
A case report of intrahepatic bile duct dilatation caused by WDR19 gene mutation and presented as Caroli syndrome
Highlight box
Key findings
• The present study reports that the child's intrahepatic bile duct dilatation and cirrhosis were considered as manifestations of Caroli syndrome, which is caused by mutations in the WDR19 gene.
What is known and what is new?
• Pathogenic mutations in the WDR19 gene have been associated with nephropathy.
• Although the cause of Caroli disease is unknown, some researchers believe that it is an autosomal recessive genetic disease related to mutations in the PKHD1 gene. But now pathogenic mutations in the WDR19 gene are also associated with Caroli syndrome or Caroli disease. We hypothesize that mutations in the WDR19 gene may contribute to the development of extrarenal phenotypes such as Caroli disease or syndrome.
What is the implication, and what should change now?
• The application of whole exome sequencing holds significant value in the diagnosis of liver diseases with unknown etiology. Currently, the patient presents with cirrhosis and abnormal hepatic function, necessitating future liver transplantation treatment.
Introduction
Congenital intrahepatic biliary dilatation (Caroli disease), a rare congenital biliary disorder, is characterized by segmental and cystic dilation of nonobstructive intrahepatic bile ducts in the absence of other liver abnormalities. Whereas Caroli syndrome represents marked dilation of the bile ducts with congenital hepatic fibrosis (1,2). Although the clinical manifestations of both are different, they usually display symptoms of fever, cholangitis, jaundice, itchy skin, abdominal pain, pancreatitis, weight loss, vomiting, and diarrhea. In the presence of fibrosis or cirrhosis, portal pressure may also increase (3-5).
Although the cause of Caroli disease is unknown, some researchers believe that it is an autosomal recessive genetic disease related to mutations in the PKHD1 gene, which affects the fibrocystic protein expressed in multiple organ systems, including renal tubular and hepatic bile duct cells. Mutations in the PKHD1 gene can lead to fibrocystin abnormalities causing fibrocystic changes in the kidneys and liver. Hence, Caroli disease is often accompanied by autosomal recessive polycystic kidney disease (ARPKD). However, some studies have also reported Caroli disease to be accompanied by autosomal dominant polycystic kidney disease (1). Another study performed exome sequencing in patients with nephronophthisis and identified Caroli disease/syndrome to be a predominant extrarenal phenotype associated with WDR19 mutations (6). The WDR19 gene encodes a protein required for ciliary retro transport (7). Pathogenic mutations in the WDR19 gene are associated with nephronophthisis (NPHP), an autosomal recessive cystic kidney disease, typically progressing to end-stage renal disease (ESKD). Here, we described a case of intrahepatic bile duct dilatation caused by a WDR19 gene mutation, which was manifested as Caroli syndrome. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-23-574/rc).
Case presentation
On August 1, 2022, a 1-year-old boy was admitted to the hospital due to the “discovery of intrahepatic bile duct dilatation for more than 4 months”. On March 15, 2022, the child came to our hospital for a follow-up of liver disease. We performed a liver function test, and the results were as follows: alanine aminotransferase (ALT), 25 U/L; aspartate aminotransferase (AST), 109 U/L; total bile acid, 106.9 µmol/L; total bilirubin (TB), 66.2 µmol/L; direct bilirubin (DB), 60.7 µmol/L; γ-glutamyl transpeptidase (γ-GT), 242 U/L. The liver ultrasound showed substantial lesions at the first hilar of the liver, thickened liver parenchymal spot, intrahepatic bile duct dilatation, enhanced Glisson system echo, and intrahepatic cystic lesions. The child was continued under observation without any treatment. After 3 months (June 30, 2022), the patient came to our hospital for further consultation. The liver ultrasound revealed hepatomegaly with diffuse hyperechogenicity and splenomegaly. Additionally, there was dilation of the left hepatic duct and an intrahepatic cystic lesion. Although the child displayed no fever, cough, vomiting, diarrhea, or other discomforts, he developed liver cirrhosis, splenomegaly, and intrahepatic bile duct dilation for unexplained reasons. Therefore, for further diagnosis and treatment of “intrahepatic bile duct dilation”, the patient was admitted to the hospital.
Previous history
At the age of 2 months and 28 days, the child was hospitalized in our department due to “jaundice and abnormal liver function for 2 months”. Oral medication included ursodeoxycholic acid capsules, bicyclol, and compound glycyrrhizin tablets. The patient was followed up in our hospital many times after discharge. After 2 months, only bicyclol and compound glycyrrhizin tablets were continued while ursodeoxycholic acid was stopped, and after three months, all of the medications were discontinued. Also, the child had a medical history of polydactyly on the left foot. The physical examination revealed that the liver was positioned 2.5 cm below the right rib, exhibiting moderate quality. The spleen was found to be at the level of the umbilicus, while there were six toes present on the left foot.
Auxiliary examination
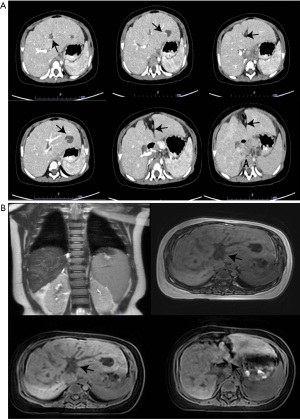
White blood cell count, 11.75×109/L; neutrophils (%), 33.2%; lymphocytes (%), 54.6%; monocytes (%), 10.6%; hemoglobin, 136.0 g/L; red blood cell count, 5.51×1012/L; platelet count, 127.0×109/L. Liver and renal function tests included the following items: creatinine, 25 µmol/L; AST, 52 U/L; uric acid, 149.0 µmol/L; γ-GT, 79 U/L; pyruvate, 46.1 µmol/L. Blood ammonia, 69 µmol/L; urine protein, 1+; virus full set of anti-CMV-IgG, 673.48 AU/mL. The procalcitonin, stool routine, lactic acid, and alpha-fetoprotein (AFP) were found to be normal. Also, ceruloplasmin was 0.435 g/L, the virus full set, the immune full set, the autoimmune hepatitis combination, and hepatitis B surface antigen quantitative tests were negative. Hepatitis C and human immunodeficiency virus (HIV) antibodies (quantitative) were also negative. CT-hepatic portal venography was performed on August 5, 2022, which showed liver cirrhosis, splenomegaly, and abnormally enhancing nodules in the lower segment of the right posterior lobe of the liver, which could be differentiated from focal hyperplastic nodules or atypical tumors (Figure 1A). Magnetic resonance-cholangiography (MRCP) (plain scan + water imaging) showed a slightly dilated intrahepatic bile duct, liver cirrhosis, and splenomegaly, with slightly scattered long T2 signal, most of which was distributed along the Glisson’s sheath, revealing the possibility of inflammatory lesions. The abnormal signals in the right posterior lobe of the liver indicated tumor lesions, while the abnormal signals in the left lobe of the liver suggested cysts or intrahepatic bile ducts, with the possibility of localized cystic dilatation. Moreover, lymphatic hyperplasia and enlargement were observed in the porta hepatis and the space between the liver and stomach (Figure 1B). Cardiac ultrasound revealed a left-to-right shunt at the atrial level, while kidney ultrasound showed slight hydrops in the right kidney.
Diagnosis and treatment process
A discussion was conducted on cirrhosis and bile duct dilatation in children. Abnormally enhanced nodules were observed in the lower segment of the right posterior lobe of the liver, most of which were considered neoplastic lesions, thus, a biopsy was recommended. Low-density lesions in the left lobe of the liver indicated cysts or cystic dilatation of the intrahepatic bile duct. A slightly dilated intrahepatic bile duct and increased lymph nodes in the hilar and liver-gastric space suggested splenomegaly, which led to the recommendation of a needle biopsy. Since the child was young, we needed to consider preoperative anesthesia and operability. Although the nature of the solid lesion in the right lobe of the liver remained unknown, the possibility of a tumor could not be ruled out, considering focal nodular hyperplasia. However, the general condition of the child was good. We communicated with the family members of the child and transferred him to the surgical treatment department. On August 17, 2022, laparoscopic liver tumor resection and liver tissue biopsy were performed in the Pediatric Surgery Department. After the operation, the child, under stable conditions, was transferred to our department for further treatment. First, various indicators were rechecked, which were as follows: white blood cell count, 3.73×109/L; neutrophils, 0.71×109/L; lymphocytes, 2.50×109/L; hemoglobin, 116.0 g/L; platelet count, 165.0×109/L; creatine kinase, 36 U/L; ALT, 15 U/L; AST, 26 U/L; γ-GT, 48 U/L; total bile acid, 2.5 µmol/L; lactic acid dehydrogenase, 263 U/L. The liver pathological results are showed in Figure 2A. All gray-yellow nodules in the liver were taken for examination and observed microscopically, which showed liver cell swelling, hydropic degeneration, and occasional hepatic cell punctate necrosis. The portal area showed fibrous tissue hyperplasia along with the local pseudolobular formation and a large number of small bile duct hyperplasia, as well as, lymphocyte infiltration, which was consistent with liver cirrhosis. Also, atypical hyperplasia of liver cells was observed in the small focal area. Electron microscopy of the liver tissue showed non-specific inflammatory damage (Figure 2B). Furthermore, we sequenced the whole genome exons (Figure 3) and found two heterozygous mutations in the WDR19 gene, namely c.2290delC (p.Q764Nfs*29) and c.2401G>C (p.G801R). One of which was derived from the father and the other from the mother. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent has been obtained from the parents of the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The patient’s condition was stable, and he was discharged from the hospital. After the discharge, outpatient follow-up visits were performed regularly. The patient’s cirrhosis has deteriorated compared to previous assessments, and there is evidence of abnormal liver function. Consequently, a liver transplant will be required at a later stage.


Discussion
The Caroli syndrome is linked to autosomal recessive polycystic kidney disease caused by pathogenic variants in the PKHD1 gene, which encodes fibrocystin, a large integral membrane protein primarily expressed the kidneys and lower level in the liver (8). Pathogenic mutations in PKD1 or PKD2 are associated with autosomal dominant polycystic kidney disease, occasionally accompanied by Caroli’s disease (9). Mutations in the WDR19 gene cause nephronophthisis that often leads to ESKD and Caroli syndrome or disease. NPHP is caused by mutations in a variety of genes encoding the proteins involved in the function of fibrils, basal bodies, and centrosomes, further causing renal disease and extrarenal manifestations, including retinal degeneration, cerebellar ataxia, and hepatic fibrosis (10). The causative gene for about 70% of NPHP cases can be found through molecular genetic screening. Halbritter et al. performed whole exome sequencing of patients clinically diagnosed with NPHP and combined it with genetic testing. To date, more than 13 NPHP genes are implied, which account for only about 40% of all cases, including NPHP1, INVS/NPHP2, NPHP3, NPHP4, IQCB1/NPHP5, CEP290/NPHP6, GLIS2/NPHP7, RPGRIP1L/NPHP8, NEK8/NPHP9, SDCCAG8/NPHP10, TMEM67/NPHP11, TTC21B/NPHP12, and WDR19/NPHP13 (11). The NPHP13 gene is also called the WRD19, SRDT5, ATD5, or CED4 gene, and it encodes the IFT144 protein. This gene mutation is present in patients with simple NPHP, NPHP with liver fibrosis, Senior-Loke syndrome, Caroli disease/syndrome, Sensenbrenner syndrome, Joubert syndrome, and Jeune syndrome (6,11-14).
Although the exact mechanism of how multiple NPHP gene defects cause kidney disease is unknown, ciliary dysfunction remains a common mechanism proposed so far. This hypothesis is based on the association between most of the proteins and various human cystic diseases located in the fibrils, basal bodies, and centrosomes, including the nephrocystin protein (15-20). Nephrocystins interact with each other and also with other proteins involved in the cell-cell and cell-matrix signaling (e.g., tensin, filamin, and tubulin) (19,21,22). Some scholars believe that the mutations in the NPHP genes change the functioning of cilia through the defects in the intracellular signaling pathway, resulting in ciliary mechanoreceptors not being able to accurately sense the fluid velocity in the renal cyst cavity. Multiple gene mutations have been found in the patients with NPHP, with their protein products expressed in the primary cilia, basal bodies, and centrosomes. These gene products may play roles in the cell-cell and cell-matrix signaling involved in the sensing of intratubular fluid velocity by ciliary mechanoreceptors (23,24). As abnormal kidney cells fail to sense fluid flow and try to compensate, the genetic defects cause uncontrolled tissue growth and cyst formation (25). The underlying liver disease in ciliopathies is manifested as congenital hepatic fibrosis, Caroli disease, and polycystic liver disease. Targeted exome sequencing (TES) was used to identify a series of nph13 cases, all of which were presented with Caroli syndrome or Caroli disease and characterized by focally dilated intrahepatic bile ducts of the liver associated with or without congenital hepatic fibrosis, respectively. Some clinical cases of nph13 were also reported in four studies (6,7,10,11). Comprehensive analysis showed that 13 of the 27 NPHP patients had liver disease while nine exhibited Caroli disease or syndrome. Since Caroli disease or syndrome may be another major phenotype associated with WDR19 mutation, screening for the presence of intrahepatic ductal dilatation may be helpful for the diagnosis of NPHP patients.
Caroli disease or syndrome may be the predominant extrarenal phenotype associated with the WDR19 gene mutation (6). During the follow-up of liver disease in our case, the intrahepatic bile duct was significantly dilated, accompanied by cirrhosis and splenomegaly. Therefore, we considered the diagnosis as Caroli syndrome. The diagnosis of Caroli syndrome does not require a liver biopsy, which is also risky in the case of a cystically dilated biliary system. However, if the patient is presented with an enlarged, firm liver with features of portal hypertension and without biliary dilatation or renal disease, histologic analysis on liver biopsy may be helpful to support the diagnosis. The final genetic results revealed that our patient’s disease was not related to the Caroli syndrome gene, but rather an extrarenal manifestation of Caroli syndrome in nephropathy caused by WDR19 mutations. Mutations in these genes led to extrarenal manifestations of NPHP, including the Caroli disease or Caroli syndrome. Furthermore, Caroli disease, a rare genetic disorder characterized by intrahepatic bile duct dilation, was found to be manifested by two other patients with homozygous WDR19 mutation (11).
Conclusions
Although the exact mechanism of how the WDR19 gene defects cause Caroli disease or Caroli syndrome is unknown, ciliary dysfunction remains a common mechanism proposed so far. Also, no specific treatment is available for NPHP. In children with early NPHP and intact renal function, supportive care is given, focusing on maintaining fluid and electrolyte balance, treating anemia, and promoting normal growth. The optimal management in the advanced stages of the disease may entail the consideration of organ transplantation.
Acknowledgments
We are grateful to the patient and his family for their contributions to this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-23-574/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-574/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-574/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent has been obtained from the parents of the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Umar J, Kudaravalli P, John S. Caroli Disease. Treasure Island (FL): StatPearls Publishing; 2022.
- Acevedo E, Laínez SS, Cáceres Cano PA, et al. Caroli's Syndrome: An Early Presentation. Cureus 2020;12:e11029. [PubMed]
- Ulrich F, Pratschke J, Pascher A, et al. Long-term outcome of liver resection and transplantation for Caroli disease and syndrome. Ann Surg 2008;247:357-64. [Crossref] [PubMed]
- Kassahun WT, Kahn T, Wittekind C, et al. Caroli's disease: liver resection and liver transplantation. Experience in 33 patients. Surgery 2005;138:888-98. [Crossref] [PubMed]
- Mabrut JY, Partensky C, Jaeck D, et al. Congenital intrahepatic bile duct dilatation is a potentially curable disease: long-term results of a multi-institutional study. Ann Surg 2007;246:236-45. [Crossref] [PubMed]
- Lee JM, Ahn YH, Kang HG, et al. Nephronophthisis 13: implications of its association with Caroli disease and altered intracellular localization of WDR19 in the kidney. Pediatr Nephrol 2015;30:1451-8. [Crossref] [PubMed]
- Yoshikawa T, Kamei K, Nagata H, et al. Diversity of renal phenotypes in patients with WDR19 mutations: Two case reports. Nephrology (Carlton) 2017;22:566-71. [Crossref] [PubMed]
- Burgmaier K, Broekaert IJ, Liebau MC. Autosomal Recessive Polycystic Kidney Disease: Diagnosis, Prognosis, and Management. Adv Kidney Dis Health 2023;30:468-76. [Crossref] [PubMed]
- Yang H, Sieben CJ, Schauer RS, et al. Genetic Spectrum of Polycystic Kidney and Liver Diseases and the Resulting Phenotypes. Adv Kidney Dis Health 2023;30:397-406. [Crossref] [PubMed]
- Wolf MT. Nephronophthisis and related syndromes. Curr Opin Pediatr 2015;27:201-11. [Crossref] [PubMed]
- Halbritter J, Porath JD, Diaz KA, et al. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 2013;132:865-84. [Crossref] [PubMed]
- Bredrup C, Saunier S, Oud MM, et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet 2011;89:634-43. [Crossref] [PubMed]
- Coussa RG, Otto EA, Gee HY, et al. WDR19: an ancient, retrograde, intraflagellar ciliary protein is mutated in autosomal recessive retinitis pigmentosa and in Senior-Loken syndrome. Clin Genet 2013;84:150-9. [Crossref] [PubMed]
- Fehrenbach H, Decker C, Eisenberger T, et al. Mutations in WDR19 encoding the intraflagellar transport component IFT144 cause a broad spectrum of ciliopathies. Pediatr Nephrol 2014;29:1451-6. [Crossref] [PubMed]
- Otto EA, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 2005;37:282-8. [Crossref] [PubMed]
- Sayer JA, Otto EA, O'Toole JF, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 2006;38:674-81. [Crossref] [PubMed]
- Fliegauf M, Horvath J, von Schnakenburg C, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol 2006;17:2424-33. [Crossref] [PubMed]
- Nürnberger J, Kribben A, Opazo Saez A, et al. The Invs gene encodes a microtubule-associated protein. J Am Soc Nephrol 2004;15:1700-10. [Crossref] [PubMed]
- Mollet G, Silbermann F, Delous M, et al. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet 2005;14:645-56. [Crossref] [PubMed]
- Wolf MTF, Bonsib SM, Larsen CP, et al. Nephronophthisis: a pathological and genetic perspective. Pediatr Nephrol 2023; Epub ahead of print. [Crossref] [PubMed]
- Mollet G, Salomon R, Gribouval O, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet 2002;32:300-5. [Crossref] [PubMed]
- Otto EA, Schermer B, Obara T, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 2003;34:413-20. [Crossref] [PubMed]
- Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol 2009;20:23-35. [Crossref] [PubMed]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007;8:880-93. [Crossref] [PubMed]
- Huber C, Cormier-Daire V. Ciliary disorder of the skeleton. Am J Med Genet C Semin Med Genet 2012;160C:165-74. [Crossref] [PubMed]


