
Advancing necrotizing enterocolitis prediction through iterative monitoring
Highlight box
Key findings
• This retrospective study, conducted at a single center over the past 7 years, identified “antenatal steroids, antenatal antibiotics, probiotics treatment before necrotizing enterocolitis (NEC), anion gap (AG, day 7), and mean corpuscular volume (MCV, day 7)” as independent risk factors for NEC. Moreover, the combination of these five indicators can predict NEC with reasonable accuracy.
What is known and what is new?
• The incidence of NEC in neonates ranges from 2% to 7%, with mortality rates reaching up to 21.9–38%. In neonatal clinical practice, early identification of NEC is crucial for the treatment and prognosis of premature infants.
• This study not only validates findings consistent with previous research but also highlights early elevated AG values as a risk factor for NEC development. Additionally, the data in this study are derived from multiple time-point repeated measurements, further enhancing the accuracy of the research.
What is the implication, and what should change now?
• We should take measures to improve the management and prevention strategies for preterm infants to reduce the incidence of NEC. This may include optimizing prenatal care measures, such as the judicious use of prenatal steroids and antibiotics, and standardizing the application of probiotic treatment. Additionally, dynamic monitoring of AG levels, along with other relevant indicators, is particularly crucial, as they could facilitate early identification and intervention of NEC.
Introduction
Background
Necrotizing enterocolitis (NEC) is a critical inflammatory bowel condition, particularly affecting preterm infants born before 32 weeks of gestation (1). Histologically, NEC involves the disruption of the intestinal epithelium and coagulative necrosis in portions of the ileum and colon (2). The incidence of NEC in neonates ranges from 2% to 7%, with mortality rates reaching up to 21.9–38% (3). Surviving infants often face poor growth, developmental issues, and gastrointestinal disorders like short bowel syndrome (4), placing a significant burden on families and society.
Rationale and knowledge gap
Understanding the mechanisms underlying NEC development remains challenging due to the nonspecific symptoms and diagnostic complexities. Infants diagnosed with NEC undergo cessation of enteral feeds, receive broad-spectrum antibiotics, and may require surgical interventions (5). Reported risk factors include prematurity, asphyxia, mechanical ventilation, patent ductus arteriosus (PDA), maternal gestational diabetes, intrahepatic cholestasis during pregnancy, preeclampsia, anemia, blood transfusion, formula feeding, antibiotic use, and infections (6-9). NEC’s multifaceted pathophysiology involves a complex interplay of maternal, neonatal, and therapeutic elements, making prediction challenging (10). Considering the rapid progression and nonspecific onset of NEC, early prediction and intervention are essential to prevent severe consequences such as necrosis, perforation, and total abdominal peritonitis.
Objective
Through a retrospective analysis of clinical data from premature infants in our center over the past 7 years, we aim to identify high-risk factors associated with NEC occurrence. Significant factors identified through univariate analysis will undergo multivariate logistic regression analysis to select relevant clinical indicators and construct a predictive model for NEC. This model aims to provide guidance for early clinical recognition and intervention. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-15/rc).
Methods
Study design
This retrospective case-control study enrolled preterm infants diagnosed with NEC in the Neonatology Department of the First Affiliated Hospital of Anhui Medical University between January 2016 and December 2023. Out of 1,679 preterm infants, 150 were diagnosed with NEC (Bell’s stage ≥ II). The control group consisted of 150 preterm infants without NEC, carefully matched for gestational age (GA) and year of birth. We enrolled participants who met the following criteria: (I) born with a GA less than 34 weeks; (II) born in the delivery room or obstetric operating room of the First Affiliated Hospital of Anhui Medical University and immediately transferred to the Neonatal Intensive Care Unit (NICU) after birth; (III) diagnosed with NEC within 30 days after birth. Participants meeting the following criteria were excluded from the study: (I) diagnosed with Bell’s stage I NEC; (II) significant abnormalities in vital organ development (such as congenital diaphragmatic hernia, anencephaly, Tetralogy of Fallot, etc.), or comorbid genetic/metabolic diseases; (III) incomplete clinical data; (IV) during the matching process, cases that died before the onset of NEC, using the time of NEC onset as the boundary, were not included in the study. This applies to both the NEC and non-NEC groups. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 2023-14-25) and individual consent for this retrospective analysis was waived.
Data collection
We collected clinical characteristics, including: (I) basic information and perinatal conditions of the patient; (II) information related to the mother’s perinatal period; (III) treatment measures before NEC; and (IV) auxiliary examination results, specifically relevant blood test parameters before NEC.
Statistical analysis
All clinical data were analyzed using SPSS statistical software (version 27; Chicago, USA). For quantitative data following a normal distribution, results are presented as mean ± standard deviation (SD) and were assessed using a correlation t-test. Non-normally distributed quantitative data were expressed as interquartile ranges (IQRs) and analyzed with the Wilcoxon signed-rank sum test. Categorical data were evaluated using Fisher’s exact test.
For data collected at multiple time points, such as laboratory results, Repeated Measures Analysis of Variance (RM-ANOVA) was employed and presented as mean ± SD. To address any violations of the sphericity assumption, Greenhouse-Geisser test was applied when necessary. The RM-ANOVA in this study is used to identify differences between two groups across multiple time points at an overall level. Significant indicators identified through RM-ANOVA were visually represented in bar graphs using GraphPad Prism 9. This graphical representation aimed to illustrate variations between groups at each specific time point.
Include significant indicators identified from univariate logistic regression analysis and RM-ANOVA in the multivariate logistic regression analysis, choosing relevant indicators as predictive factors for the model. In univariate logistic regression analysis, we applied the Bonferroni correction method to adjust for multiple testing. We conducted a total of 25 comparisons, considering P<0.002 as statistically significant. Subsequently, we utilized the R programming language to construct a nomogram. Participants in our study were divided into a training cohort (n=120) and a validation cohort (n=30), with internal validation conducted using bootstrap. Finally, calibration plots and ROC curves were employed to visually demonstrate the accuracy of the predictive model.
Results
General data analysis
No statistically significant differences were observed in gender, GA, birth weight (BW), mode of delivery (cesarean section), multiple births, in vitro fertilization (IVF), and Apgar scores at 1 and 5 minutes when comparing the NEC group with the non-NEC group (P>0.05) (see Table 1). Based on matching principles, this study’s consistent baseline data reaffirms its high matching quality, thus boosting its scientific validity and credibility.
Table 1
| Characteristics | NEC (n=150) | Non-NEC (n=150) | χ2/Z/t |
|---|---|---|---|
| Male | 78 (52.0) | 78 (52.0) | 0.000 |
| GA (week) | 30 [29–31] | 30 [29–31] | 0.897 |
| BW (g) | 1,351.28±277.75 | 1,338.30±316.18 | 0.913 |
| Caesarean section | 102 (68.0) | 112 (74.7) | 1.630 |
| Multiple births | 50 (33.3) | 45 (30.0) | 0.385 |
| IVF | 34 (22.7) | 26 (17.3) | 1.333 |
| 1-min Apgar score | 7 [6–8] | 7 [6–8] | 0.984 |
| 5-min Apgar score | 8 [7–9] | 9 [8–9] | 1.130 |
Values are presented as n (%), median [IQR] or mean ± SD. GA, gestational age; BW, birth weight; IVF, in vitro fertilization; NEC, necrotizing enterocolitis; IQR, interquartile range; SD, standard deviation.
Maternal and perinatal factors
Demographics of infants
We included a total of 150 matched cases. Of these infants (see Table 2), they had a median GA of 30 weeks with IQR of 29.00–31.75 weeks, had a median BW of 1,340 grams with an IQR of 1,180–1,520 grams. One hundred fifty-six (52.00%) infants were males, 214 (71.33%) were cesarean delivery, 95 (31.67%) were multiple births, 60 (20.00%) were assisted reproduction. The mothers of 62 (20.67%) infants were classified as advanced maternal age.
Table 2
| Characteristics | Values (N=300) | Univariate regression analysis | |
|---|---|---|---|
| Odds ratio (95% CI) | P value | ||
| Demographics | |||
| Male sex | 156 (52.00) | 0.88 (0.34, 2.27) | 0.79 |
| GA (week) | 30.00 [29.00, 31.75] | 0.91 (0.80, 1.05) | 0.19 |
| BW (g) | 1,340 [1,180, 1,520] | 1.00 (1.00, 1.00) | 0.23 |
| Cesarean delivery | 214 (71.33) | 1.28 (0.42, 3.88) | 0.67 |
| Multiple births | 95 (31.67) | 1.01 (0.26, 3.88) | 0.99 |
| IVF | 60 (20.00) | 1.22 (0.34, 4.42) | 0.77 |
| Perinatal characteristics | |||
| Elderly maternal | 62 (20.67) | 0.76 (0.22, 2.64) | 0.67 |
| Hypertensive disorder complicating pregnancy | 79 (26.33) | 2.21 (0.95, 3.12) | 0.06 |
| GDM | 72 (24.00) | 0.64 (0.21, 2.00) | 0.44 |
| Antenatal vaginal bleeding | 64 (21.33) | 0.35 (0.11, 1.17) | 0.09 |
| Antenatal magnesium sulfate | 83 (27.67) | 0.43 (0.13, 1.38) | 0.16 |
| Antenatal steroids | 178 (59.33) | 2.88 (1.51, 3.23) | <0.001* |
| PROM >18 hours | 104 (34.67) | 1.53 (0.34, 7.01) | 0.04 |
| Antenatal antibiotics | 126 (42.00) | 2.91 (1.17, 3.67) | 0.002* |
| 1-min Apgar score | 7 [6, 8] | 1.09 (0.74, 1.62) | 0.66 |
| 5-min Apgar score | 9 [8, 10] | 0.69 (0.43, 1.11) | 0.13 |
| Pulmonary surfactant treatmenta | 192 (64.00) | 3.20 (0.97, 10.51) | 0.04 |
| Age of starting enteral feeding (day) | 3 [2, 4] | 0.96 (0.62, 1.48) | 0.84 |
| Probiotics treatmenta | 163 (54.33) | 0.28 (0.10, 0.84) | 0.001* |
| PDAa | 71 (23.67) | 0.88 (0.24, 3.20) | 0.85 |
| Erythrocyte transfusiona (mL/kg) | 0 [0, 0] | 0.95 (0.91, 0.98) | 0.003* |
| Plasma transfusiona (mL/kg) | 0 [0, 15] | 1.08 (1.04, 1.13) | <0.001* |
| EOS | 7 (2.33) | 0.16 (0.04, 6.09) | 0.33 |
| PHb | 7.27 [7.23, 7.35] | 0.33 (0.01, 1.28) | 0.71 |
| BEb | –4 [–6, –2] | 0.91 (0.81, 1.02) | 0.11 |
Values are presented as n (%) or median [IQR]. a, all events preceded the onset of NEC; b, all first postnatal measurements; *, P≤0.003. NEC, necrotizing enterocolitis; GA, gestational age; BW, birth weight; IVF, in vitro fertilization; Elderly maternal, mother’s age ≥35 years old; GDM, gestational diabetes mellitus; PROM, premature rupture of membranes; PDA, patent ductus arteriosus; EOS, early onset sepsis; PH, potential of hydrogen; BE, base excess; CI, confidence interval; IQR, interquartile range.
Key factors and treatments in preterm infants
We conducted univariate logistic regression analysis on potential factors associated with NEC, including maternal and infant clinical characteristics, as well as treatments received by infants postnatally. The results indicated statistically significant associations with the following indicators: antenatal steroids, premature rupture of membranes (PROM) >18 hours, antenatal antibiotics, pulmonary surfactant (PS) treatment, probiotics treatment, erythrocyte transfusion, and plasma transfusion. However, after Bonferroni correction, we concluded that there may be potential correlations between antenatal steroids [95% confidence interval (CI): 1.51–3.23], antenatal antibiotics (95% CI: 1.17–3.67), and probiotics treatment (95% CI: 0.10–0.84) with NEC.
Laboratory indicators
We compared the auxiliary examination indicators at specific time points between the two groups using RM-ANOVA, with a focus on identifying indicators that exhibited inter-group differences across multiple time points. For hematological parameters, we investigated three time points (day 1, day 7, and day NEC). Due to limitations at our center, hepatic function indicators were only studied on the 7th day after birth and on the day of NEC onset.
Among the laboratory indicators, we observed inter-group variations in overall levels of anion gap (AG), lymphocyte count, mean corpuscular volume (MCV), red cell distribution width (RDW), RDW to platelet ratio (RPR), and eosinophil count at various time points (see Table 3). The MCV exhibited statistically significant differences within 24 hours after birth. AG, MCV, RDW, and RPR showed statistical distinctions on the 7th postnatal day. AG, lymphocyte count, MCV, RDW, and eosinophil count demonstrated statistical differences on the day of NEC onset (see Figure 1). Subsequently, logistic regression analysis was used to determine the predictive sensitivity of these hematological indicators and their corresponding cut-off values (see Table 4).
Table 3
| Characteristics | NEC (n=150) | Non-NEC (n=150) | F |
|---|---|---|---|
| AG (mmol/L) | 5.886* | ||
| Day 1 | 10.82±3.62 | 10.96±4.00 | 1.357 |
| Day 7 | 13.15±3.61 | 11.75±3.46 | 3.432* |
| Day NEC | 13.37±3.86 | 10.57±3.17 | 13.574*** |
| Lymphocyte count (109/L) | 14.185*** | ||
| Day 1 | 4.15±1.79 | 4.05±2.01 | 0.012 |
| Day 7 | 3.66±1.22 | 3.78±1.29 | 1.582 |
| Day NEC | 2.87±1.61 | 4.06±1.23 | 59.745*** |
| HB (g/L) | 0.131 | ||
| Day 1 | 159.72±21.24 | 165.51±21.18 | 0.687 |
| Day 7 | 128.72±23.49 | 134.98±22.48 | 0.548 |
| Day NEC | 119.27±23.02 | 118.49±18.54 | 2.154 |
| RBC (1012/L) | 0.479 | ||
| Day 1 | 4.27±0.65 | 4.22±0.63 | 2.188 |
| Day 7 | 3.68±0.71 | 4.78±1.76 | 0.772 |
| Day NEC | 3.47±0.62 | 3.34±0.58 | 5.882* |
| MCV (fL) | 10.982*** | ||
| Day 1 | 117.62±7.18 | 114.40±7.22 | 5.875* |
| Day 7 | 112.61±7.59 | 108.96±6.21 | 9.764** |
| Day NEC | 108.39±5.92 | 105.40±6.11 | 8.512* |
| MPV (fL) | 0.352 | ||
| Day 1 | 10.31±0.81 | 10.25±0.67 | 0.157 |
| Day 7 | 11.28±1.96 | 11.73±0.85 | 0.275 |
| Day NEC | 11.99±1.02 | 12.01±0.91 | 2.986 |
| PDW (fL) | 0.296 | ||
| Day 1 | 11.33±1.61 | 11.10±1.36 | 0.774 |
| Day 7 | 15.35±3.67 | 14.90±2.93 | 0.441 |
| Day NEC | 15.21±3.15 | 15.77±3.04 | 1.313 |
| RDW-SD (fL) | 7.857** | ||
| Day 1 | 16.29±1.12 | 16.56±1.37 | 1.872 |
| Day 7 | 16.01±1.59 | 16.69±1.69 | 6.692* |
| Day NEC | 16.23±1.52 | 16.76±1.87 | 9.732** |
| PLT (109/L) | 3.976 | ||
| Day 1 | 233.59±59.81 | 216.9±58.83 | 5.121* |
| Day 7 | 242.15±81.23 | 225.19±87.63 | 4.875* |
| Day NEC | 269.12±89.13 | 258.41±101.38 | 1.152 |
| RPR | 8.676** | ||
| Day 1 | 0.08±0.04 | 0.08±0.03 | 3.941 |
| Day 7 | 0.07±0.05 | 0.09±0.05 | 8.624** |
| Day NEC | 0.07±0.06 | 0.08±0.07 | 2.912 |
| Eosinophil count (109/L) | 6.498* | ||
| Day 1 | 0.15±0.17 | 0.15±0.13 | 0.798 |
| Day 7 | 0.45±0.48 | 0.40±0.33 | 0.377 |
| Day NEC | 0.21±0.39 | 0.46±0.37 | 19.151*** |
| Eosinophil percentage (%) | 3.188 | ||
| Day 1 | 1.78±1.37 | 1.70±1.53 | 0.028 |
| Day 7 | 4.67±3.50 | 4.11±2.89 | 1.579 |
| Day NEC | 3.05±3.94 | 4.87±3.73 | 13.175*** |
| TBA (ìmol/L) | 0.896 | ||
| Day 7 | 13.15±8.25 | 14.11±7.61 | 1.877 |
| Day NEC | 16.12±14.59 | 17.67±12.56 | 0.128 |
| DB (ìmol/L) | 0.433 | ||
| Day 7 | 11.25±3.49 | 12.21±4.51 | 0.523 |
| Day NEC | 13.85±3.99 | 13.94±6.37 | 0.196 |
| DB to TB ratio | 0.197 | ||
| Day 7 | 0.15±0.05 | 0.12±0.11 | 3.132 |
| Day NEC | 0.29±0.12 | 0.20±0.17 | 1.531 |
| Pre-albumin (mg/L) | 3.180 | ||
| Day 7 | 82.15±28.77 | 76.92±28.03 | 3.596 |
| Day NEC | 85.18±33.33 | 80.04±28.35 | 1.770 |
| ALB (g/L) | 0.490 | ||
| Day 7 | 31.17±3.25 | 32.12±3.40 | 1.441 |
| Day NEC | 32.29±4.17 | 32.45±3.02 | 0.255 |
Values are presented as mean ± SD. *, P<0.05; **, P<0.01; ***, P<0.001. AG, anion gap; HB, hemoglobin; RBC, red blood cell; MCV, mean corpuscular volume; MPV, mean platelet volume; PDW, platelet distribution width; RDW-SD, red cell distribution width standard deviation; PLT, platelet; RPR, RDW to PLT ratio; TBA, total bile acid; DB, direct bilirubin; TB, total bilirubin; ALB, albumin; NEC, necrotizing enterocolitis; SD, standard deviation.

Table 4
| Characteristics | Cut-off value | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|
| AG (day 7) (mmol/L) | >11.82 | 61 | 65 | 0.648 (0.582, 0.701) |
| AG (day NEC) (mmol/L) | >12.06 | 65 | 74 | 0.682 (0.581, 0.717) |
| Lymphocyte count (day NEC) (109/L) | <3.12 | 71 | 79 | 0.762 (0.712, 0.834) |
| MCV (day 1) (fL) | >121.25 | 58 | 78 | 0.642 (0.551, 0.722) |
| MCV (day 7) (fL) | >116.50 | 49 | 80 | 0.660 (0.552, 0.731) |
| MCV (day NEC) (fL) | >102.30 | 75 | 62 | 0.582 (0.501, 0.657) |
| RDW-SD (day 7) (fL) | <16.55 | 64 | 57 | 0.624 (0.518, 0.697) |
| RDW-SD (day NEC) (fL) | <16.17 | 59 | 70 | 0.633 (0.543, 0.746) |
| RPR (day 7) | <0.08 | 71 | 47 | 0.588 (0.519, 0.672) |
| Eosinophil count (day NEC) (109/L) | <0.17 | 67 | 85 | 0.755 (0.682, 0.821) |
AG, anion gap; NEC, necrotizing enterocolitis; MCV, mean corpuscular volume; RDW-SD, red cell distribution width standard deviation; RPR, RDW to platelet ratio; AUC, area under the curve; CI, confidence interval.
Prediction model for NEC
Firstly, we included the above statistically significant factors in a multivariate logistic regression analysis (see Table 5). The results showed that the combined indicators of “antenatal steroids, antenatal antibiotics, probiotics treatment, AG day 7, MCV day 7” had a good fit for predicting NEC.
Table 5
| Variables | β | S.E. | Wald | P |
|---|---|---|---|---|
| Antenatal steroids | 1.703 | 0.337 | 5.051 | <0.001* |
| Antenatal antibiotics | 0.948 | 0.337 | 2.816 | 0.005* |
| Probiotics | −0.542 | 0.323 | −1.683 | 0.04* |
| AG day 7 | 0.094 | 0.047 | 2.004 | 0.045* |
| MCV day 1 | 0.050 | 0.030 | 1.634 | 0.10 |
| MCV day 7 | 0.069 | 0.032 | 2.157 | 0.03* |
| RDW day 7 | −0.209 | 0.135 | −1.552 | 0.12 |
| PLT day 1 | 0.000 | 0.003 | 0.053 | 0.96 |
| PLT day 7 | 0.000 | 0.004 | 0.082 | 0.93 |
| RPR day 7 | −8.475 | 8.330 | −1.022 | 0.31 |
*, P<0.05. β (Beta), regression coefficient; S.E. (standard error), standard error of the regression coefficient; AG, anion gap; MCV, mean corpuscular volume; RDW, red cell distribution width; PLT, platelet; RPR, RDW to platelet ratio.
Secondly, out of 150 matched cases, we randomly selected 120 cases as the training cohort and 30 cases as the validation cohort. We used the data from the training cohort to construct a nomogram for NEC prediction (see Figure 2). The C-index for the training and validation cohorts was 0.835 and 0.848 respectively, indicating good predictive performance (see Figure 3A,3B).


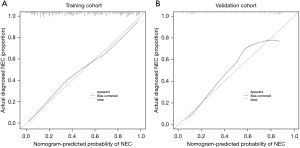
Lastly, to reduce over-fitting bias, the training cohort was calibrated using 1,000 bootstrapped samples (see Figure 4). The calibration plot also showed a high consistency between the predicted probability of NEC and the actual observed values, indicating good calibration of the model.
Discussion
NEC is a severe gastrointestinal disorder affecting premature infants, with an incidence ranging from 7% to 11% and a mortality rate of 10% to 30%, particularly when surgical intervention is necessary (11). Clinical guidelines for NEC, developed worldwide, provide different levels of evidence-based recommendations for its prevention, diagnosis, and treatment. Many studies usually collect auxiliary examination data for NEC at a single time point. In contrast, our approach involved collecting data at multiple time points before NEC onset, which enhances the credibility of the study and reduces errors.
This study found that the combined indicators of “antenatal steroids, antenatal antibiotics, probiotics treatment, AG day 7, MCV day 7” demonstrated good predictive accuracy for NEC through RM-ANOVA, univariate, and multivariate logistic regression analyses.
Before our study, many researchers had tried to identify potential predictors for NEC. Lin et al., in a retrospective study spanning 8 years, identified acidosis as an important predictor in the prediction model of NEC with the presence of portal venous gas (PVG) (12). Early correction of acidosis may reduce the risk of NEC. Additionally, Chen et al., in a study conducted in 2023, suggested that elevated lactate levels may indicate rapid progression of NEC (13). Our study also suggests that higher AG values are associated with NEC. In a multi-center retrospective study by Kordasz et al., severe anemia was identified as an important indicator for predicting NEC-related mortality risk (14). While this study did not incorporate hemoglobin as a predictive factor, possibly due to the small sample size of a single-center, the elevated MCV levels on the 7th day after birth were found to be correlated with NEC, indirectly suggesting a potential relationship between anemia and NEC. Arciero et al. established a predictive model for NEC through mathematical modeling and found that early probiotic administration was beneficial for preterm infants, reducing the incidence of critical illnesses (15). Similarly, the nomogram in our study corroborated this finding. The relationship between antenatal steroid administration and NEC remains unclear. Study has suggested a reduction in NEC mortality rates with antenatal steroid administration in predictive models (16), but our model suggests that antenatal steroid administration may increase the risk of NEC. Further prospective studies are needed to validate this finding. Below, we will explain how potential predictors are linked to NEC.
Antenatal steroids
In clinical practice, doctors often administer steroid hormones during pregnancy to expedite lung development in babies, especially in cases where premature delivery is anticipated (17). Many studies suggest that administering hormones before birth reduces the incidence of NEC, possibly by alleviating inflammation in the gut (18), aiding in gut development, and affecting the types of bacteria that live in the intestines (19). However, a study by Anthony Walters pointed out that the available evidence doesn’t definitively determine whether using prenatal steroids is beneficial or harmful for preventing NEC (RR: 0.84, 95% CI: 0.59 to 1.22; 5,736 infants) (20). Therefore, more prospective studies are needed to confirm the relationship between prenatal steroid hormone use and NEC.
PROM
PROM refers to the spontaneous rupture of fetal, amniotic, and chorionic membranes in pregnant before delivery (21). Infection can be both a cause and consequence of PROM. The potential mechanism linking infection and PROM involves the invasion of bacteria into the uterus, which prompts the decidua and fetal membranes to produce pro-inflammatory cytokines. Consequently, this results in the release of prostaglandins, metalloproteases, and other bioactive substances. Prostaglandins stimulate uterine contractions, while metalloproteases aid in softening the cervix and targeting the membranes, ultimately leading to rupture (22).
In neonates born to mothers with PROM, NEC may be associated with an exaggerated inflammatory response involving the release of cytokines and chemokines, which can cause intestinal mucosal injury. A prospective case-control study has demonstrated elevated levels of IL-6, IL-10, and ENA-78 in the venous blood of newborns from mothers with PROM compared to normal controls (23).
Antenatal antibiotics
To date, the pathogenesis of NEC is considered multi-factorial, with the disturbance of normal intestinal flora and overgrowth of potentially pathogenic bacteria implicated in its development. The maternal microbiota serves as the initial microbial inoculum for the infant gut, and perinatal factors such as diet and antibiotic use during pregnancy, as well as neonatal factors like intra-partum antibiotics, GA, and mode of delivery, can influence microbial colonization (24).
In preterm infants, antibiotic administration may contribute to microecological dysregulation. Study has indicated that antibiotic treatment within the first 14 days of life or continued for more than five days is associated with an increased risk of NEC or mortality (25). Unfortunately, the effects of early empiric antibiotic therapy on intestinal flora and its impact on the risk of NEC remain unclear. Large-sample multicenter studies are necessary to explore the relationship with NEC.
PS
PS is commonly administered to address respiratory distress syndrome (RDS), effectively reducing alveolar surface tension and enhancing lung compliance and gas exchange (26). Research indicates a correlation between NEC and compromised mesenteric perfusion and hypoxia (27). The use of PS not only enhances pulmonary ventilation but also facilitates improved mesenteric circulation and oxygen delivery, consequently lowering the incidence of NEC.
Pre-morbidity use of probiotics
Preterm infants, being particularly susceptible to intestinal dysbiosis, face the risk of aberrant microbial colonization. The initial microbiome of newborns is typically dominated by Bifidobacterium species, but various exogenous factors such as delivery method, formula feeding, and antibiotic exposure can disrupt this balance (28).
In a mouse model investigating epithelial barrier function, Abdulqadir et al. observed that enteral administration of the probiotic Lactobacillus rhamnosus GG led to enhanced expression of tight junction protein (claudin 3), resulting in reduced intestinal permeability persisting for up to 3 weeks post-supplementation, as evidenced by serum FD4 measurements (29). Beyond fortifying the structural integrity of the intestinal barrier, probiotics have shown the capacity to augment microbiome diversity with beneficial bacteria and diminish colonization by enteric pathogens (30).
Blood transfusion
To date, the relationship between blood transfusions and NEC has been investigated in various studies, yielding conflicting results (31). Proposed pathogenetic pathways include immunologic dysfunction, the direct impact of blood product storage, and reperfusion injury (32). Conversely, some studies found no conclusive association between transfusions and NEC (33), while others have even proposed a potential protective effect of transfusions against NEC (34). A more in-depth investigation is warranted to elucidate these associations.
AG
Building on previous research investigating risk factors for NEC, our study examined the correlation between serum AG (SAG) levels and NEC simultaneously. SAG is derived by subtracting the concentration of serum chloride ions and bicarbonate ions from the concentration of serum sodium ions (35). Elevated SAG levels are typically associated with metabolic acidosis, which may stem from factors such as excessive acid production, reduced excretion, laboratory errors, severe volume depletion (hypoproteinemia), metabolic alkalosis, respiratory alkalosis, severe hyperphosphatemia, and increased levels of anionic polymeric proteins (36). Although SAG can be calculated using serum or plasma electrolytes, we primarily focused on serum values in our study while recognizing that both serum and plasma AG values are acceptable.
The AG plays a crucial role in distinguishing the etiology of metabolic acidosis, categorizing it into hyperchloremic acidosis with a standard AG and normochloremic metabolic acidosis with an increased AG. Common causes of high AG metabolic acidosis include conditions such as renal failure, diabetic ketoacidosis, and lactic acidosis (37), with a standard AG typically falling within the range of 8 to 12 mmol/L (38). While existing literature extensively covers AG in various contexts, its association with NEC has received limited attention. This study found that elevated early AG values indicate an increased risk of NEC onset.
Lymphocyte count
In recent years, there has been a growing focus on T-cell subsets and their role in NEC. A novel population of cells within the innate immune system, known as innate lymphoid cells (ILCs), has garnered increased attention for their potential contribution to immunity. ILCs are capable of producing cytokines such as INF-γ and TNF, which play a crucial role in safeguarding the intestinal epithelium against invasive viruses, bacteria, or other intracellular microorganisms (39). Earlier studies have demonstrated a reduction in the number of regulatory T cells (Tregs), which serve as a key source of anti-inflammatory cytokines, in the ileum of NEC-afflicted rats and human infants compared to their healthy counterparts (40). This suggests that, although Tregs are present in the intestines, their quantity may not be sufficient to mitigate the excessive inflammatory state observed in NEC (41). In our study, we observed lymphocyte counts on the day of NEC diagnosis were notably lower in the NEC group compared to the control group.
MCV, RDW, RPR, eosinophil count
The pathogenesis of NEC is intricately linked to intestinal hypoxia-ischemia. Anemia is considered a potential etiological factor, contributing to altered mesenteric blood flow and, ultimately, compromised tissue perfusion and intestinal injury (42). With anemia diminishing the oxygen-carrying capacity of blood below the requirements of developing tissues, it may intensify anaerobic metabolism and the generation of byproducts like lactic acid. Gutierrez et al. have demonstrated that in the context of tissue hypoxia, the imbalance between cellular adenosine triphosphate (ATP) requirements and aerobic ATP production is partially compensated by anaerobic sources, including glycolysis, the creatine kinase reaction, and the adenylate kinase reaction (43). However, these processes may trigger cellular mechanisms that contribute to cellular dysfunction and, ultimately, cell death. This could potentially serve as a causal factor for the onset of NEC in preterm infants (44).
Although previous studies have explored the association between RPR and NEC (45), our study found differences between the groups only on the seventh day postnatal. Watanabe et al. demonstrated a significant increase in eosinophil counts at two weeks of age among infants with NEC (46). However, our study revealed lower eosinophil counts in the NEC group compared to the control group on the day of NEC diagnosis. Hence, further prospective studies are necessary to confirm the association between eosinophil count and NEC.
Limitations
As a single-center retrospective study, this research may contain some selection bias. The sample size is limited due to the relatively low incidence of stage II/III NEC in premature infants, which constrains the scope of our findings. Future investigations should aim to increase the number of cases or conduct prospective studies to further elucidate these findings.
Conclusions
NEC is a severe inflammatory intestinal disease in preterm infants, characterized by significant morbidity and mortality. Our study suggested that antenatal steroids, antenatal antibiotics, probiotics treatment, AG day 7, and MCV day 7 were the key factors for NEC. Based on our research, we conclude that the following measures might reduce the rate of NEC: (I) optimizing antenatal care: ensure judicious use of antenatal steroids and antibiotics to reduce the risk of NEC; (II) enhancing probiotic administration: implement standardized protocols for probiotic treatment before NEC onset; Monitor the administration and effectiveness of probiotics to ensure optimal outcomes; (III) monitoring AG and MCV levels: raise awareness among healthcare professionals about the importance of early elevated AG values as potential indicators of NEC; dynamically monitor AG and MCV levels for the early identification of NEC patients.
In conclusion, the risk estimation nomogram for NEC offers clinical value by guiding early prediction, targeted prevention, and early intervention strategies for NEC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-15/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-15/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 2023-14-25) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masi AC, Stewart CJ. The role of the preterm intestinal microbiome in sepsis and necrotising enterocolitis. Early Hum Dev 2019;138:104854. [Crossref] [PubMed]
- Zhang Y, Yan M, Xia Y, et al. Glutaredoxin-1 modulates the NF-κB signaling pathway to activate inducible nitric oxide synthase in experimental necrotizing enterocolitis. Mol Ther Methods Clin Dev 2024;32:101214. [Crossref] [PubMed]
- Battersby C, Santhalingam T, Costeloe K, et al. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103:F182-9. [Crossref] [PubMed]
- Liu S, Liu Y, Lai S, et al. Values of serum intestinal fatty acid-binding protein, fecal calprotectin, and fecal human β-defensin 2 for predicting necrotizing enterocolitis. BMC Pediatr 2024;24:183. [Crossref] [PubMed]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. [Crossref] [PubMed]
- Eaton S, Rees CM, Hall NJ. Current Research on the Epidemiology, Pathogenesis, and Management of Necrotizing Enterocolitis. Neonatology 2017;111:423-30. [Crossref] [PubMed]
- Xiong T, Maheshwari A, Neu J, et al. An Overview of Systematic Reviews of Randomized-Controlled Trials for Preventing Necrotizing Enterocolitis in Preterm Infants. Neonatology 2020;117:46-56. [Crossref] [PubMed]
- Lin H, Mao S, Shi L, et al. Clinical characteristic comparison of low birth weight and very low birth weight preterm infants with neonatal necrotizing enterocolitis: a single tertiary center experience from eastern China. Pediatr Surg Int 2018;34:1201-7. [Crossref] [PubMed]
- Rose AT, Saroha V, Patel RM. Transfusion-related Gut Injury and Necrotizing Enterocolitis. Clin Perinatol 2020;47:399-412. [Crossref] [PubMed]
- Huang P, Luo N, Shi X, et al. Risk factor analysis and nomogram prediction model construction for NEC complicated by intestinal perforation. BMC Pediatr 2024;24:143. [Crossref] [PubMed]
- Alganabi M, Lee C, Bindi E, et al. Recent advances in understanding necrotizing enterocolitis. F1000Res 2019;8:F1000 Faculty Rev-107.
- Lin X, Zeng HP, Fang YF, et al. Predictive Indicators for Necrotizing Enterocolitis With the Presence of Portal Venous Gas and Outcomes of Surgical Interventions. Front Pediatr 2021;9:683510. [Crossref] [PubMed]
- Chen J, Zhong W, Hou L, et al. Predictive factors for rapid progression in preterm neonates with necrotizing enterocolitis. Front Pediatr 2022;10:970998. [Crossref] [PubMed]
- Kordasz M, Racine M, Szavay P, et al. Risk factors for mortality in preterm infants with necrotizing enterocolitis: a retrospective multicenter analysis. Eur J Pediatr 2022;181:933-9. [Crossref] [PubMed]
- Arciero JC, Ermentrout GB, Upperman JS, et al. Using a mathematical model to analyze the role of probiotics and inflammation in necrotizing enterocolitis. PLoS One 2010;5:e10066. [Crossref] [PubMed]
- Garg PM, Bernieh A, Hitt MM, et al. Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis. Pediatr Res 2021;89:163-70. [Crossref] [PubMed]
- Roberts D, Brown J, Medley N, et al. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;3:CD004454. [PubMed]
- Rautava S, Walker WA, Lu L. Hydrocortisone-induced anti-inflammatory effects in immature human enterocytes depend on the timing of exposure. Am J Physiol Gastrointest Liver Physiol 2016;310:G920-9. [Crossref] [PubMed]
- Chawanpaiboon S, Chukaew R, Pooliam J. A comparison of 2 doses of antenatal dexamethasone for the prevention of respiratory distress syndrome: an open-label, noninferiority, pragmatic randomized trial. Am J Obstet Gynecol 2024;230:260.e1-260.e19. [Crossref] [PubMed]
- Walters A, McKinlay C, Middleton P, et al. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev 2022;4:CD003935. [PubMed]
- Liang Y, Li M, Lyu Q, et al. The relationship between maternal exposure to ambient air pollutants and premature rupture of membranes: A systematic review and meta-analysis. Environ Pollut 2024;347:123611. [Crossref] [PubMed]
- Li MD, Lu JW, Zhang F, et al. ADAMTS4 is a crucial proteolytic enzyme for versican cleavage in the amnion at parturition. Commun Biol 2024;7:301. [Crossref] [PubMed]
- Zaharie GC, Drugan T, Crivii C, et al. Postpartum assessment of fetal inflammatory response syndrome in a preterm population with premature rupture of membranes: A Romanian study. Exp Ther Med 2021;22:1427. [Crossref] [PubMed]
- Suárez-Martínez C, Santaella-Pascual M, Yagüe-Guirao G, et al. Infant gut microbiota colonization: influence of prenatal and postnatal factors, focusing on diet. Front Microbiol 2023;14:1236254. [Crossref] [PubMed]
- Rina P, Zeng Y, Ying J, et al. Association of initial empirical antibiotic therapy with increased risk of necrotizing enterocolitis. Eur J Pediatr 2020;179:1047-56. [Crossref] [PubMed]
- Yi Z, Tan Y, Liu Y, et al. A systematic review and meta-analysis of pulmonary surfactant combined with budesonide in the treatment of neonatal respiratory distress syndrome. Transl Pediatr 2022;11:526-36. [Crossref] [PubMed]
- Bubberman JM, van Zoonen A, Bruggink JLM, et al. Necrotizing Enterocolitis Associated with Congenital Heart Disease: a Different Entity? J Pediatr Surg 2019;54:1755-60. [Crossref] [PubMed]
- Nolan LS, Rimer JM, Good M. The Role of Human Milk Oligosaccharides and Probiotics on the Neonatal Microbiome and Risk of Necrotizing Enterocolitis: A Narrative Review. Nutrients 2020;12:3052. [Crossref] [PubMed]
- Abdulqadir R, Engers J, Al-Sadi R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr Dev Nutr 2023;7:102026. [Crossref] [PubMed]
- Underwood MA, Kalanetra KM, Bokulich NA, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr 2013;163:1585-1591.e9. [Crossref] [PubMed]
- Howarth C, Banerjee J, Aladangady N. Red Blood Cell Transfusion in Preterm Infants: Current Evidence and Controversies. Neonatology 2018;114:7-16. [Crossref] [PubMed]
- Salem A, Patel RM. Red Blood Cell Transfusion, Anemia, Feeding, and the Risk of Necrotizing Enterocolitis. Clin Perinatol 2023;50:669-81. [Crossref] [PubMed]
- Wallenstein MB, Arain YH, Birnie KL, et al. Red blood cell transfusion is not associated with necrotizing enterocolitis: a review of consecutive transfusions in a tertiary neonatal intensive care unit. J Pediatr 2014;165:678-82. [Crossref] [PubMed]
- Sood BG, Rambhatla A, Thomas R, et al. Decreased hazard of necrotizing enterocolitis in preterm neonates receiving red cell transfusions. J Matern Fetal Neonatal Med 2016;29:737-44. [Crossref] [PubMed]
- Fenves AZ, Emmett M. Approach to Patients With High Anion Gap Metabolic Acidosis: Core Curriculum 2021. Am J Kidney Dis 2021;78:590-600. [Crossref] [PubMed]
- Haber LA, Dhaliwal G, Lo L, et al. Evaluating a low anion gap: A practical approach. Cleve Clin J Med 2023;90:619-23. [Crossref] [PubMed]
- Funes S, de Morais HA. A Quick Reference on High Anion Gap Metabolic Acidosis. Vet Clin North Am Small Anim Pract 2017;47:205-7. [Crossref] [PubMed]
- Mara MA, Good M, Weitkamp JH. Innate and adaptive immunity in necrotizing enterocolitis. Semin Fetal Neonatal Med 2018;23:394-9. [Crossref] [PubMed]
- Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015;517:293-301. [Crossref] [PubMed]
- Liu Y, Fatheree NY, Dingle BM, et al. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One 2013;8:e56547. [Crossref] [PubMed]
- Weitkamp JH, Koyama T, Rock MT, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 2013;62:73-82. [Crossref] [PubMed]
- Crabtree CS, Pakvasa M, Radmacher PG, et al. Retrospective case-control study of necrotizing enterocolitis and packed red blood cell transfusions in very low birth weight infants. J Neonatal Perinatal Med 2018;11:365-70. [Crossref] [PubMed]
- Gutierrez MW, Arrieta MC. The intestinal mycobiome as a determinant of host immune and metabolic health. Curr Opin Microbiol 2021;62:8-13. [Crossref] [PubMed]
- Singh R, Shah BL, Frantz ID 3rd. Necrotizing enterocolitis and the role of anemia of prematurity. Semin Perinatol 2012;36:277-82. [Crossref] [PubMed]
- Kasirer Y, Shchors I, Hammerman C, et al. Platelet Indices: Universally Available Clinical Adjunct for Diagnosing Necrotizing Enterocolitis. Am J Perinatol 2023; Epub ahead of print. [Crossref] [PubMed]
- Watanabe H, Washio Y, Tamai K, et al. Postnatal longitudinal analysis of serum Nitric oxide and eosinophil counts in extremely preterm infants. Pediatr Neonatol 2023; Epub ahead of print. [Crossref] [PubMed]


