effective in controlling one seizure type it will be effective in the control of other types (30). This study provides new evidence supporting the effectiveness of PER in reducing multiple forms of seizure in patients with DS. However, further research is needed to understand the variation in efficacy rates and individual responses to PER. This is the largest study conducted on PER’s effects in DS patients and the first in our region. It has limitations, including its retrospective design, small sample size, and limited prior literature for comparison. Efficacy reporting may also be influenced by other interventions and clinical observations, not solely PER.
Table 5
| Author | Study design | Patient response | Study date |
|---|---|---|---|
| Current study | Observational, retrospective | 7 pediatric patients with PER efficacy of >50% | 2023 |
| Nissenkorn et al. (24) | Observational, retrospective | 11 patients with PER efficacy of >50% (6 patients had >90% reduction in seizure) | 2023 |
| Chang et al. (29) | Observational, retrospective | 2 pediatric patients with PER efficacy of >50% | 2020 |
| Yoshitomi et al. (30) | Observational, retrospective | 5 pediatric patients with PER efficacy of >50% | 2019 |
| Lin et al. (28) | Observational, retrospective | 4 pediatric patients with PER efficacy of >50% (2 patients had >90% reduction in seizure) | 2018 |
| Swiderska et al. (27) | Prospective and retrospective | The study enrolled 1 pediatric patient with early discontinuation due to lack of seizure control | 2017 |
| De Liso et al. (26) | Observational, retrospective | 1 patient with PER efficacy of >50% | 2016 |
| Biró et al. (25) | Observational, retrospective | 1 patient with PER efficacy of >50% | 2015 |
PER, perampanel.
Conclusions
Our study aimed to assess the efficacy of PER among pediatric patients with DS. The results of our study revealed a significant relationship between the younger aged patients and the increase in the efficacy of PER. Moreover, other factors such as the dose given had some effect on the efficacy as well. In conclusion, this study presented evidence of promising therapeutic potentials for PER among some patients with DS, with data supporting the value of this treatment. However, additional studies are still required to confirm and verify the current findings. We recommend that a double blinded clinical trial with a control and an experimental group to be conducted in order to further support the current evidence on the use of PER to treat DS in pediatric population.
Acknowledgments
The abstract of this work has been presented as a poster at the 8th annual conference of the Saudi Pediatric Neurology Society.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-581/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-581/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-581/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Unit of Biomedical Ethics at the Faculty of Medicine at King Abdulaziz University approved this study (reference number: 244-23) on May 2, 2023. Informed consent was obtained from the parents or legal guardians of all patients. Informed consent was also obtained for off-label use of PER on the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522-30. [Crossref] [PubMed]
- Dravet C. The core Dravet syndrome phenotype. Epilepsia 2011;52:3-9. [Crossref] [PubMed]
- Anwar A, Saleem S, Patel UK, et al. Dravet Syndrome: An Overview. Cureus 2019;11:e5006. [PubMed]
- Harkin LA, McMahon JM, Iona X, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain 2007;130:843-52. [Crossref] [PubMed]
- Sugawara T, Mazaki-Miyazaki E, Fukushima K, et al. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology 2002;58:1122-4. [Crossref] [PubMed]
- Steel D, Symonds JD, Zuberi SM, et al. Dravet syndrome and its mimics: Beyond SCN1A. Epilepsia 2017;58:1807-16. [Crossref] [PubMed]
- Meng H, Xu HQ, Yu L, et al. The SCN1A mutation database: updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum Mutat 2015;36:573-80. [Crossref] [PubMed]
- Harkin LA, Bowser DN, Dibbens LM, et al. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet 2002;70:530-6. [Crossref] [PubMed]
- McIntosh AM, McMahon J, Dibbens LM, et al. Effects of vaccination on onset and outcome of Dravet syndrome: a retrospective study. Lancet Neurol 2010;9:592-8. [Crossref] [PubMed]
- Bender AC, Morse RP, Scott RC, et al. SCN1A mutations in Dravet syndrome: impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy Behav 2012;23:177-86. [Crossref] [PubMed]
- Hurst DL. Epidemiology of severe myoclonic epilepsy of infancy. Epilepsia 1990;31:397-400. [Crossref] [PubMed]
- Yakoub M, Dulac O, Jambaqué I, et al. Early diagnosis of severe myoclonic epilepsy in infancy. Brain Dev 1992;14:299-303. [Crossref] [PubMed]
- Wang YQ, Fang ZX, Zhang YW, et al. Efficacy of the ketogenic diet in patients with Dravet syndrome: A meta-analysis. Seizure 2020;81:36-42. [Crossref] [PubMed]
- Tong X, Deng Y, Liu L, et al. Clinical implementation of ketogenic diet in children with drug-resistant epilepsy: Advantages, disadvantages, and difficulties. Seizure 2022;99:75-81. [Crossref] [PubMed]
- Lattanzi S, Trinka E, Russo E, et al. Pharmacotherapy for Dravet Syndrome: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Drugs 2023;83:1409-24. [Crossref] [PubMed]
- Hanada T, Hashizume Y, Tokuhara N, et al. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 2011;52:1331-40. [Crossref] [PubMed]
- Food and Drug Administration (FDA). Fycompa® Prescribing Information [Internet]. [cited 2024 Jan 5]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202834s011lbl.pdf
- Agency EM. Fycompa® Annex I: Summary of Product Characteristics [Internet]. [cited 2024 Jan 5]. Available online: https://www.ema.europa.eu/en/documents/product-information/fycompa-epar-product-information_en.pdf
- De Liso P, Moavero R, Coppola G, et al. Current role of perampanel in pediatric epilepsy. Ital J Pediatr 2017;43:51. [Crossref] [PubMed]
- Matricardi S, Cesaroni E, Bonanni P, et al. Long-term effectiveness of add-on perampanel in patients with Lennox-Gastaut syndrome: A multicenter retrospective study. Epilepsia 2023;64:e98-e104. [Crossref] [PubMed]
- Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 2012;78:1408-15. [Crossref] [PubMed]
- French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 2013;54:117-25. [Crossref] [PubMed]
- French JA, Krauss GL, Biton V, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 2012;79:589-96. [Crossref] [PubMed]
- Nissenkorn A, Kluger G, Schubert-Bast S, et al. Perampanel as precision therapy in rare genetic epilepsies. Epilepsia 2023;64:866-74. [Crossref] [PubMed]
- Biró A, Stephani U, Tarallo T, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: first experiences. Neuropediatrics 2015;46:110-6. [Crossref] [PubMed]
- De Liso P, Vigevano F, Specchio N, et al. Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies-An Italian observational multicenter study. Epilepsy Res 2016;127:93-100. [Crossref] [PubMed]
- Swiderska N, Tan HJ, Rajai A, et al. Effectiveness and tolerability of Perampanel in children, adolescents and young adults with refractory epilepsy: A UK national multicentre study. Seizure 2017;52:63-70. [Crossref] [PubMed]
- Lin KL, Lin JJ, Chou ML, et al. Efficacy and tolerability of perampanel in children and adolescents with pharmacoresistant epilepsy: The first real-world evaluation in Asian pediatric neurology clinics. Epilepsy Behav 2018;85:188-94. [Crossref] [PubMed]
- Chang FM, Fan PC, Weng WC, et al. The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure 2020;75:82-6. [Crossref] [PubMed]
- Yoshitomi S, Takahashi Y, Yamaguchi T, et al. Efficacy and tolerability of perampanel in pediatric patients with Dravet syndrome. Epilepsy Res 2019;154:34-8. [Crossref] [PubMed]
- Dixon-Salazar TJ, Silhavy JL, Udpa N, et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med 2012;4:138ra78. [Crossref] [PubMed]
- Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res 2005;63:141-9. [Crossref] [PubMed]
- Samanta D. Changing Landscape of Dravet Syndrome Management: An Overview. Neuropediatrics 2020;51:135-45. [Crossref] [PubMed]
- Heron SE, Scheffer IE, Iona X, et al. De novo SCN1A mutations in Dravet syndrome and related epileptic encephalopathies are largely of paternal origin. J Med Genet 2010;47:137-41. [Crossref] [PubMed]
- Berg AT, Shinnar S, Levy SR, et al. Early development of intractable epilepsy in children: a prospective study. Neurology 2001;56:1445-52. [Crossref] [PubMed]
- Berg AT, Vickrey BG, Testa FM, et al. How long does it take for epilepsy to become intractable? A prospective investigation. Ann Neurol 2006;60:73-9. [Crossref] [PubMed]
- Geerts A, Arts WF, Stroink H, et al. Course and outcome of childhood epilepsy: a 15-year follow-up of the Dutch Study of Epilepsy in Childhood. Epilepsia 2010;51:1189-97. [Crossref] [PubMed]
- Fernandes M, Dainese F, Operto F, et al. Perampanel effectiveness and tolerability in patients with epilepsy at long-term follow-up. Epilepsy Behav 2021;121:108069. [Crossref] [PubMed]
- Hwang SK, Lee YJ, Nam SO, et al. Real-Life Effectiveness and Tolerability of Perampanel in Pediatric Patients Aged 4 Years or Older with Epilepsy: A Korean National Multicenter Study. J Clin Neurol 2020;16:53-9. [Crossref] [PubMed]
- Rohracher A, Zimmermann G, Villanueva V, et al. Perampanel in routine clinical use across Europe: Pooled, multicenter, observational data. Epilepsia 2018;59:1727-39. [Crossref] [PubMed]
- Gao L, Shi L, Liu Q. Effectiveness and tolerability of adjunctive perampanel in the treatment of pediatric patients with uncontrolled epilepsy: A retrospective, single-center, real-world study. Epilepsy Behav 2022;137:108961. [Crossref] [PubMed]


